Degradation of Fatty Acid Phase-Change Materials (PCM): New Approach for Its Characterization
Abstract
:1. Introduction
2. Results
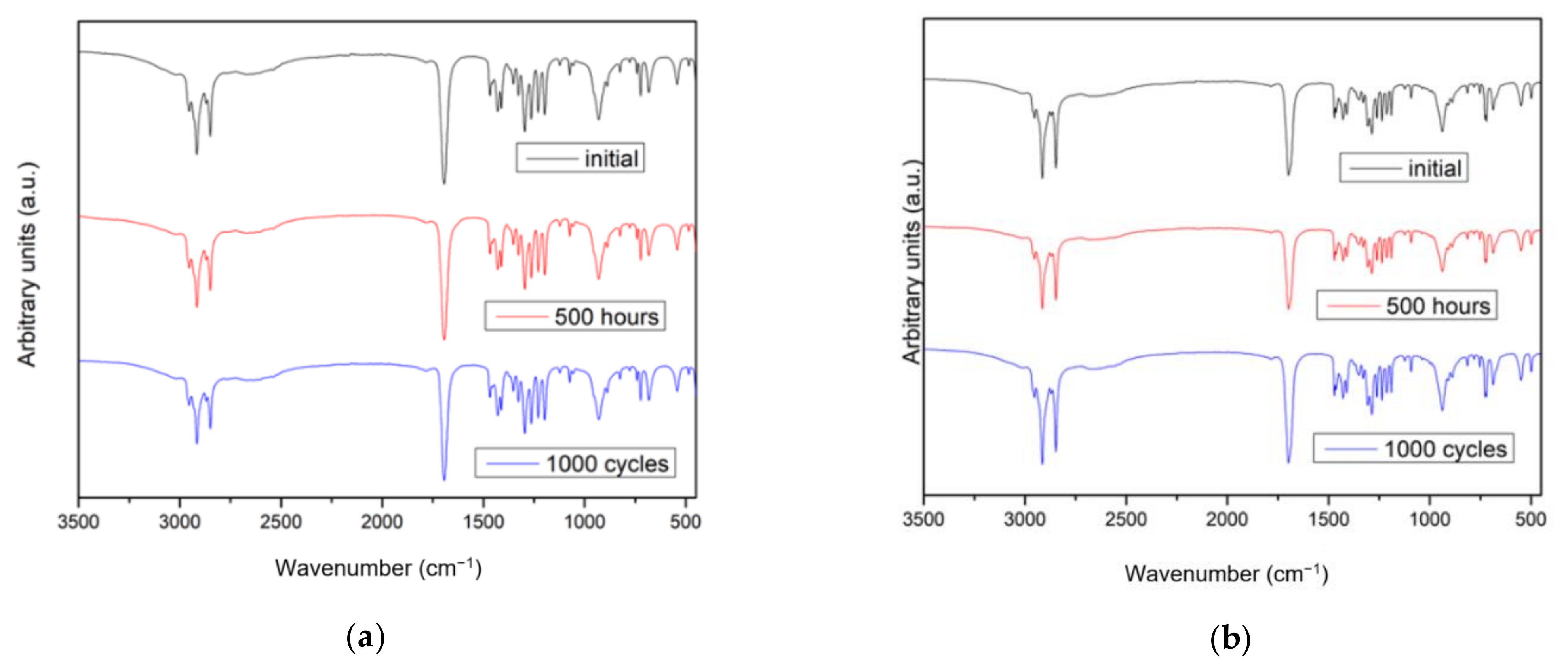
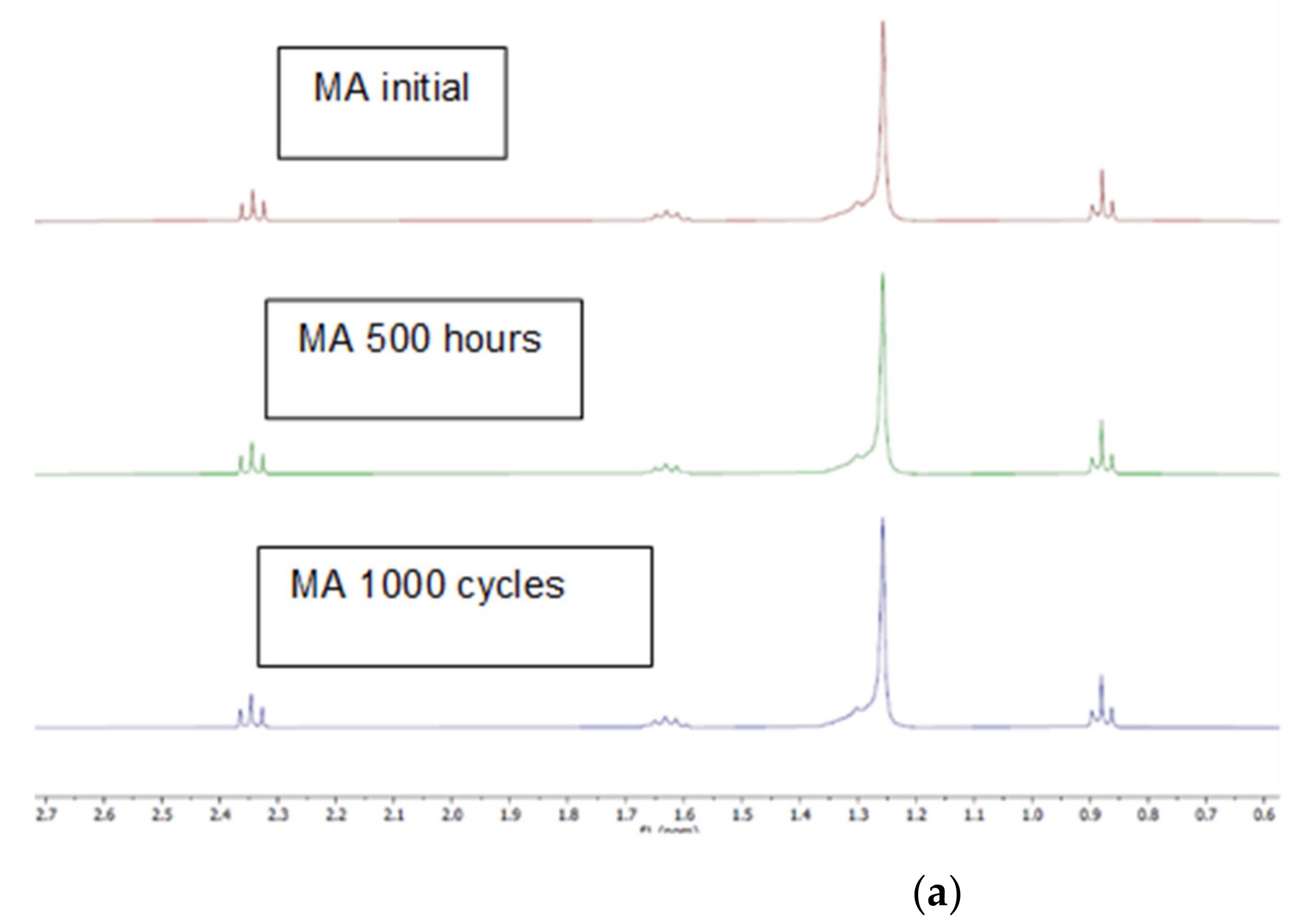
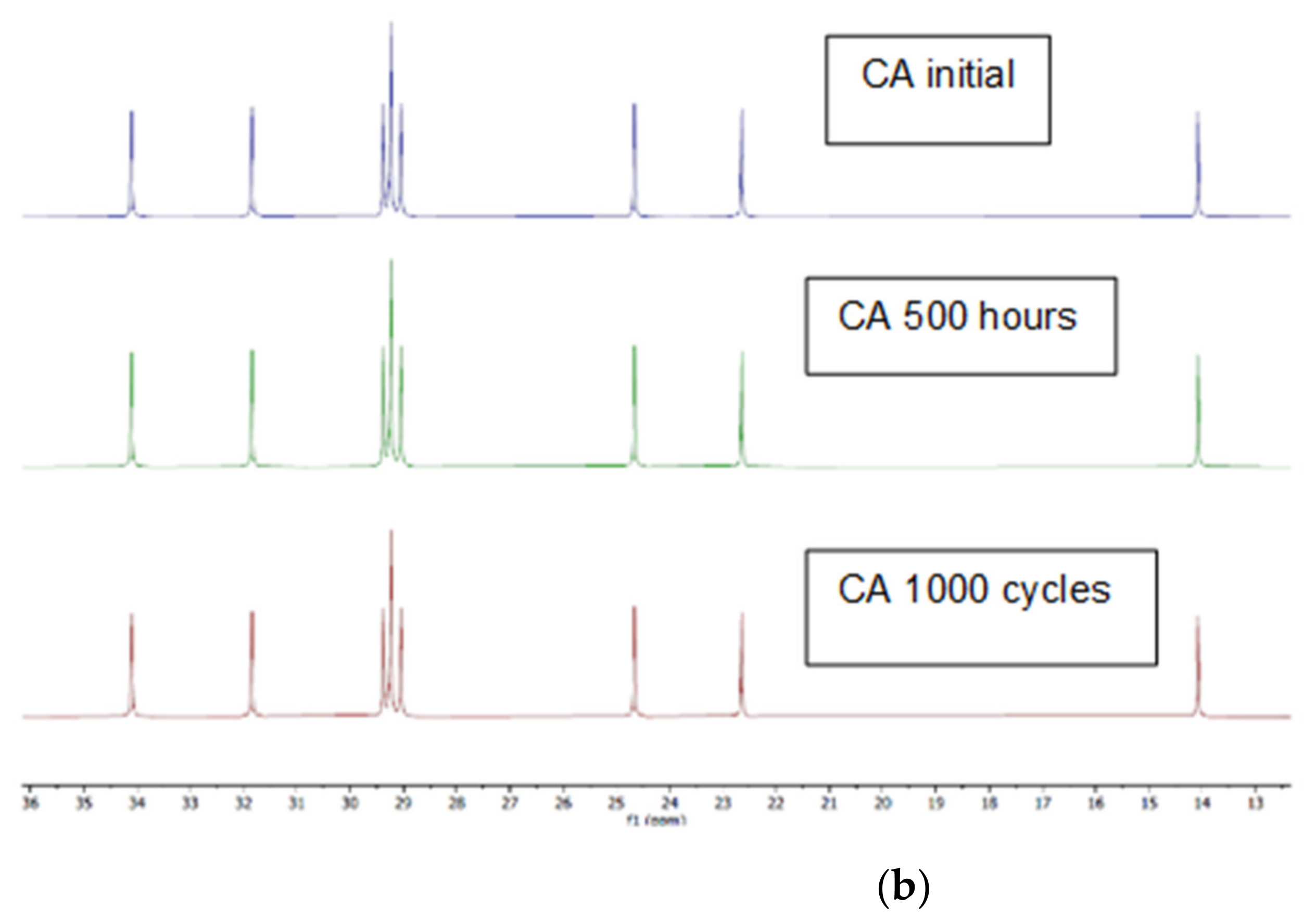
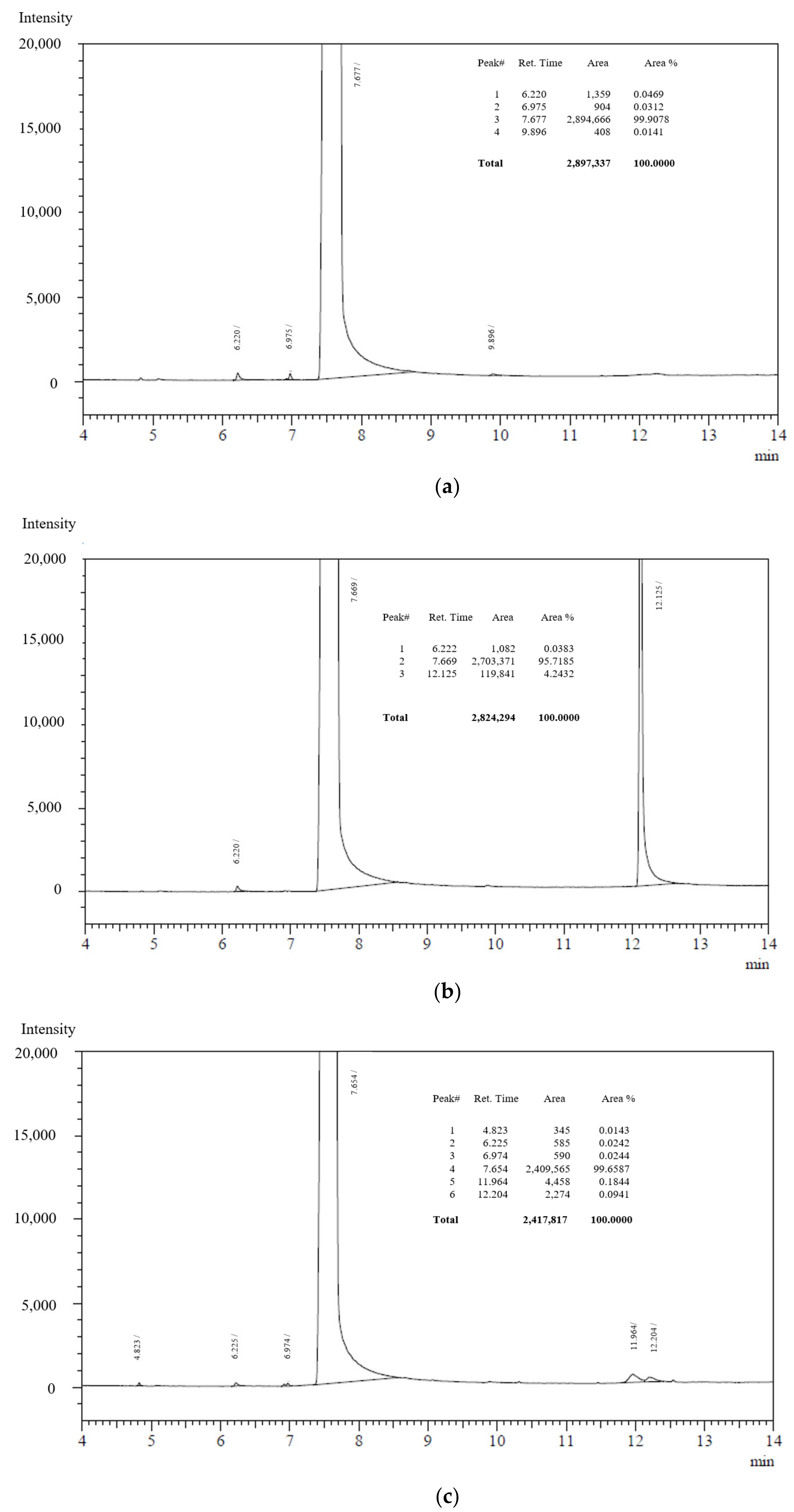
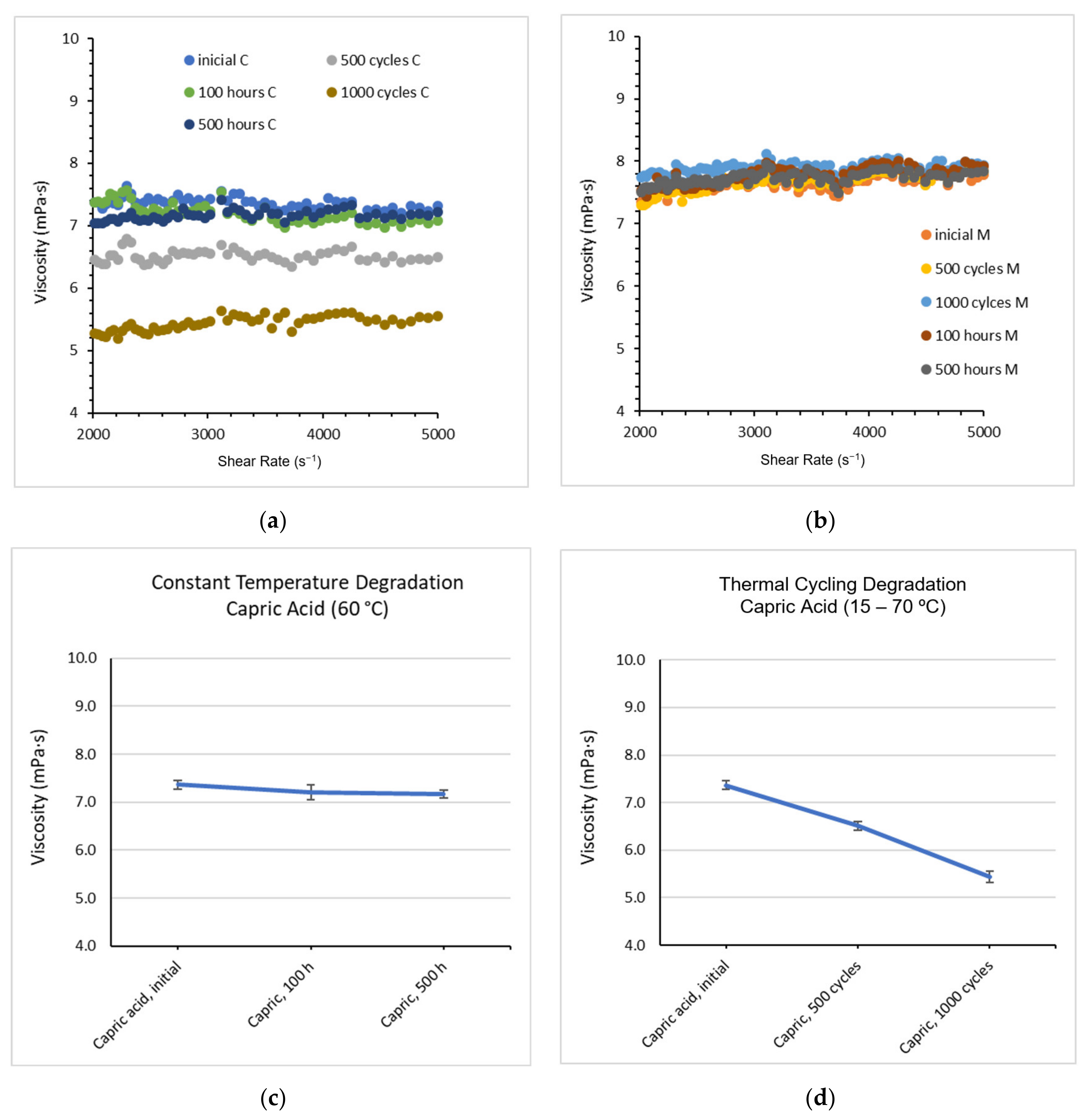
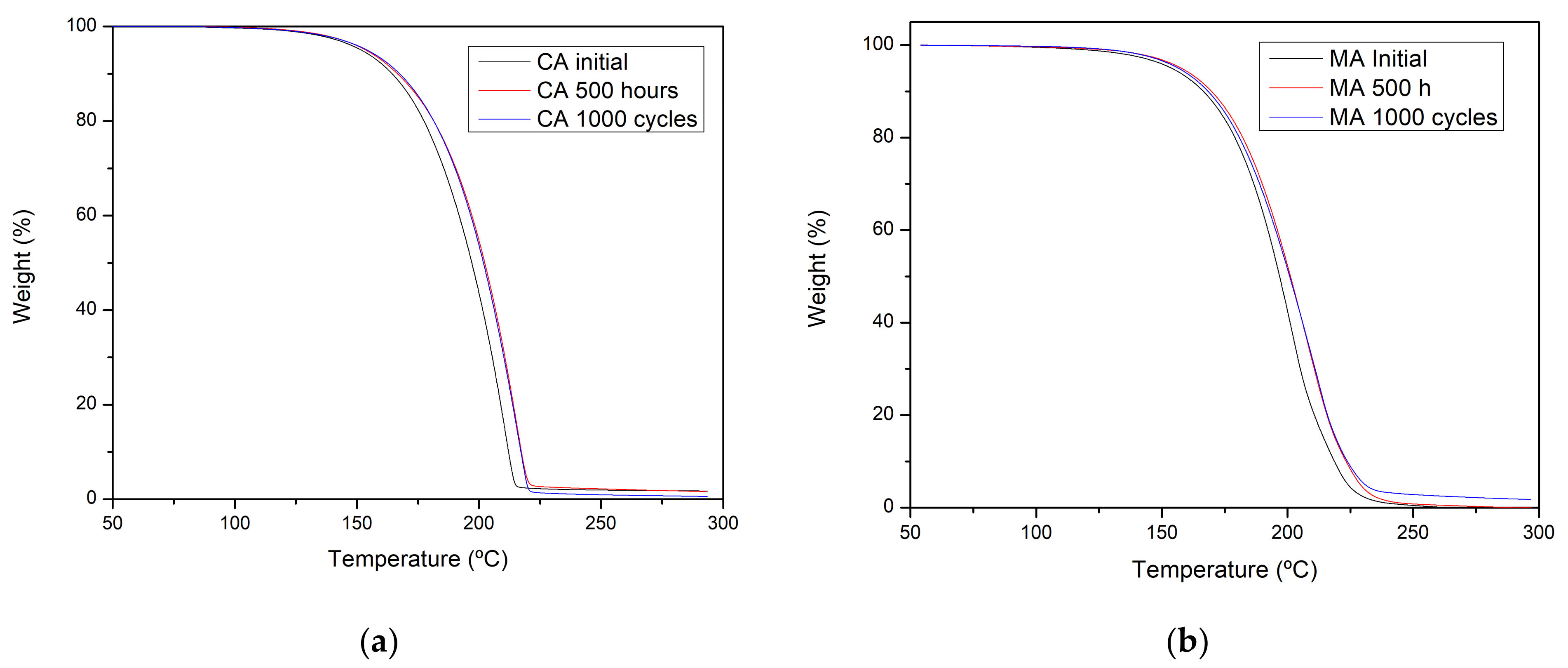
2.1. Changes in Chemical Composition or Chemical Structure
2.2. Latent Heat Stored
3. Materials and Methods
3.1. Materials and Thermal Treatments
3.2. Fatty Acid Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- International Energy Agency (IEA). CO2 emissions from fuel combustion. Available online: https://webstore.iea.org/download/tableofcontents/5 (accessed on 2 February 2021).
- International Energy Agency (IEA). Total Energy Consumption 2020. Available online: https://yearbook.enerdata.net/total-energy/world-consumption-statistics.html (accessed on 2 February 2021).
- Diarce, G.; Campos-Celador, Á.; Martin, K.; Urresti, A.; García-Romero, A.; Sala, J. A comparative study of the CFD modeling of a ventilated active façade including phase change materials. Appl. Energy 2014, 126, 307–317. [Google Scholar] [CrossRef]
- Navarro, L.; De Gracia, A.; Colclough, S.; Browne, M.; McCormack, S.J.; Griffiths, P.; Cabeza, L.F. Thermal energy storage in building integrated thermal systems: A review. Part 1. active storage systems. Renew. Energy 2016, 88, 526–547. [Google Scholar] [CrossRef] [Green Version]
- Cabeza, L.F.; Castell, A.; Barrenechea, C.; de Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- De Gracia, A.; Cabeza, L.F. Phase change materials and thermal energy storage for buildings. Energy Build. 2015, 103, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Chen, Y. Improving the energy discharging performance of a latent heat storage (LHS) unit using fractal-tree-shaped fins. Appl. Energy 2020, 259, 114102. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Lohrasbi, S.; Ganji, D.D. Numerical analysis of discharging process acceleration in LHTESS by immersing innovative fin configuration using finite element method. Appl. Therm. Eng. 2016, 107, 154–166. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, Y.; Cheng-Bin, Z.; Huang, Y.; Chen, Y.-P. Melting behaviors of PCM in porous metal foam characterized by fractal geometry. Int. J. Heat Mass Transf. 2017, 113, 1031–1042. [Google Scholar] [CrossRef]
- Barreneche, C.; Martín, M.; La Rosa, J.C.-D.; Majó, M.; Fernandez, A. Own-Synthetize Nanoparticles to Develop Nano-Enhanced Phase Change Materials (NEPCM) to Improve the Energy Efficiency in Buildings. Molecules 2019, 24, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaipekli, A.; San, A. Capric–myristic acid/vermiculite composite as form-stable phase change material for thermal energy storage. Sol. Energy 2009, 83, 323–332. [Google Scholar] [CrossRef]
- Sari, A.; San, H.; Onal, A.; Sari, A. Thermal properties and thermal reliability of eutectic mixtures of some fatty acids as latent heat storage materials. Energy Convers. Manag. 2004, 45, 365–376. [Google Scholar] [CrossRef]
- Acree, W.; Chickos, J.S. Phase Transition Enthalpy Measurements of Organic and Organometallic Compounds. Sublimation, Vaporization and Fusion Enthalpies From 1880 to 2010. J. Phys. Chem. Ref. Data 2010, 39, 043101. [Google Scholar] [CrossRef]
- Lazaro, A.; Peñalosa, C.; Solé, A.; Serras, P.; Haussmann, T.; Fois, M.; Zalba, B.; Gschwander, S.; Cabeza, L.F. Intercomparative tests on phase change materials characterisation with differential scanning calorimeter. Appl. Energy 2013, 109, 415–420. [Google Scholar] [CrossRef]
- Barreneche, C.; Solé, A.; Miró, L.; Martorell, I.; Fernandez, A.; Cabeza, L.F. Study on differential scanning calorimetry analysis with two operation modes and organic and inorganic phase change material (PCM). Thermochim. Acta 2013, 553, 23–26. [Google Scholar] [CrossRef]

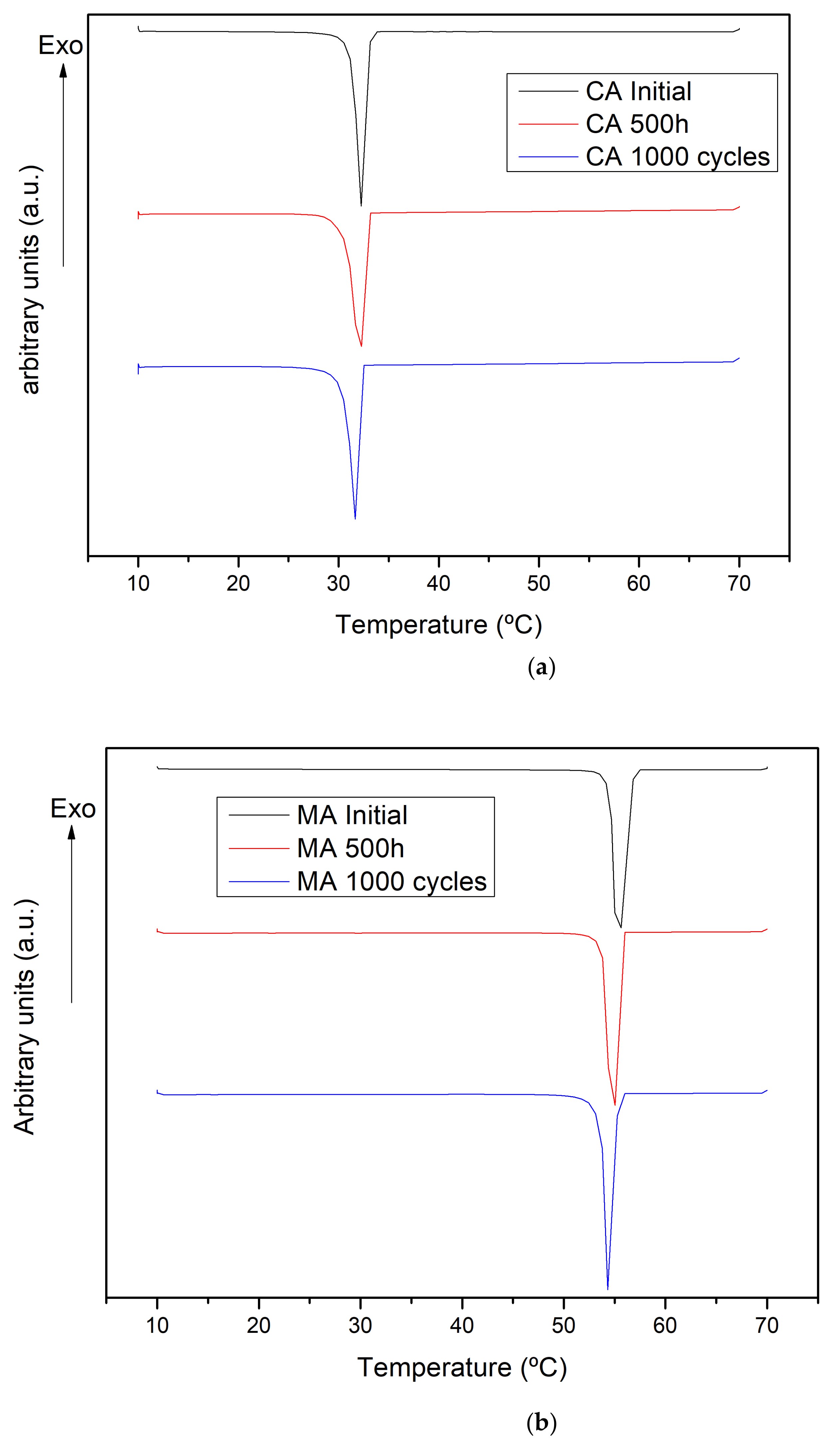
| Sample | N° Hours/Cycles | ΔHm [J·g−1] | ΔHm [%] | Tm [°C] | Tm [°C] |
|---|---|---|---|---|---|
| Capric acid | Initial | 159 ± 1 | 32.3 ± 0.0 | ||
| 500 h | 149 ± 1 | −7 | 32.1 ± 0.0 | −0.19 | |
| 1000 cycles | 153 ± 1 | −4 | 31.8 ± 0.0 | −0.46 | |
| Myristic acid | Initial | 190 ± 1 | 55.3 ± 0.0 | ||
| 500 h | 182 ± 1 | −4 | 54.6 ± 0.0 | −0.66 | |
| 1000 cycles | 184 ± 1 | −3 | 54.6 ± 0.0 | −0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majó, M.; Sánchez, R.; Barcelona, P.; García, J.; Fernández, A.I.; Barreneche, C. Degradation of Fatty Acid Phase-Change Materials (PCM): New Approach for Its Characterization. Molecules 2021, 26, 982. https://doi.org/10.3390/molecules26040982
Majó M, Sánchez R, Barcelona P, García J, Fernández AI, Barreneche C. Degradation of Fatty Acid Phase-Change Materials (PCM): New Approach for Its Characterization. Molecules. 2021; 26(4):982. https://doi.org/10.3390/molecules26040982
Chicago/Turabian StyleMajó, Marc, Ricard Sánchez, Pol Barcelona, Jordi García, Ana Inés Fernández, and Camila Barreneche. 2021. "Degradation of Fatty Acid Phase-Change Materials (PCM): New Approach for Its Characterization" Molecules 26, no. 4: 982. https://doi.org/10.3390/molecules26040982






