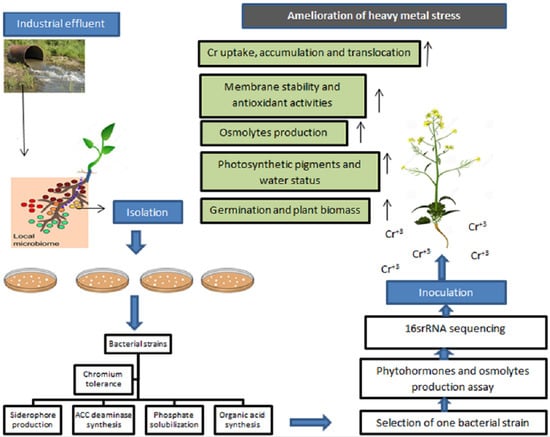
Role of Bacillus cereus in Improving the Growth and Phytoextractability of Brassica nigra (L.) K. Koch in Chromium Contaminated Soil
Abstract
:1. Introduction
2. Results
2.1. Isolation, Heavy Metal Tolerance, and Plant Growth-Promoting Characteristics of Bacterial Strains
2.2. Phytohormones and Osmolyte Production by B. cereus in the Presence of Chromium Stress
2.3. Selection and Identification of Bacterial Strain by 16S rRNA Sequencing
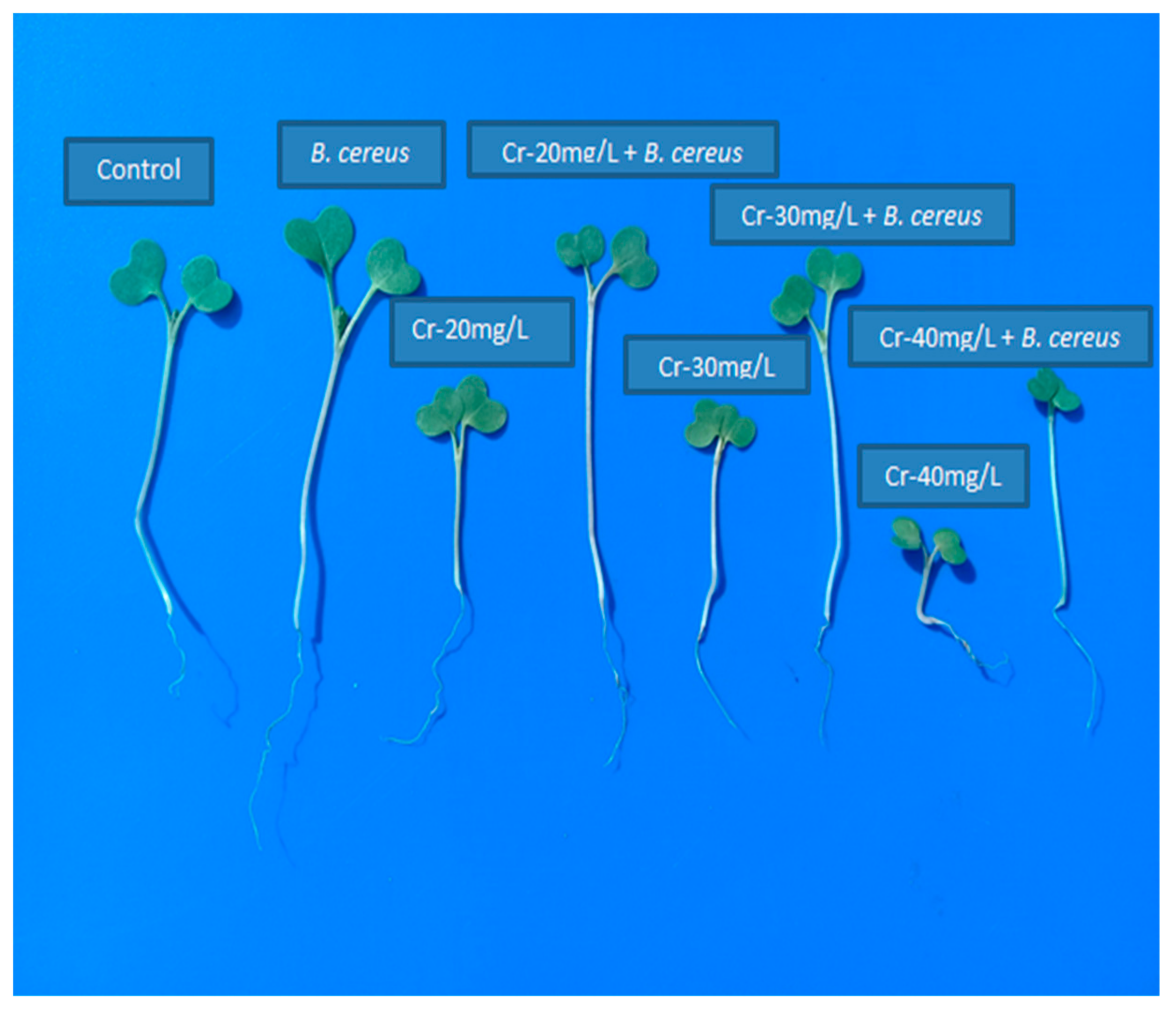
2.4. Role of B. cereus on Germination of B. nigra
2.5. Role of B. cereus on Plant Growth and Biomass Production
2.6. Role of B. cereus on the Content of Photosynthetic Pigments of Plant
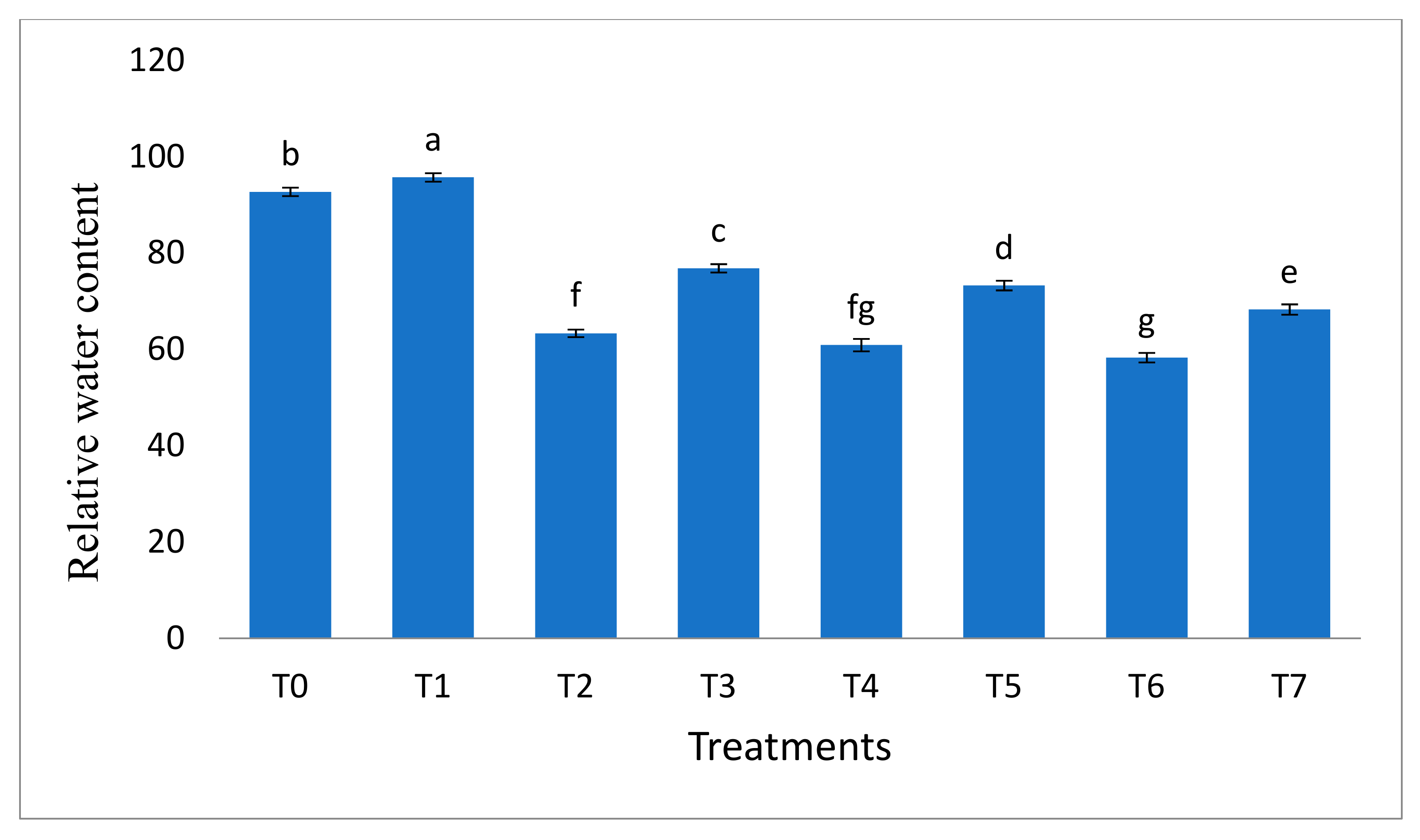
2.7. Role of B. cereus on the Water Status of Plant
2.8. Role of B. cereus on Proline and Sugar Content of Plant
2.9. Role of B. cereus on Malondialdehyde Content (MDA) and Membrane Stability Index (MSI) of Plant
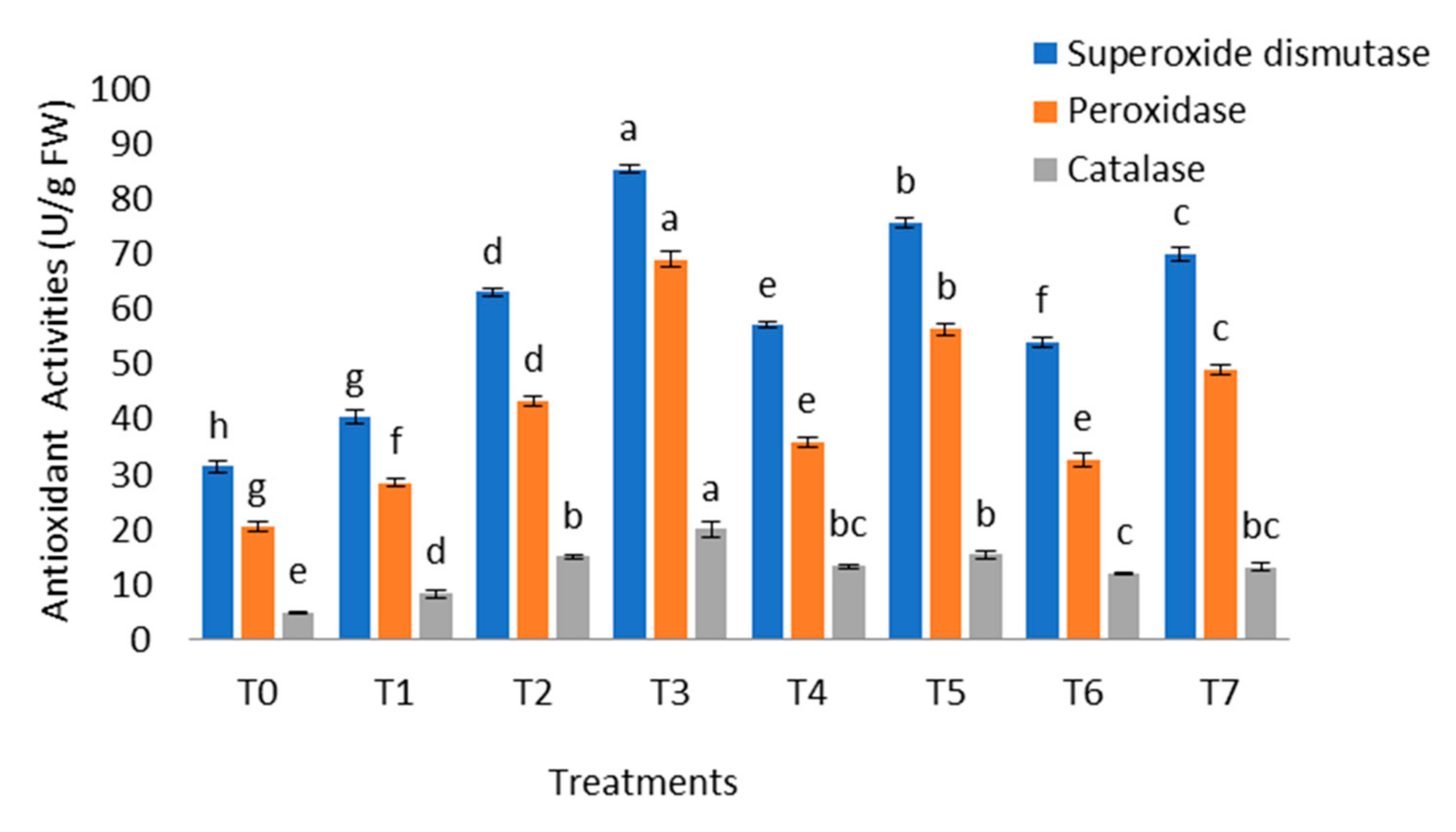
2.10. Role of B. cereus on Antioxidant Enzymes Activities of Plant
2.11. Role of B. cereus on Uptake, Bioaccumulation, and Translocation of Chromium in Plant
3. Discussion
4. Materials and Methods
4.1. Soil Sampling and Analysis
4.2. Isolation, Heavy Metal Tolerance, and Plant Growth-Promoting Characteristics of Bacterial Strains
4.3. Analysis of Phytohormones and Osmolyte Production
4.4. Identification of Bacterial Strain by 16S rRNA Sequencing
4.5. Germination Experiment
4.6. Pot Experiment
4.7. Growth Parameters
4.8. Chlorophyll and Carotenoid Content
4.9. Estimation of Relative Water Content
4.10. Estimation of Proline and Sugar Content
4.11. Malondialdehyde Content (MDA) and Membrane Stability Index (MSI)
4.12. Antioxidant Enzymes Activities
4.13. Determination of Chromium Its Bioaccumulation and Translocation
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, R.; Jha, A.B.; Misra, A.N.; Sharma, P. Adaption mechanisms in plants under heavy metal stress conditions during phytoremediation. Phytomanag. Pollut. Sites. 2019, 329–360. [Google Scholar] [CrossRef]
- Ahmad, N.; Akhtar, M.S.; Ahmed, R.; Zafar, R.; Hussain, S.; Ishaqe, M.; Naeem, M. Assessment of heavy metals in vegetables, sewage, and soil-grown near Babu Sabu Toll Plaza of Lahore, Pakistan. Pakistan J. Anal. Environ. Chem. 2019, 20, 82–87. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and, ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, B.; Rizvi, A.; Zaidi, A.; Khan, M.S.; Musarrat, J. Understanding the phyto-interaction of heavy metal oxide bulk and nanoparticles: Evaluation of seed germination, growth, bioaccumulation, and metallothionein production. RSC Adv. 2019, 9, 4210–4225. [Google Scholar] [CrossRef] [Green Version]
- Hejna, M.; Moscatelli, A.; Stroppa, N.; Onelli, E.; Pilu, S.; Baldi, A.; Rossi, L. Bioaccumulation of heavy metals from wastewater through a Typha latifolia and Thelypteris palustris phytoremediation system. Chemosphere 2020, 241, 125018. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, Z.; Asghar, H.N.; Zahir, Z.A. Comparative growth analysis of okra (Abelmoschus esculentus (L.) Moench)) in the presence of PGPR and press mud in chromium contaminated soil. Chemosphere 2020, 262, 127865. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.N. Phytohormonal Roles in Plant Responses to Heavy Metal Stress—Implications for Using Macrophytes in Phytoremediation of Aquatic Ecosystems. Environ. Toxicol. Chem. 2020, 40, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, Q. Phytoremediation of cadmium-contaminated wetland soil with Typha latifolia L. and the underlying mechanisms involved in the heavy-metal uptake and removal. Environ. Sci. Pollut. Res. 2020, 27, 4905–4916. [Google Scholar] [CrossRef] [PubMed]
- Raklami, A.; Oufdou, K.; Tahiri, A.I.; Mateos-Naranjo, E.; Navarro-Torre, S.; Rodríguez-Llorente, I.D.; Pajuelo, E. Safe cultivation of Medicago sativa in metal-polluted soils from semi-arid regions assisted by heat-and metallo-resistant PGPR. Microorganisms 2019, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Long, C.; Wang, D.; Yang, J. Phytoremediation of cadmium (Cd) and uranium (U) contaminated soils by Brassica juncea L. enhanced with exogenous application of plant growth regulators. Chemosphere 2020, 242, 125112. [Google Scholar] [CrossRef] [PubMed]
- Zaier, H.; Ghnaya, T.; Rejeb, K.B.; Lakhdar, A.; Rejeb, S.; Jemal, F. Effects of EDTA on phytoextraction of heavy metals (Zn, Mn, and Pb) from sludge-amended soil with Brassica napus L. Bioresour. Technol. 2010, 101, 3978–3983. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A review on heavy metals contamination in soil: Effects, sources, and remediation techniques. Soil Sediment Cont. Intl. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Cevher-Keskin, B.; Yıldızhan, Y.; Yüksel, B.; Dalyan, E.; Memon, A.R. Characterization of differentially expressed genes to Cu stress in B. nigra by Arabidopsis genome arrays. Environ. Sci. Pollut. Res. 2019, 26, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; Lassueur, S.; Ponzio, C.; Gols, R.; Dicke, M.; Reymond, P. Combined biotic stresses trigger similar transcriptomic responses but contrasting resistance against a chewing herbivore in Brassica nigra. BMC Plant Biol. 2017, 17, 1–14. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Khan, N.; Zandi, P.; Ali, S.; Mehmood, A.; Adnan Shahid, M.; Yang, J. Impact of salicylic acid and PGPR on the drought tolerance and phytoremediation potential of Helianthus annus L. Front. Microbial. 2018, 9, 2507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, T.U.; Bano, A.; Naz, I. Alleviation of heavy metals toxicity by the application of plant growth-promoting rhizobacteria and effects on wheat grown in the saline sodic field. Int. J. Phytoremediation 2017, 19, 522–529. [Google Scholar] [CrossRef]
- Abdelkrim, S.; Jebara, S.H.; Saadani, O.; Abid, G.; Taamalli, W.; Zemni, H.; Jebara, M. In situ effects of Lathyrus sativus-PGPR to remediate and restore quality and fertility of Pb and Cd polluted soils. Ecotoxicol. Environ. Saf. 2020, 192, 110260. [Google Scholar] [CrossRef]
- Singh, S.K.; Singh, P.P.; Gupta, A.; Singh, A.K.; Keshri, J. Tolerance of heavy metal toxicity using PGPR strains of Pseudomonas species. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 239–252. [Google Scholar] [CrossRef]
- Tang, Y.; Kang, H.; Qin, Z.; Zhang, K.; Zhong, Y.; Li, H.; Mo, L. Significance of manganese resistant Bacillus cereus strain WSE01 as a bioinoculant for promotion of plant growth and manganese accumulation in Myriophyllum verticillatum. Sci. Total Environ. 2020, 707, 135867. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Cheng, K.; Sun, K.Y.; Ma, X. Microbially Mediated Remediation of Contaminated Sediments by Heavy Metals: A Critical Review. Curr. Pollut. 2021, 1–12. [Google Scholar] [CrossRef]
- Mushtaq, M.U.; Iqbal, A.; Nawaz, I.; Mirza, C.R.; Yousaf, S.; Farooq, G.; Ali, M.A.; Khan, A.H.A.; Iqbal, M. Enhanced uptake of Cd, Cr, and Cu in Catharanthus roseus (L.) G. Don by Bacillus cereus: Application of moss and compost to reduce metal availability. Environ. Sci. Pollut. Res. 2020, 27, 39807–39818. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Kandasamy, G.; He, Z.; Kandasamy, S.; Alfarhan, A.H.; Pugazhendhi, A. Phytoextraction competence of J. curcas L. on ore waste dump of the bauxite mine under the influence of multi potential Bacillus cereus. Environ. Technol. Innov. 2021, 21, 101221. [Google Scholar] [CrossRef]
- Ke, T.; Zhang, J.; Tao, Y.; Zhang, C.; Zhang, Y.; Xu, Y.; Chen, L. Individual and combined application of Cu-tolerant Bacillus spp. enhance the Cu phytoextraction efficiency of perennial ryegrass. Chemosphere 2021, 263, 127952. [Google Scholar] [CrossRef]
- Ahemad, M. Remediation of metalliferous soils through the heavy metal resistant plant growth-promoting bacteria: Paradigms and prospects. Arab. J. Chem. 2019, 12, 1365–1377. [Google Scholar] [CrossRef] [Green Version]
- Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotoxicol. Environl. Saf. 2018, 147, 175–191. [Google Scholar] [CrossRef]
- Wang, L.; Lin, H.; Dong, Y.; Li, B.; He, Y. Effects of endophytes inoculation of rhizosphere and endosphere micrology ecology of Indian mustarad (Brassica juncea) grown in vanadium-contaminated soil and its encenement on phytoremediation. Chemosphere 2020, 124891. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.I.; Abdullah, S.R.S.; Idris, M.; Kurniawan, S.B.; Halmi, M.I.E.; Sbani, N.H.A.; Hasan, H.A. Applying rhizobacteria consortium for the enhancement of Scirpus grossus growth and phytoaccumulation of Fe and Al in pilot constructed wetlands. J. Environ. Manag. 2020, 267, 110643. [Google Scholar] [CrossRef]
- Taie, H.A.; El-Yazal, M.A.S.; Ahmed, S.M.; Rady, M.M. Polyamines modulate growth, antioxidant activity, and genomic DNA in heavy metal–stressed wheat plant. Environ. Sci. Pollut. Res. 2019, 26, 22338–22350. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.J.; Kim, K.M. Metal resistant endophytic bacteria reduces cadmium, nickel toxicity, and enhances expression of metal stress-related genes with improved growth of Oryza Sativa, via regulating its antioxidant machinery and endogenous hormones. Plants 2019, 8, 363. [Google Scholar] [CrossRef] [Green Version]
- Oladele, E.O.; Adewumi, O.O.; Yahaya, T.; Taiwo, I.A. Response of Bambara groundnut (Vigna subterranean L.) and Maize (Zea mays L.) to heavy metal stress. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Cai, M.; Hu, C.; Wang, X.; Zhao, Y.; Jia, W.; Sun, X.; Zhao, X. Selenium induces changes of rhizosphere bacterial characteristics and enzyme activities affecting chromium/selenium uptake by pak choi (Brassica campestris L. ssp. Chinensis Makino) in chromium contaminated soil. Environ. Pollut. 2019, 249, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Shreya, D.; Jinal, H.N.; Kartik, V.P.; Amaresan, N. Amelioration effect of chromium-tolerant bacteria on growth, physiological properties and chromium mobilization in chickpea (Cicer arietinum) under chromium stress. Arch. Microbiol. 2020, 202, 1–8. [Google Scholar] [CrossRef]
- Subiramani, S.; Ramalingam, S.; Muthu, T.; Nile, S.H.; Venkidasamy, B. Development of abiotic stress tolerance in crops by plant growth-promoting rhizobacteria (PGPR). In Phyto-Microbiome in Stress Regulation; Kumar, M., Kumar, V., Prasad, R., Eds.; Springer: Singapore, 2020; pp. 125–145. [Google Scholar] [CrossRef]
- Gupta, P.; Kumar, V.; Usmani, Z.; Rani, R.; Chandra, A.; Gupta, V.K. A comparative evaluation towards the potential of Klebsiella sp. and Enterobacter sp. in plant growth promotion, oxidative stress tolerance and chromium uptake in Helianthus annuus (L.). J. Hazard. Mater. 2019, 377, 391–398. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Ali, S.; Refay, Y.; Rizwan, M.; Alhammad, B.A.; El-Hendawy, S.E. Chromium resistant microbes and melatonin reduced Cr uptake and toxicity, improved physio-biochemical traits and yield of wheat in contaminated soil. Chemosphere 2020, 250, 126239. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chi, J. Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 2014, 375, 205–214. [Google Scholar] [CrossRef]
- Abdollahi, S.; Golchin, A.; Shahryari, F.; Alamdari, P. PGPR inoculation of a contaminated soil affects plant growth and phytoavailability of Cd and Pb. Arch. Agron. Soil Sci. 2020, 1–18. [Google Scholar] [CrossRef]
- Konkolewska, A.; Piechalak, A.; Ciszewska, L.; Antos-Krzemińska, N.; Skrzypczak, T.; Hanc, A.; Małecka, A. Combined use of companion planting and PGPR for the assisted phytoextraction of trace metals (Zn, Pb, Cd). Environ. Sci. Pollut. Res. 2020, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Sharma, N.L.; Singh, C.K.; Sarkar, S.K.; Singh, I.; Dotaniya, M.L. Effect of chromium (VI) toxicity on morpho-physiological characteristics, yield, and yield components of two chickpea (Cicer arietinum L.) varieties. PLoS ONE 2020, 15, e0243032. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Ahmad, P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant. 2020, 168, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Khanna, K.; Jamwal, V.L.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Reports. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Odiyi, B.O.; Giwa, G.O.; Abiya, S.E.; Babatunde, O.S. Effects of crude oil pollution on the morphology, growth and heavy metal content of maize (Zea mays Linn.). J. Appl. Sci. Environ. Manag. 2020, 24, 119–125. [Google Scholar] [CrossRef]
- Guo, J.; Muhammad, H.; Lv, X.; Wei, T.; Ren, X.; Jia, H.; Hua, L. Prospects and applications of plant growth promoting rhizobacteria to mitigate soil metal contamination: A review. Chemosphere 2020, 246, 125823. [Google Scholar] [CrossRef]
- Zafar-ul-Hye, M.; Naeem, M.; Danish, S.; Khan, M.J.; Fahad, S.; Datta, R.; El-Esawi, M.A. Effect of Cadmium-Tolerant Rhizobacteria on Growth Attributes and Chlorophyll Contents of Bitter Gourd under Cadmium Toxicity. Plants 2020, 9, 1386. [Google Scholar] [CrossRef]
- Hayat, K.; Menhas, S.; Bundschuh, J.; Zhou, P.; Niazi, N.K.; Khan, A.A. Plant growth promotion and enhanced uptake of Cd by combinatorial application of Bacillus pumilus and EDTA on Zea mays L. Int. J. Phytoremediation 2020, 22, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Rajkumar, M.; Zhang, C.; Freitas, H. Inoculation of Brassica oxyrrhina with plant growth promoting bacteria for the improvement of heavy metal phytoremediation under drought conditions. J. Hazard. Mater. 2016, 320, 36–44. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Naveed, M.; Mitter, B.; Sessitsch, A. Cadmium-tolerant bacteria induce metal stress tolerance in cereals. Environ. Sci. Pollut. Res. 2014, 21, 11054–11065. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.; Pallavi, K.P. Effect of plant growth-promoting rhizobacteria (PGPR) on growth and physiological parameters in chickpea. J. Pharmcogn. Phytochem. 2020, 9, 29–34. [Google Scholar]
- Khanna, K.; Jamwal, V.L.; Sharma, A.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Ahmad, P. Supplementation with plant growth promoting rhizobacteria (PGPR) alleviates cadmium toxicity in Solanum lycopersicum by modulating the expression of secondary metabolites. Chemosphere 2019, 230, 628–639. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Imran, K.; Tanveer, M.; Shahid, M.; Shakoor, A.; Longchang, W. Phyto-toxicity of chromium in maize: Oxidative damage, osmolyte accumulation, anti-oxidative defense and chromium uptake. Pedosphere 2017, 27, 262–273. [Google Scholar] [CrossRef]
- Singh, H.P.; Mahajan, P.; Kaur, S.; Batish, D.R.; Kohli, R.K. Chromium toxicity and tolerance in plants. Environ. Chem. Lett. 2013, 11, 229–254. [Google Scholar] [CrossRef]
- Din, B.U.; Rafique, M.; Javed, M.T.; Kamran, M.A.; Mehmood, S.; Khan, M.; Chaudhary, H.J. Assisted phytoremediation of chromium spiked soils by Sesbania Sesban in association with Bacillus xiamenensis PM14: A biochemical analysis. Plant Physiol. Biochem. 2020, 146, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Nemat, H.; Shah, A.A.; Akram, W.; Ramzan, M.; Yasin, N.A. Ameliorative effect of co-application of Bradyrhizobium japonicum EI09 and Se to mitigate chromium stress in Capsicum annum L. Int. J. Phytoremediation 2020, 22, 1396–1407. [Google Scholar] [CrossRef]
- Dash, B.; Soni, R.; Goel, R. Rhizobacteria for reducing heavy metal stress in plant and soil. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Singapore, 2019; pp. 179–203. [Google Scholar] [CrossRef]
- Kamran, M.A.; Bibi, S.; Xu, R.K.; Hussain, S.; Mehmood, K.; Chaudhary, H.J. Phyto-extraction of chromium and influence of plant growth promoting bacteria to enhance plant growth. J. Geochem. Explor. 2017, 182, 269–274. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Luo, Y.; Freitas, H. Inoculation of endophytic bacteria on host and non-host plants—effects on plant growth and Ni uptake. J. Hazard. Mater. 2011, 195, 230–237. [Google Scholar] [CrossRef]
- Chen, L.; Luo, S.; Xiao, X.; Guo, H.; Chen, J.; Wan, Y.; Liu, C. Application of plant growth-promoting endophytes (PGPE) isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Appl. Soil Ecol. 2010, 46, 383–389. [Google Scholar] [CrossRef]
- Braud, A.; Jézéquel, K.; Bazot, S.; Lebeau, T. Enhanced phytoextraction of an agricultural Cr-and Pb-contaminated soil by bioaugmentation with siderophore-producing bacteria. Chemosphere 2009, 74, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.G.; Kim, J.D.; Oh, B.T. Enhancement of heavy metal phytoremediation by Alnus firma (Siebold & Zuccarini with endophytic Bacillus thuringiensis GDB-1. J. Hazard. Mater. 2013, 250, 477–483. [Google Scholar] [CrossRef]
- Ndeddy Aka, R.J.; Babalola, O.O. Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (Cd, Cr, and Ni) uptake of Brassica juncea (L.) Czern. Int. J. Phytoremed 2016, 18, 200–209. [Google Scholar] [CrossRef]
- Sharma, P.; Tripathi, S.; Chaturvedi, P.; Chaurasia, D.; Chandra, R. Newly isolated Bacillus sp. PS-6 assisted phytoremediation of heavy metals usingphtagmites communis, Potential application in wastewater treatment. Bioresour. Technol. 2021, 320, 124353. [Google Scholar] [CrossRef] [PubMed]
- Suciu, I.; Cosma, C.; Todică, M.; Bolboacă, S.D.; Jäntschi, L. Analysis of soil heavy metal pollution and pattern in Central Transylvania. Int. J. Mol. Sci. 2008, 9, 434–453. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.K.; Panda, S.S.; Basu, A.; Dhal, N.K. Enhancement of toxic Cr (VI), Fe, and other heavy metals phytoremediation by the synergistic combination of native Bacillus cereus strain and Vetiveria zizanioides L. Int. J. Phytoremediation 2018, 20, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta. 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Yasin, M.; El-Mehdawi, A.F.; Anwar, A.; Pilon-Smits, E.A.; Faisal, M. Microbial-enhanced selenium and iron biofortification of wheat (Triticum aestivum L.)-applications in phytoremediation and biofortification. Int. J. Phytoremediation 2015, 17, 341–347. [Google Scholar] [CrossRef]
- Tien, T.M.; Gaskins, M.H.; Hubbell, D. Plant growth substances produced by Azospirillum brasilense and their effect on the growth of pearl millet (Pennisetum americanum L.). Appl. Environ. Microbial. 1979, 37, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, S.K.; Singh, J.S.; Singh, D.P. Exopolysaccharide-producing plant growth-promoting rhizobacteria under salinity condition. Pedosphere 2011, 21, 214–222. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Chen, W.P.; Kuo, T.T. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 1993, 21, 2260. [Google Scholar] [CrossRef]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacterial. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Ramadoss, D.; Lakkineni, V.K.; Bose, P.; Ali, S.; Annapurna, K. Mitigation of salt stress in wheat seedlings by halotolerant bacteria isolated from saline habitats. Springer Plus 2013, 2, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Sammar, R.; Saleem, M.F.; Khan, I.H.; Jamil, M.; Ijaz, M.; Khan, M.A. Evaluating the drought stress tolerance efficiency of wheat (Triticum aestivum L.) cultivars. Russ. J. Agric. Soc. Econ. Sci. 2012, 12, 41–46. [Google Scholar] [CrossRef]
- Gee, G.; Bauder, J. Particle size analysis by hydrometer: A simplified method for routine textural analysis and a sensitivity test of measurement parameters 1. Soil Sci. Society. Am. J. 1979, 43, 1004–1007. [Google Scholar] [CrossRef]
- Song, Y.N.; Zhang, F.S.; Marschner, P.; Fan, F.L.; Gao, H.M.; Bao, X.G.; Li, L. Effect of intercropping on crop yield and chemical and microbiological properties in rhizosphere of wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L.). Biol. Fertil. Soils. 2007, 43, 565–574. [Google Scholar] [CrossRef]
- Alia, N.; Sardar, K.; Said, M.; Salma, K.; Sadia, A.; Sadaf, S.; Toqeer, A.; Miklas, S. Toxicity and bioaccumulation of heavy metals in spinach (Spinacia oleracea) grown in a controlled environment. Int. J. Environm. Res. Public Health 2015, 12, 7400–7416. [Google Scholar] [CrossRef] [Green Version]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Anuradha, C.; Selvarajan, R.; Vasantha, S.; Suresha, G.S. Biochemical Characterization of Compatible Plant Virus Interaction: A Case Study with Bunchy Top Virus-Banana Host-Pathosystem. Plant Pathol. J. 2015, 14, 212–222. [Google Scholar] [CrossRef] [Green Version]
- Sofy, A.R.; Dawoud, R.A.; Sofy, M.R.; Mohamed, H.I.; Hmed, A.A.; El-Dougdoug, N.K. Improving regulation of enzymatic and non-enzymatic antioxidants and stress-related gene stimulation in Cucumber mosaic cucumovirus-infected cucumber plants treated with glycine betaine, chitosan and combination. Molecules 2020, 25, 2341. [Google Scholar] [CrossRef] [PubMed]
- Weatherley, P.E. Studies in the water relations of the cotton plant. I. The field measurement of water deficits in leaves. New Phytol. 1950, 49, 81–97. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A colorimetric method for the determination of sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Sairam, R.K.; Saxena, D.C. Oxidative stress and antioxidants in wheat genotypes: Possible mechanism of water stress tolerance. J. Agron. Crop Sci. 2000, 184, 55–61. [Google Scholar] [CrossRef]
- Ullah, N.; Haq, I.U.; Safdar, N.; Mirza, B. Physiological and biochemical mechanisms of allelopathy mediated by the allelochemical extracts of Phytolacca latbenia (Moq.) H. Walter. Toxicol. Ind. Health 2015, 31, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Lagrimini, L.M. Wound-induced deposition of polyphenols in transgenic plants overexpressing peroxidase. Plant Physiol. 1991, 96, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar] [CrossRef]
- Gill, R.A.; Zhang, N.; Ali, B.; Farooq, M.A.; Xu, J.; Gill, M.B.; Zhou, W. Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black-and yellow-seeded Brassica napus L. Environ. Sci. Pollut. Res. 2016, 23, 20483–20496. [Google Scholar] [CrossRef] [PubMed]
- Shahabivand, S.; Parvaneh, A.; Aliloo, A.A. Different response of Alyssum montanum and Helianthus annuus to cadmium bioaccumulation mediated by the endophyte fungus Serendipita indica. Acta Ecol. Sin. 2020, 40, 315–322. [Google Scholar] [CrossRef]

| Treatments | IAA (µg/mL) | CK (µg/mL) | ABA (µg/mL) | Proline (µg/mL) | Sugar (µg/mL) |
|---|---|---|---|---|---|
| Cr-0 mg/L | 81.33 ± 1.76 a | 1.55 ± 0.01 a | 2.57 ± 0.10 c | 10.16 ± 0.35 d | 12.76 ± 0.23 d |
| Cr-20 mg/L | 62.66 ± 0.88 b | 1.22 ± 0.03 b | 6.48 ± 0.20 a | 27.00 ± 1.15 a | 43.40 ± 1.22 a |
| Cr-30 mg/L | 55.00 ± 1.73 c | 0.81 ± 0.00 c | 5.84 ± 0.56 a | 22.66 ± 1.20 b | 34.00 ± 1.52 b |
| Cr-40 mg/L | 48.66 ± 2.02 d | 0.62 ± 0.00 d | 4.38 ± 0.07 b | 17.66 ± 1.45 c | 19.66 ± 1.45 c |
| Treatments | Germination Percentage (%) | Seedling Length (cm) | VI |
|---|---|---|---|
| T0 | 85.33 ± 1.45 b | 8.13 ± 0.42 b | 692.83 ± 25.32 b |
| T1 | 92.33 ± 0.88 a | 10.43 ± 0.72 a | 964.20 ± 73.68 a |
| T2 | 63.00 ± 1.15 d | 4.72 ± 0.00 de | 297.76 ± 4.91 de |
| T3 | 73.00 ± 1.52 c | 6.52 ± 0.49 c | 477.29 ± 45.64 c |
| T4 | 57.00 ± 1.00 e | 4.22 ± 0.00 ef | 240.74 ± 4.60 ef |
| T5 | 66.00 ± 0.57 d | 5.74 ± 0.01 cd | 379.26 ± 2.48 d |
| T6 | 51.33 ± 0.66 f | 3.62 ± 0.01 f | 185.84 ± 2.98 f |
| T7 | 55.00 ± 0.57 e | 4.24 ± 0.01 ef | 233.21 ± 3.08 ef |
| Treatments | Shoot Length (cm) | Root Length (cm) | Fresh Weight (mg) | Dry Weight (mg) |
|---|---|---|---|---|
| T0 | 19.00 ± 1.52 b | 15.70 ± 0.95 b | 1016.00 ± 21.07 b | 35.00 ± 1.15 b |
| T1 | 21.70 ± 1.37 a | 18.40 ± 0.55 a | 1287.70 ± 16.75 a | 47.66 ± 0.88 a |
| T2 | 9.20 ± 0.73 d | 7.50 ± 0.20 d | 533.33 ± 16.49 e | 19.03 ± 0.38 ef |
| T3 | 12.50 ± 0.81 c | 8.93 ± 0.088 c | 789.33 ± 14.49 c | 30.86 ± 0.87 c |
| T4 | 8.16 ± 0.72 d | 7.13 ± 0.23 d | 472.67 ± 6.17 f | 17.06 ± 0.24 fg |
| T5 | 9.76 ± 0.35 d | 7.96 ± 0.12 cd | 681.67 ± 14.14 d | 27.40 ± 0.37 d |
| T6 | 7.46 ± 0.55 d | 7.00 ± 0.05 d | 402.33 ± 15.49 g | 14.90 ± 0.40 g |
| T7 | 8.23 ± 0.34 d | 7.59 ± 0.04 d | 571.33 ± 12.60 e | 20.33 ± 1.24 e |
| Treatments | Proline µg/mg | Sugar µg/mg |
|---|---|---|
| T0 | 1.56 ± 0.01 f | 151.33 ± 3.17 b |
| T1 | 3.20 ± 0.01 e | 185.00 ± 1.73 a |
| T2 | 6.50 ± 0.02 b | 69.66 ± 1.45 d |
| T3 | 7.07 ± 0.03 a | 89.66 ± 0.88 c |
| T4 | 5.33 ± 9.02 d | 58.66 ± 1.45 e |
| T5 | 5.76 ± 0.01 c | 69.00 ± 1.52 d |
| T6 | 5.09 ± 0.33 d | 53.33 ± 1.45 f |
| T7 | 5.37 ± 0.01 d | 58.00 ± 1.15 ef |
| Treatments | MDA (µmol/g2FW) | MSI (%) |
|---|---|---|
| T0 | 4.33 ± 0.01 f | 84.60 ± 0.98 b |
| T1 | 2.64 ± 0.00 g | 91.86 ± 1.21 a |
| T2 | 18.00 ± 0.32 c | 43.00 ± 0.87 f |
| T3 | 7.32 ± 0.01 e | 65.10 ± 0.88 c |
| T4 | 23.83 ± 0.64 b | 38.90 ± 1.30 g |
| T5 | 15.66 ± 0.59 d | 52.60 ± 0.85 d |
| T6 | 31.56 ± 0.67 a | 35.90 ± 1.26 g |
| T7 | 22.70 ± 0.51 b | 47.20 ± 0.72 e |
| Treatments | Root Cr Content (mg/g DW) | Shoot Cr Content (mg/g DW) | Bioconcentration Factor (Root) | Bioconcentration Factor (Shoot) | Translocation Factor |
|---|---|---|---|---|---|
| T0 | 1.54 ± 0.00 g | 0.41 ± 0.01 f | 0.57 ± 0.00 f | 0.13 ± 0.02 g | 0.26 ± 0.00 a |
| T1 | 2.36 ± 0.01 g | 0.67 ± 0.00 f | 0.88 ± 0.00 f | 0.24 ± 0.00 g | 0.28 ± 0.00 a |
| T2 | 314.67 ± 4.91 f | 31.70 ± 1.00 e | 15.73 ± 0.24 e | 1.58 ± 0.05 f | 0.10 ± 0.00 e |
| T3 | 855.00 ± 10.44 d | 127.33 ± 12.34 c | 42.75 ± 0.52 a | 6.36 ± 0.61 c | 0.14 ± 0.01 c |
| T4 | 607.67 ± 12.66 e | 75.30 ± 1.73 d | 19.75 ± 0.91 d | 2.50 ± 0.05 e | 0.12 ± 0.00 d |
| T5 | 1149.70 ± 8.08 b | 222.67 ± 7.68 b | 35.11 ± 3.14 b | 7.42 ± 0.25 b | 0.19 ± 0.00 b |
| T6 | 960.67 ± 10.10 c | 139.67 ± 7.31 c | 24.01 ± 0.25 c | 3.49 ± 0.18 d | 0.14 ± 0.00 cd |
| T7 | 1311.30 ± 15.98 a | 356.17 ± 6.20 a | 32.78 ± 0.39 b | 8.90 ± 0.15 a | 0.27 ± 0.00 a |
| Texture | Loam |
|---|---|
| pH | 7.20 ± 0.24 |
| Electrical conductivity (dsm−1) | 0.65 ± 0.00 |
| Organic matter (%) | 1.43 ± 0.03 |
| Saturation (%) | 46.72 ± 1.68 |
| Potassium (mg/kg) | 132.29 ± 7.42 |
| Nitrogen (mg/kg) | 61.03 ± 2.36 |
| Iron (mg/kg) | 4.76 ± 0.06 |
| Phosphorus (mg/kg) | 5.34 ± 0.02 |
| Bioavailable Cr (mg/kg) | 2.68 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhtar, N.; Ilyas, N.; Yasmin, H.; Sayyed, R.Z.; Hasnain, Z.; A. Elsayed, E.; El Enshasy, H.A. Role of Bacillus cereus in Improving the Growth and Phytoextractability of Brassica nigra (L.) K. Koch in Chromium Contaminated Soil. Molecules 2021, 26, 1569. https://doi.org/10.3390/molecules26061569
Akhtar N, Ilyas N, Yasmin H, Sayyed RZ, Hasnain Z, A. Elsayed E, El Enshasy HA. Role of Bacillus cereus in Improving the Growth and Phytoextractability of Brassica nigra (L.) K. Koch in Chromium Contaminated Soil. Molecules. 2021; 26(6):1569. https://doi.org/10.3390/molecules26061569
Chicago/Turabian StyleAkhtar, Nosheen, Noshin Ilyas, Humaira Yasmin, R. Z. Sayyed, Zuhair Hasnain, Elsayed A. Elsayed, and Hesham A. El Enshasy. 2021. "Role of Bacillus cereus in Improving the Growth and Phytoextractability of Brassica nigra (L.) K. Koch in Chromium Contaminated Soil" Molecules 26, no. 6: 1569. https://doi.org/10.3390/molecules26061569