Sugar-Based Ionic Liquids: Multifaceted Challenges and Intriguing Potential
Abstract
:1. Introduction
2. Synthesis
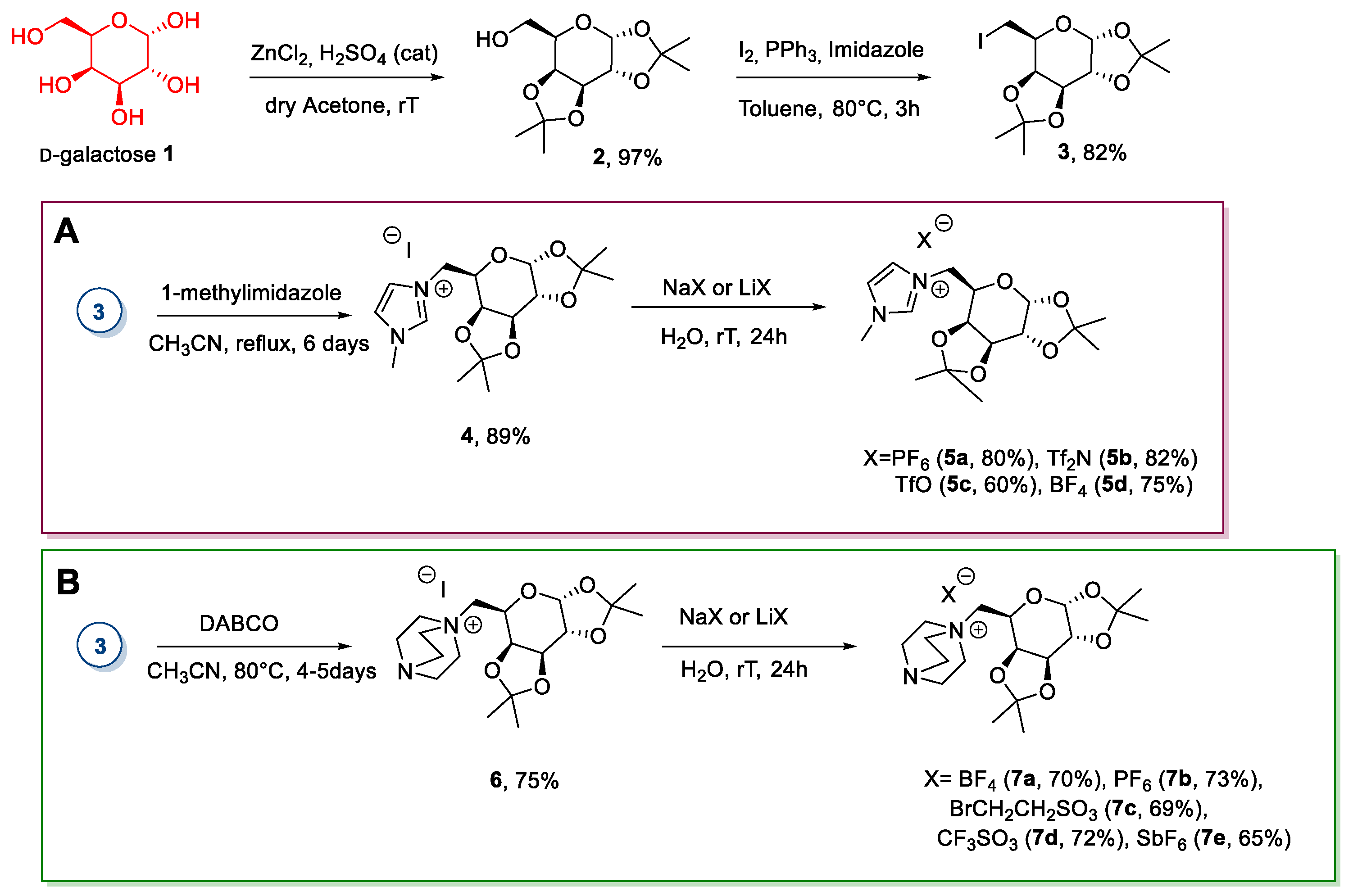
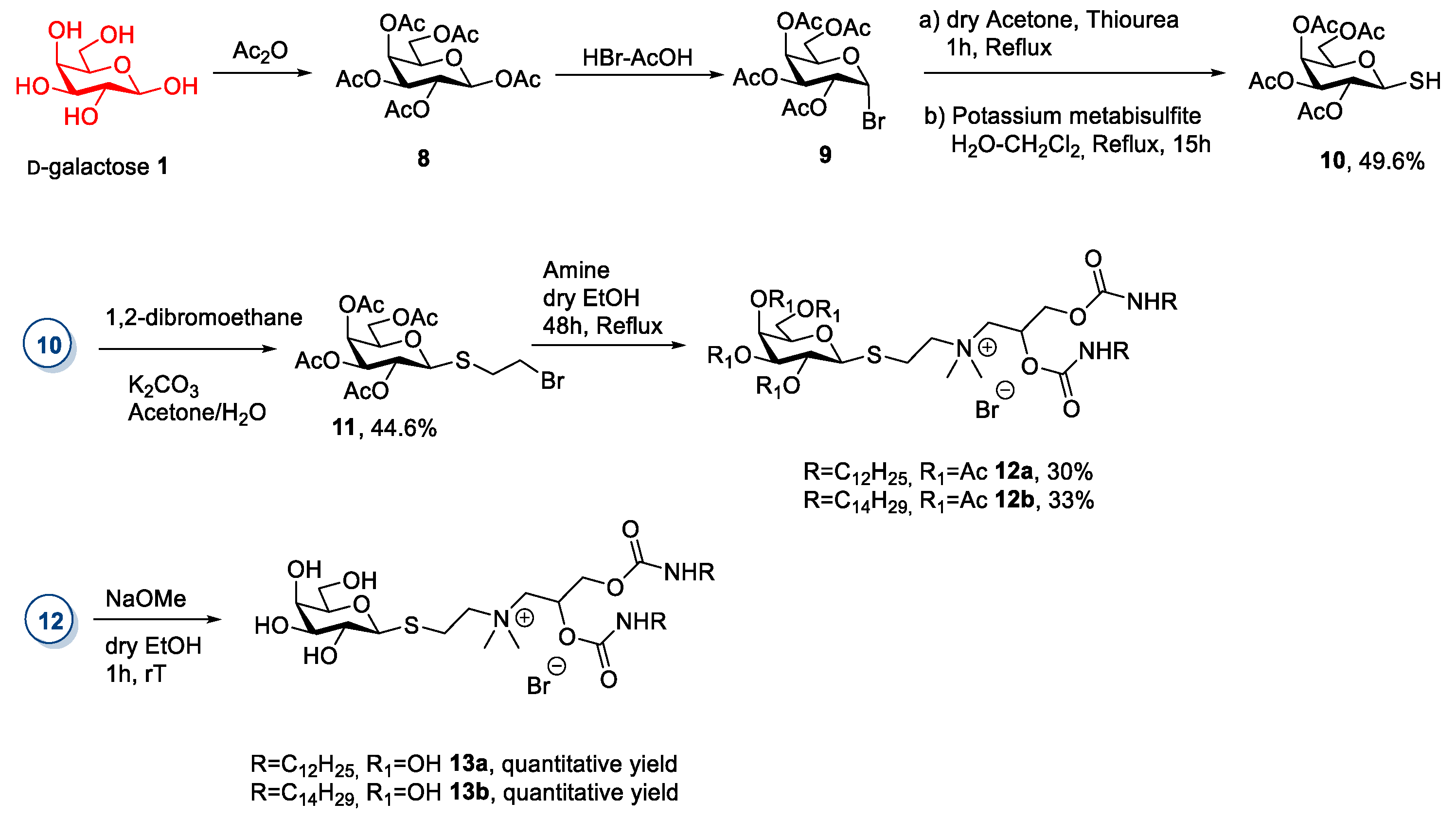
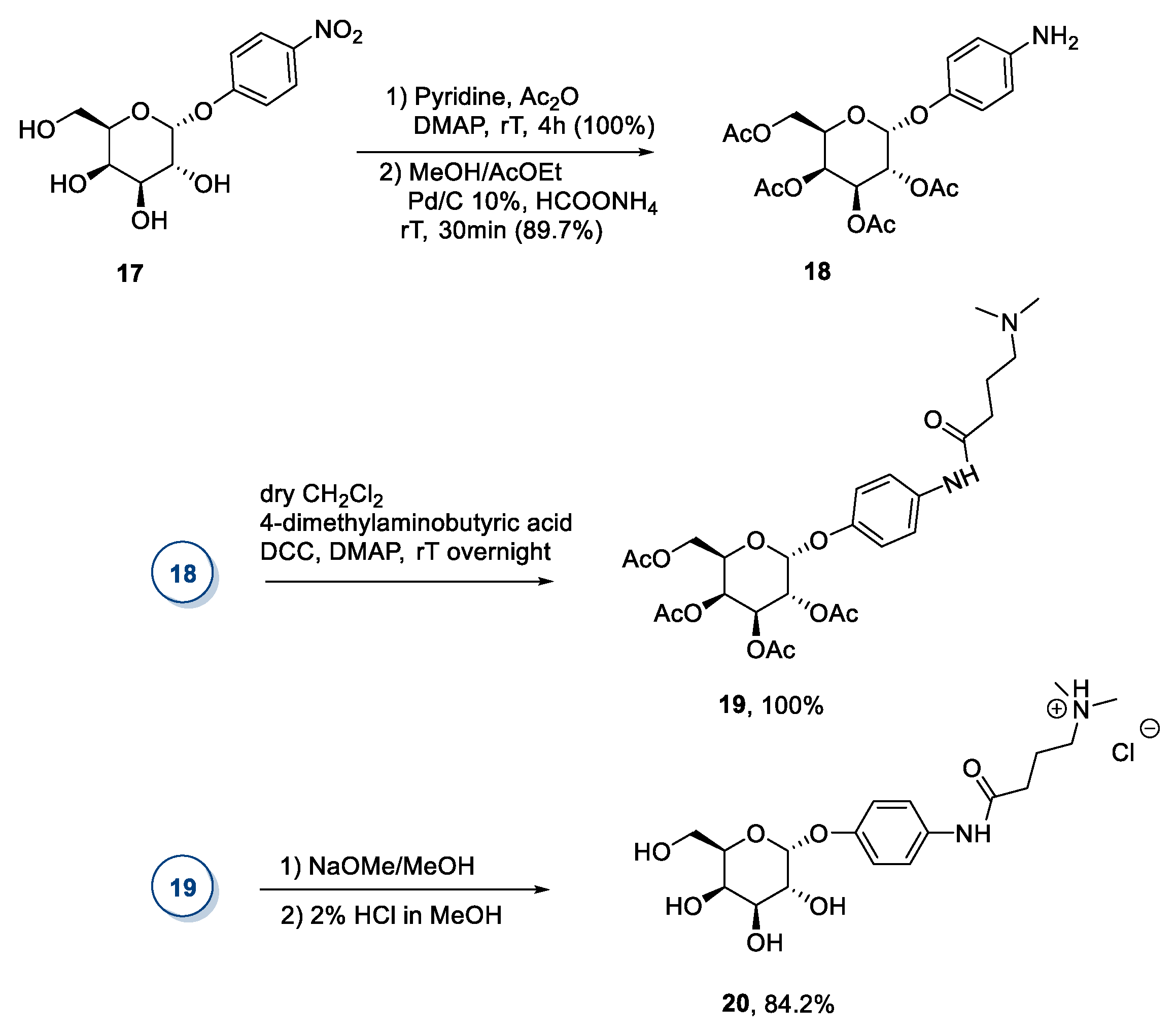
2.1. d-galactose
2.2. d-xylose
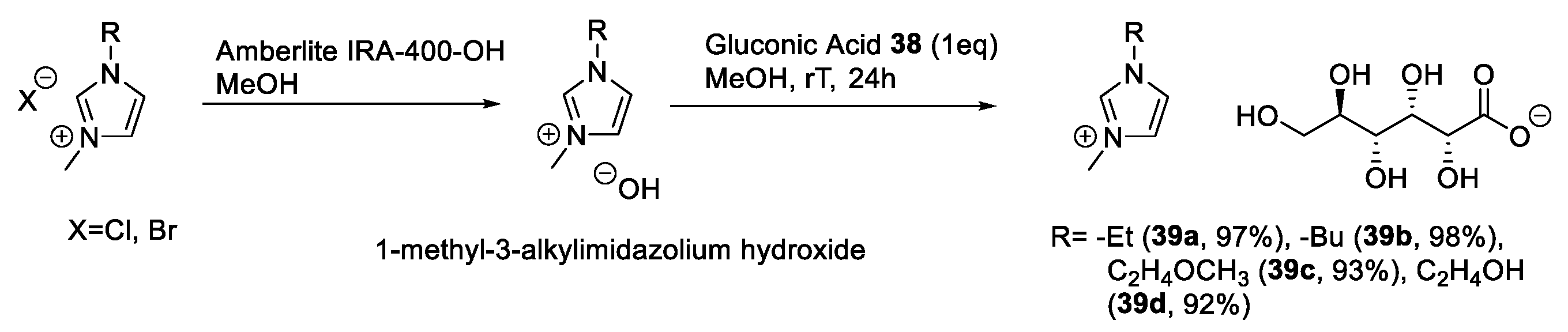
2.3. d-gluconic Acid
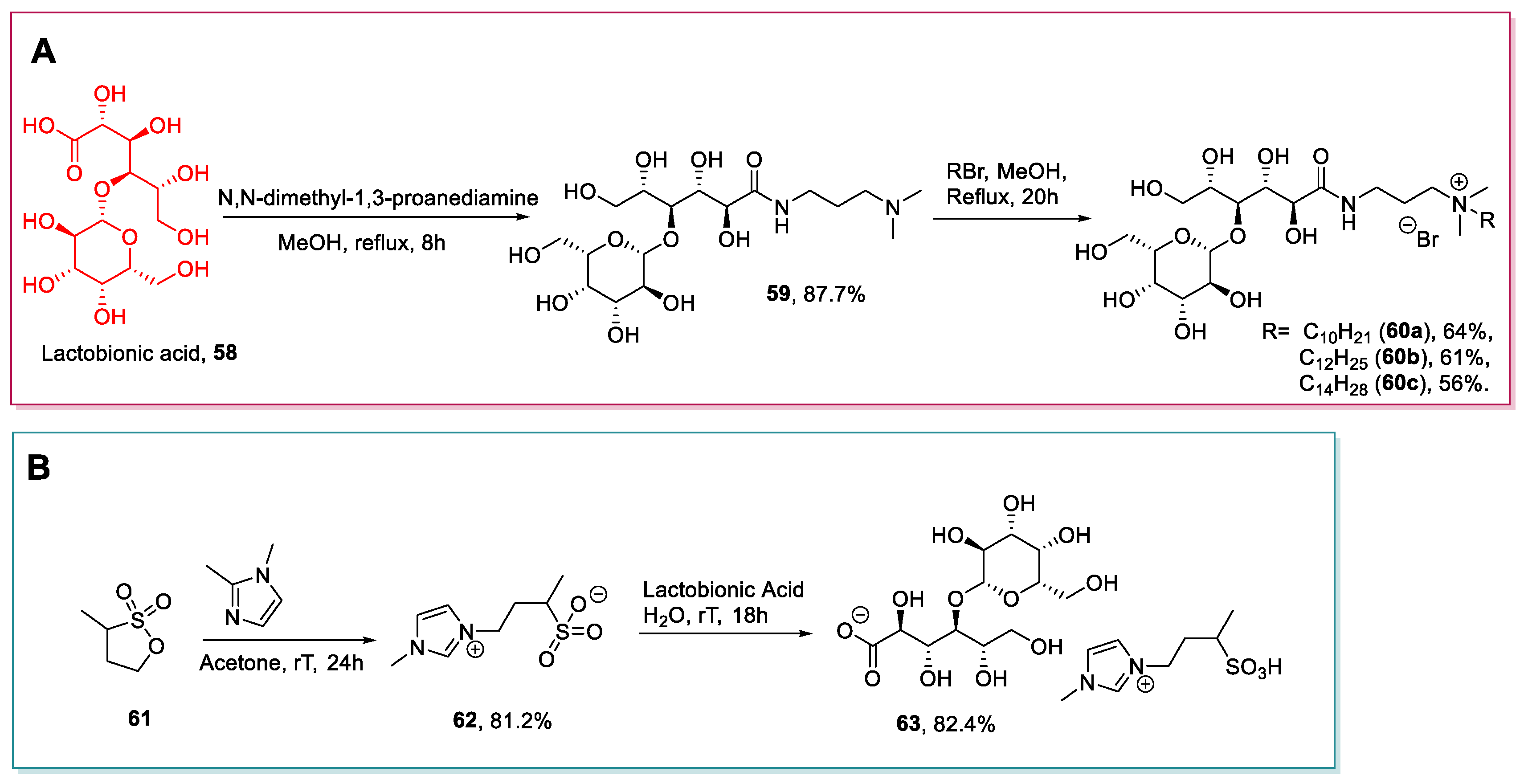
2.4. d-glucuronic Acid, d-galacturonic Acid, Lactobionic Acid and Glucosamine
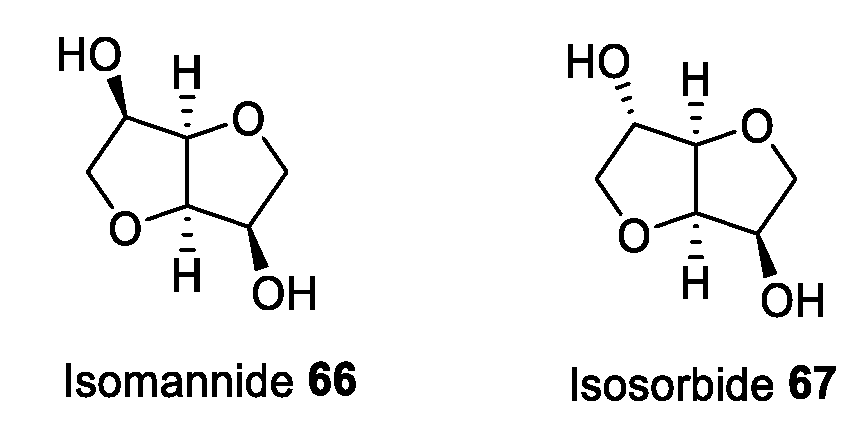
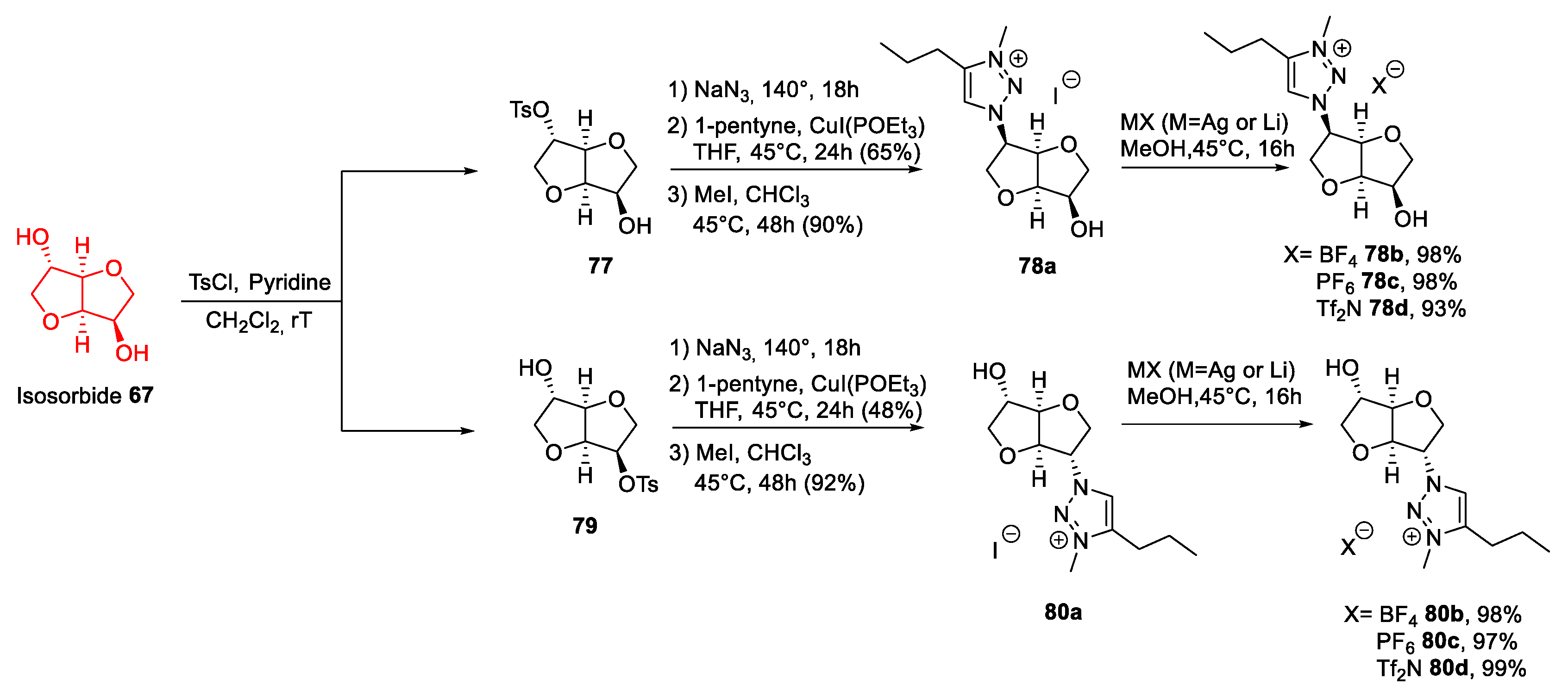
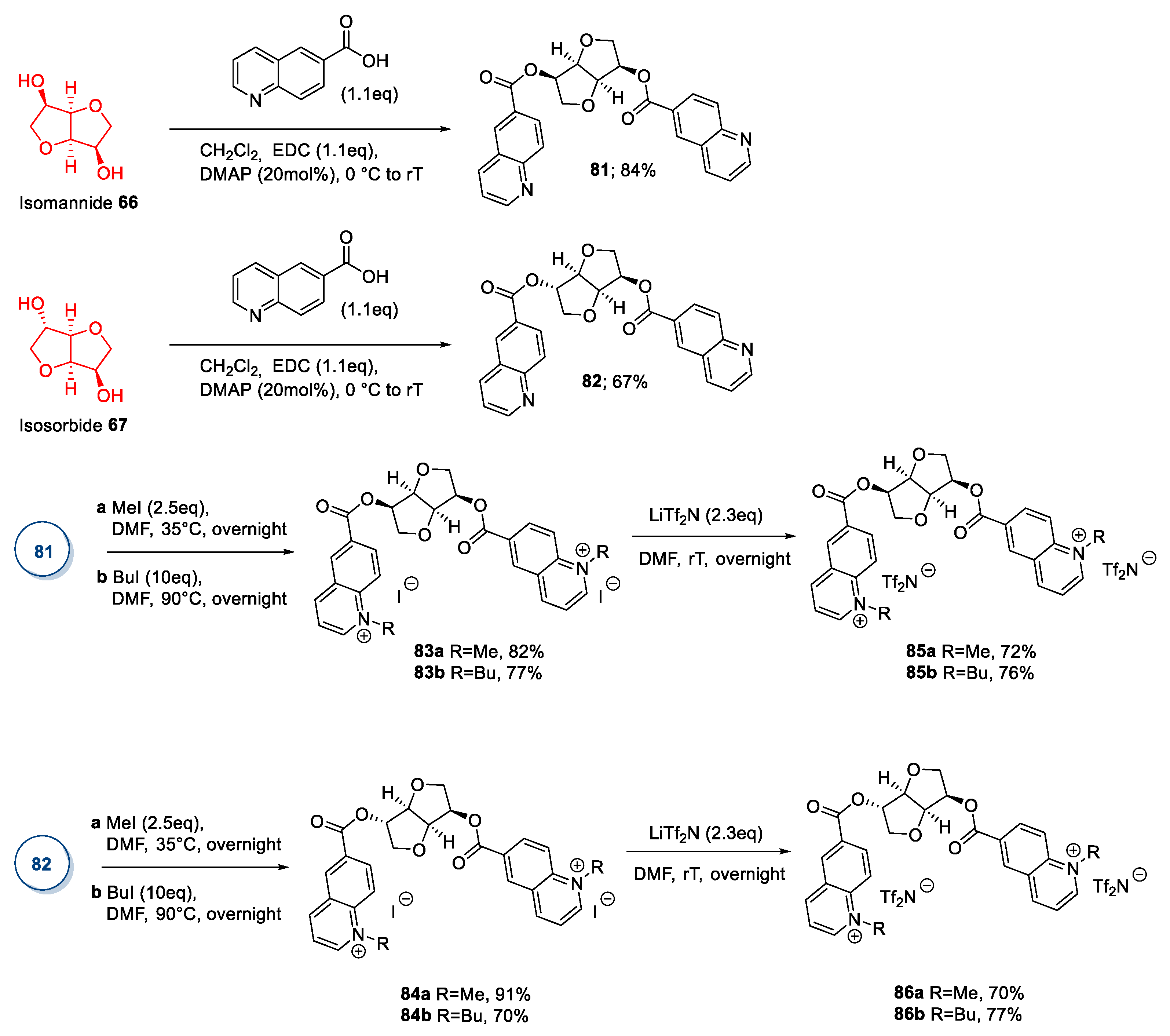
2.5. Isohexides
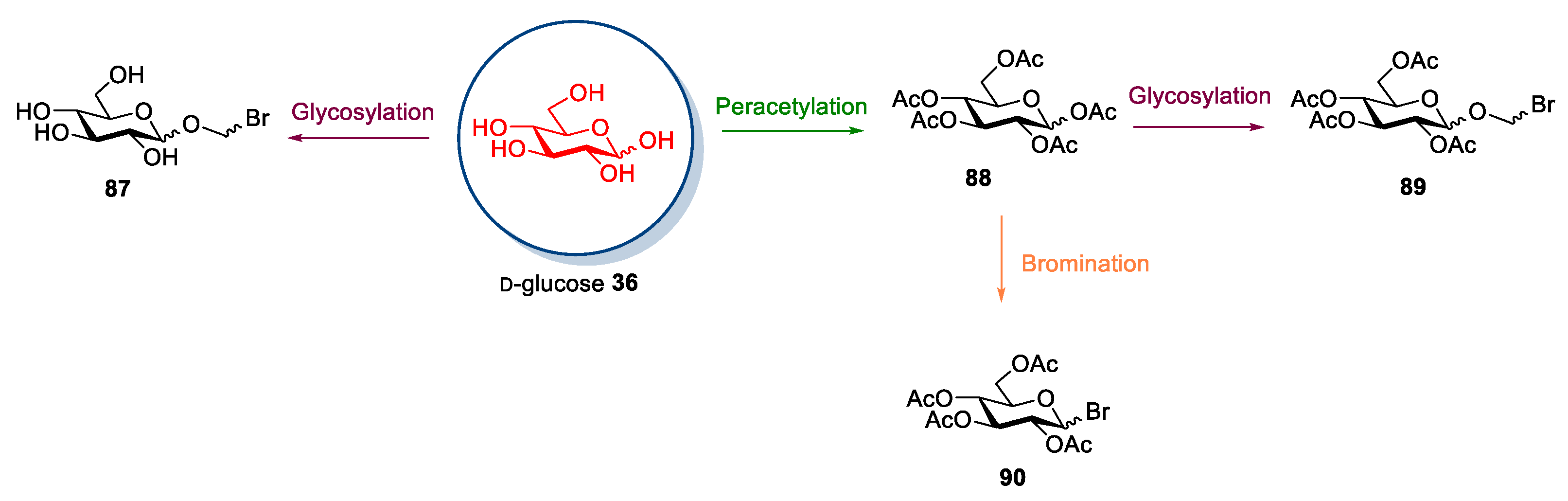
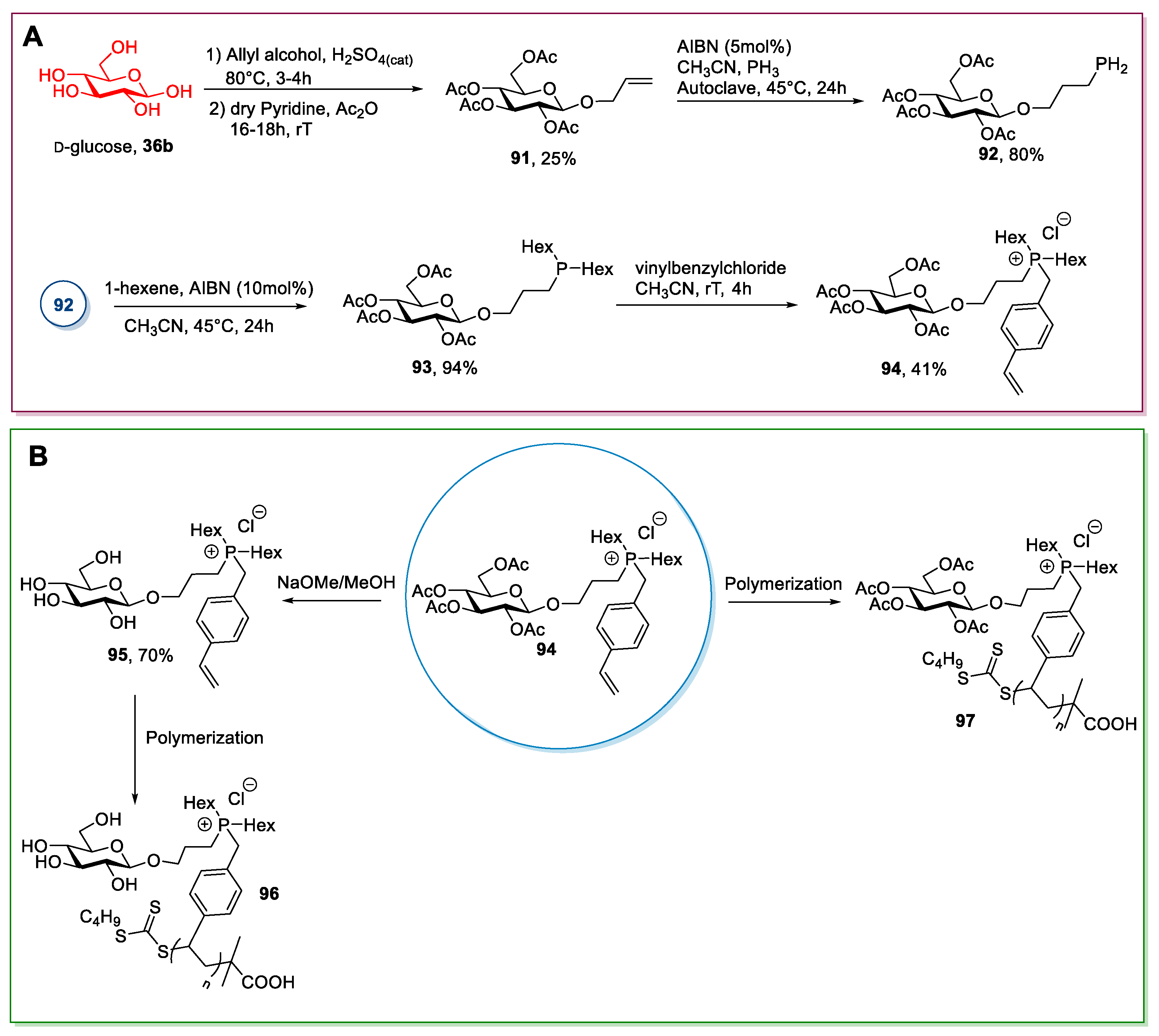
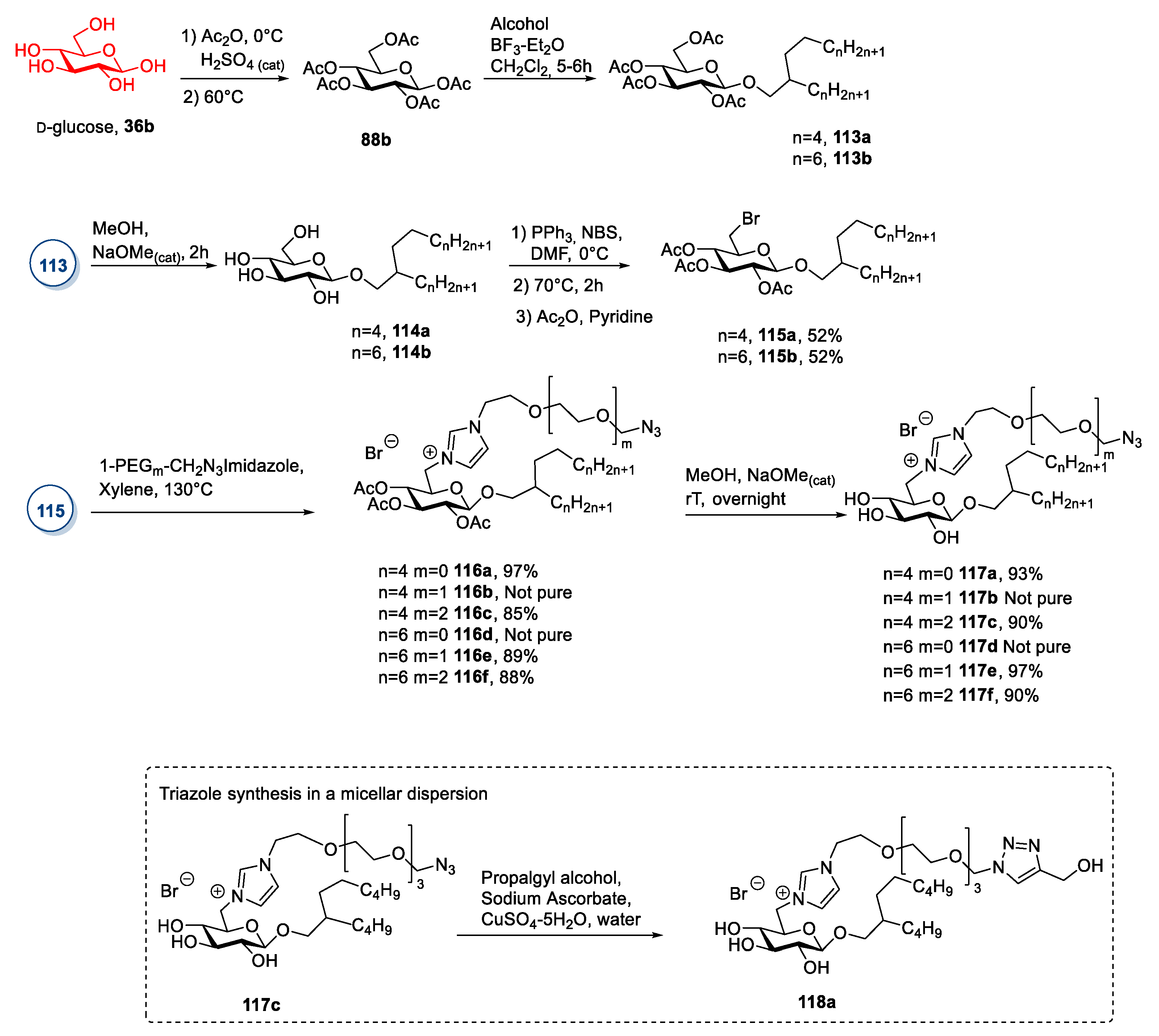
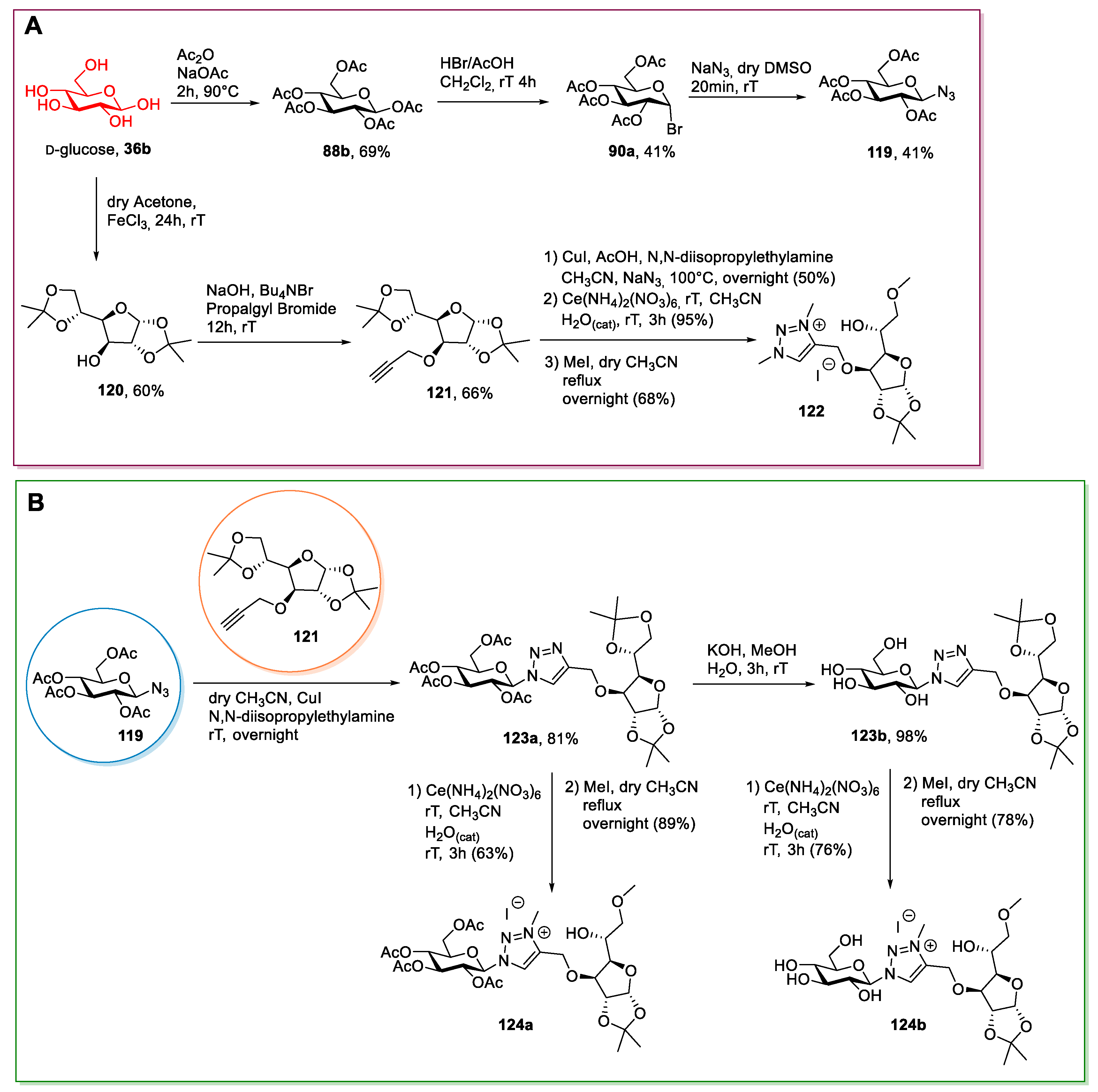
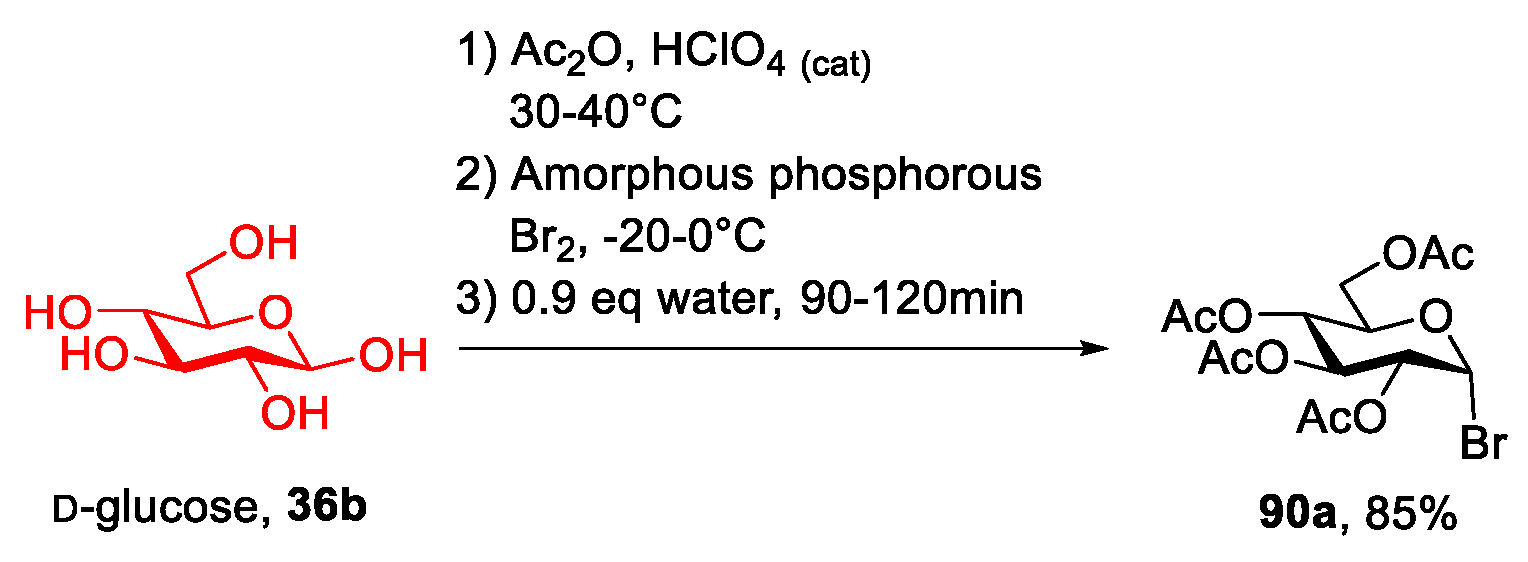
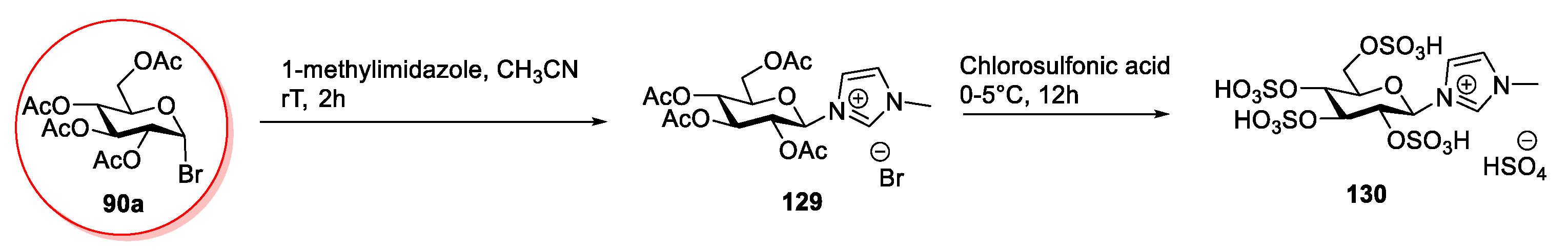
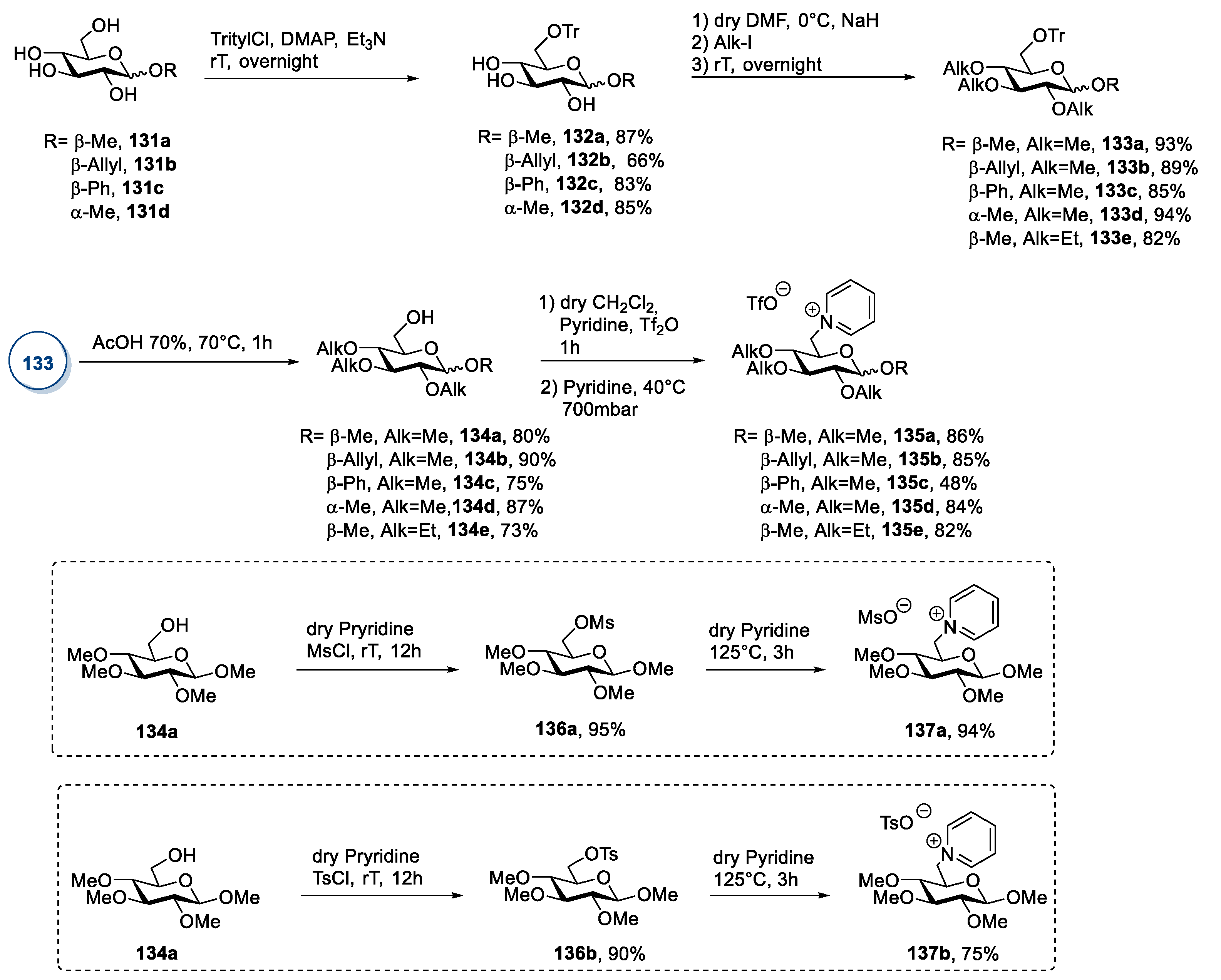
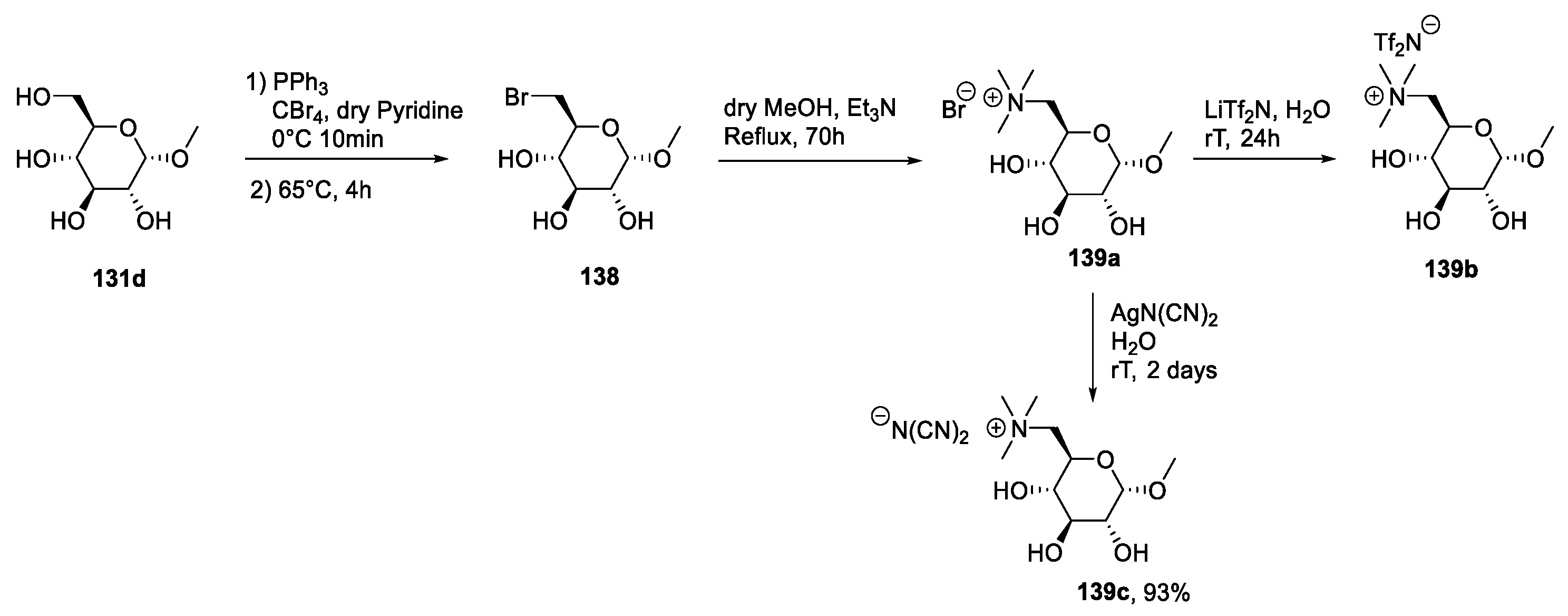
2.6. d-glucose and N-methyl-glucamine
2.7. d-mannose
2.8. Other Monosaccharides
2.9. Disaccharides
3. Thermal Analysis
3.1. d-galactose
3.2. d-xylose and Xylosides
3.3. d-gluconic Acid
3.4. d-glucuronic-, d-galacturonic- and d-quinic Acid
3.5. Isohexides
3.6. d-glucose
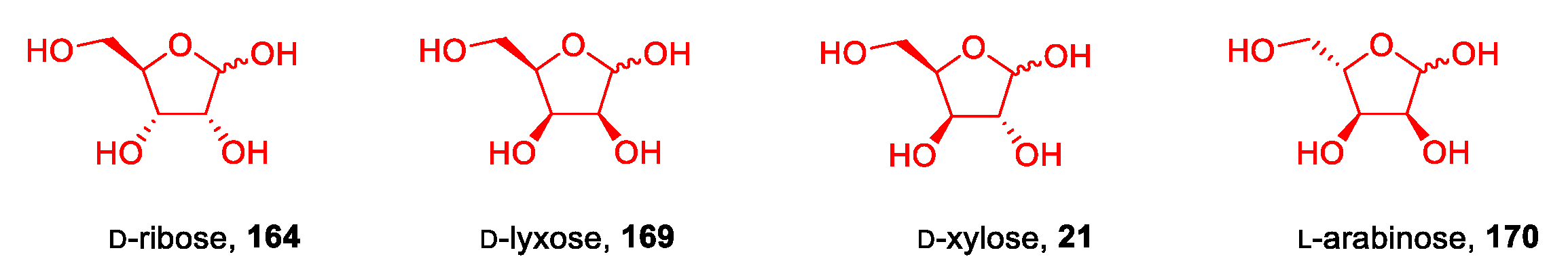
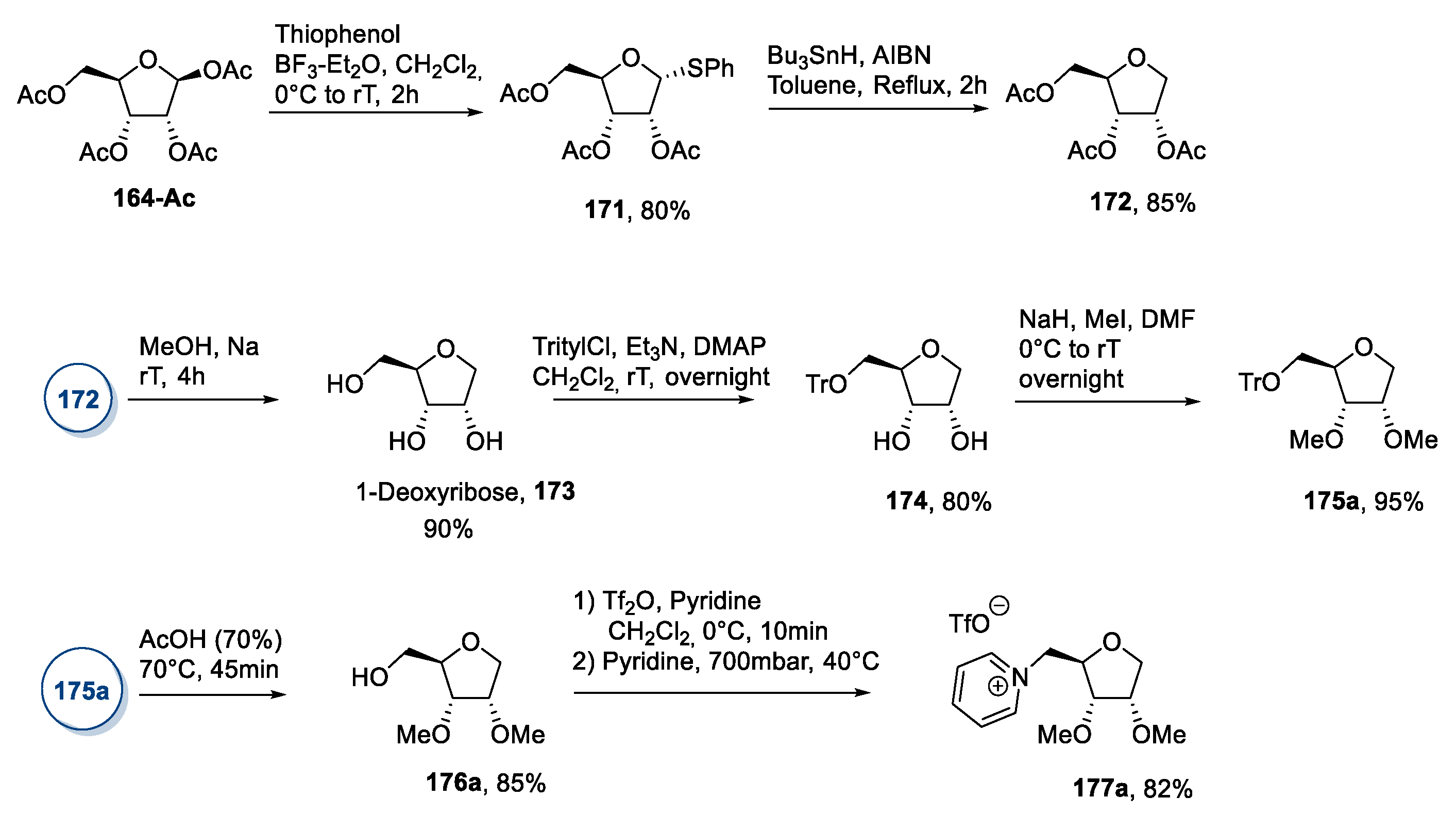
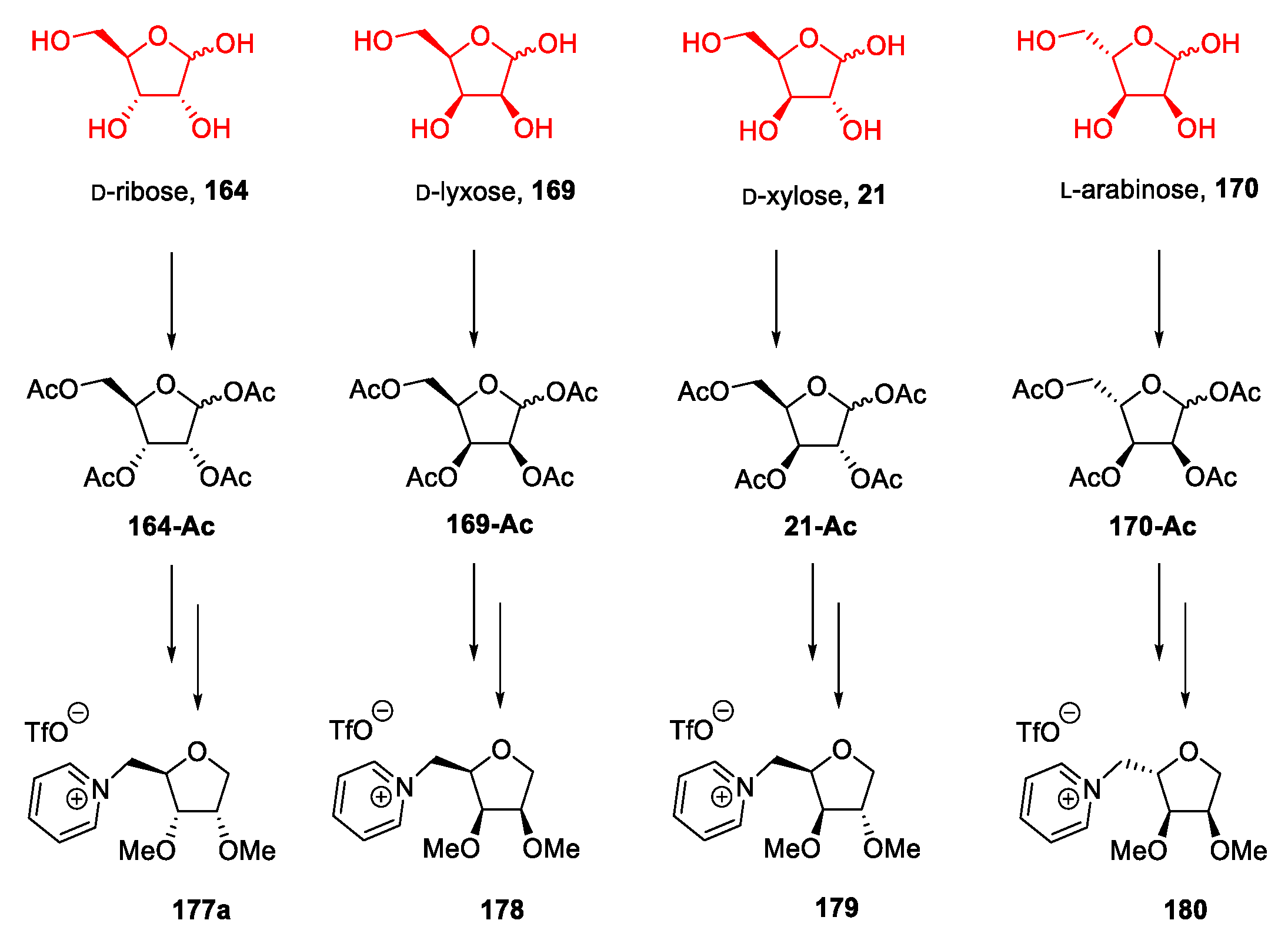
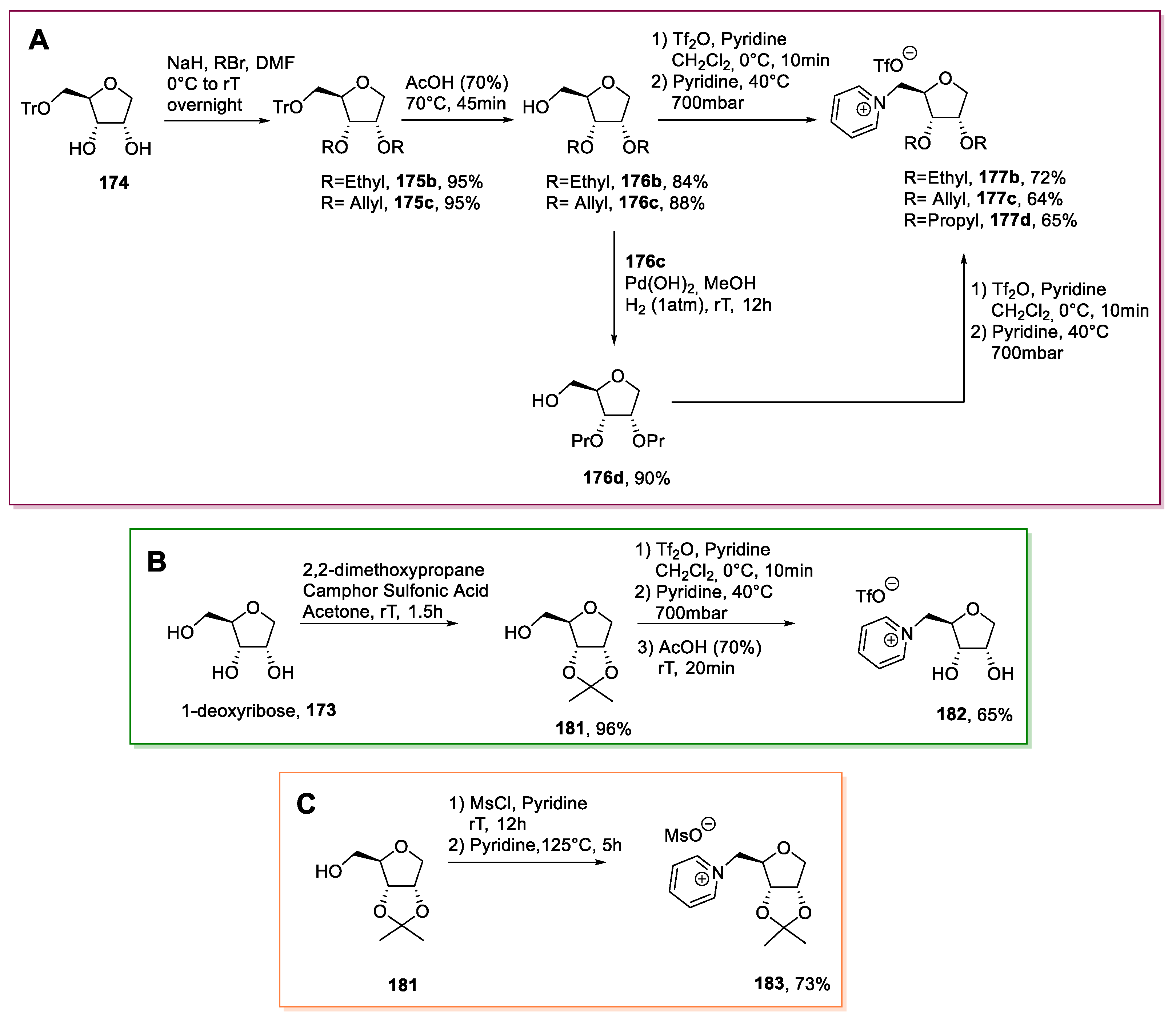
3.7. Other Monosaccharides: Pentoses
3.8. Disaccharides
3.9. Concluding Remarks
4. Applications
4.1. Solvents
4.2. Catalysts and Ligands
4.3. Asymmetric Synthesis and Enantiodiscrimination
4.4. Surfactants
4.5. Chromatography and Electrophoresis
4.6. Miscellaneous Applications
5. Future Perspective
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sugar Core | Physical State | Melting Point (°C) | Ref. | |
|---|---|---|---|---|
| 4 | d-galactose | Colorless solid | 196–198 | [21,22,23] |
| 5a | d-galactose | Colorless solid | 159–161 (142–145) | [21,23] |
| 5b | d-galactose | Colorless solid | 83–85 | [21,22] |
| 5c | d-galactose | Colorless solid | 121–123 | [21] |
| 5d | d-galactose | Colorless solid | 177–179 | [21] |
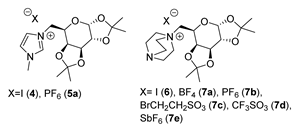
| 6 | d-galactose | White solid | Not reported | [24] |
| 7a | d-galactose | Off-white solid | Not reported | [24] |
| 7b | d-galactose | White solid | Not reported | [24] |
| 7c | d-galactose | Light green solid | Not reported | [24] |
| 7d | d-galactose | Off white solid | Not reported | [24] |
| 7e | d-galactose | White solid | Not reported | [24] |
| 12a | d-galactose | Light yellow viscous fluid | [28] | |
| 12b | d-galactose | No information | [28] | |
| 13a | d-galactose | Light yellow solid | Not reported | [28] |
| 13b | d-galactose | No information | [28] | |
| 15a | d-galactose | Oil | [32] | |
| 15b | d-galactose | Oil | [32] | |
| 16a | d-galactose | Thick syrup | [32] | |
| 16b | d-galactose | Thick syrup | [32] | |
| 20 | d-galactose | Solid | 62–63 | [33] |
| Sugar Core | Physical State | Melting Point (°C) | Ref. | |
|---|---|---|---|---|
| 25a | d-xylose | White wax | [34] | |
| 25b | d-xylose | White wax | [34] | |
| 26 | d-xylose | Oily | [38] | |
| 27a | d-xylose | White solid | 59 | [38] |
| 27b | d-xylose | Oily | [38] | |
| 27c | d-xylose | White solid | 98 | [38] |
| 28a | d-xylose | White solid | 74 | [38] |
| 28b | d-xylose | Oily | [38] | |
| 28c | d-xylose | White solid | 112 | [38] |
| 29a | d-xylose | Oily | [38] | |
| 29b | d-xylose | Oily | [38] | |
| 29c | d-xylose | Oily | [38] | |
| 35a | d-xylose | Light brown color liquid | [39,40] | |
| 35b | d-xylose | Light brown color liquid | [39,40] | |
| 35c | d-xylose | Light brown color liquid | [39,40] | |
| 35d | d-xylose | Light brown color liquid | [39,40] | |
| 35e | d-xylose | Light brown color liquid | [39,40] |
| Sugar Core | Physical State | Melting Point (°C) | Ref. | |
|---|---|---|---|---|
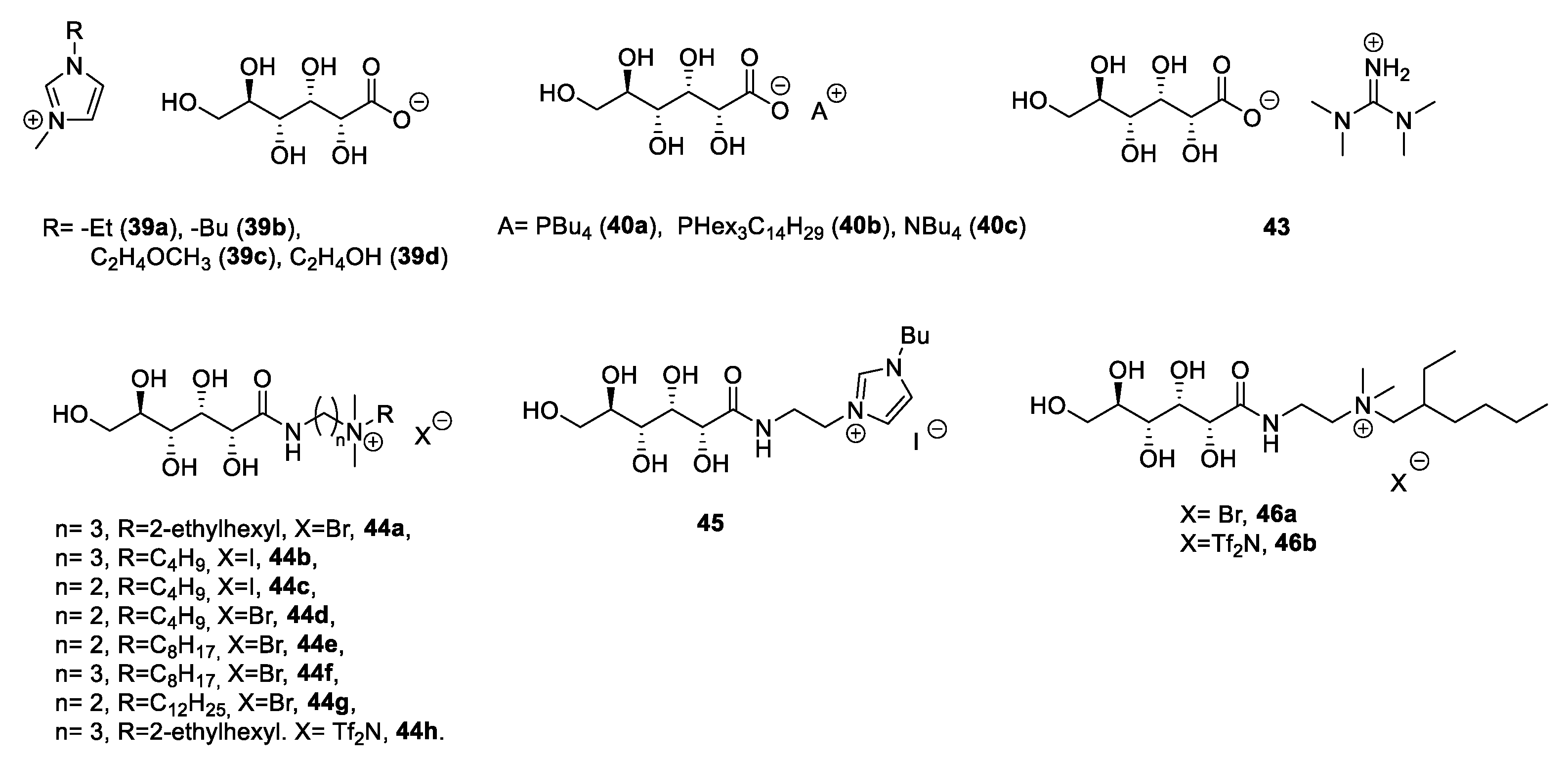
| 39a | d-gluconic acid | Yellow viscous liquid | [43] | |
| 39b | d-gluconic acid | Pale yellow gel | [43] | |
| 39c | d-gluconic acid | Yellow viscous liquid | [43] | |
| 39d | d-gluconic acid | Yellow gel | [43] | |
| 40a | d-gluconic acid | Solid | 81.5 | [17,44] |
| 40b | d-gluconic acid | Solid | 35.6 | [17,44] |
| 40c | d-gluconic acid | Solid | 134.9 | [17,44] |
| 41 | d-gluconic acid | Liquid | 25–30 | [45] |
| 42a | d-gluconic acid | Classified as IL by the authors. No physical state reported. | [46] | |
| 42b | d-gluconic acid | Classified as IL by the authors. No physical state reported. | [46] | |
| 42c | d-gluconic acid | Classified as IL by the authors. No physical state reported. | [46] | |
| 42d | d-gluconic acid | Classified as IL by the authors. No physical state reported. | [46] | |
| 42e | d-gluconic acid | Classified as IL by the authors. No physical state reported. | [46] | |
| 42f | d-gluconic acid | Classified as IL by the authors. No physical state reported. | [46] | |
| 42g | d-gluconic acid | Classified as IL by the authors. No physical state reported. | [46] | |
| 43 | d-gluconic acid | Yellow viscous oil | [17] | |
| 44a | d-gluconic acid | Liquid | [17] | |
| 44b | d-gluconic acid | Light yellow oil | [17] | |
| 44c | d-gluconic acid | Light yellow oil | [17] | |
| 44d | d-gluconic acid | Light yellow oil | [17] | |
| 44e | d-gluconic acid | Light yellow oil | [17] | |
| 44f | d-gluconic acid | Light yellow oil | [17] | |
| 44g | d-gluconic acid | Light yellow oil | [17] | |
| 44h | d-gluconic acid | Light yellow oil | [17] | |
| 45 | d-gluconic acid | Light yellow oil | [17] | |
| 46a | d-gluconic acid | Light yellow oil | [48] | |
| 46b | d-gluconic acid | Yellow oil | [48] | |
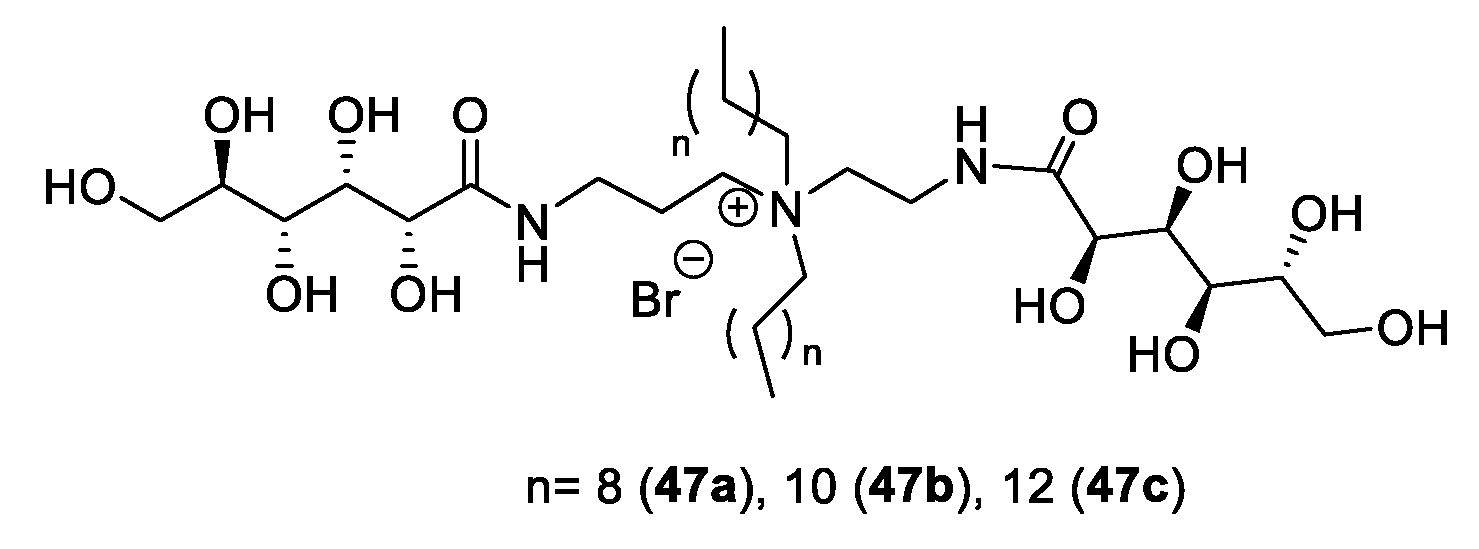
| 47a | d-gluconic acid | White solid | 88.2–90.1 | [47,49] |
| 47b | d-gluconic acid | White solid | 95.1–96.6 | [47,49] |
| 47c | d-gluconic acid | White | 96.7–98.4 | [47,49] |
| 48a | d-gluconic acid | Brown-yellow syrup | [47,50] | |
| 48b | d-gluconic acid | White solid | 76.5–78.3 | [47,50] |
| 48c | d-gluconic acid | White solid | 78.9–83.1 | [47,50] |
| 48d | d-gluconic acid | White solid | 93.5–94.6 | [47,50] |
| 49a | d-gluconic acid | Brown-yellow syrup | [47,51] | |
| 49b | d-gluconic acid | Brown-yellow syrup | [47,51] | |
| 49c | d-gluconic acid | Brown-yellow syrup | [47,51] |
| Sugar Core | Physical State | Melting Point (°C) | Ref. | |
|---|---|---|---|---|
| 52a | d-glucuronic acid | White solid | 95 | [54,55] |
| 52b | d-glucuronic acid | Viscous oil | [56] | |
| 52c | d-glucuronic acid | Viscous liquid | [54] | |
| 53a | d-galacturonic acid | White solid | 98 | [55] |
| 53b | d-galacturonic acid | Wax | [56] | |
| 53c | d-galacturonic acid | Solid | 50.1 | [57] |
| 56 | (−)-quinic acid | Liquid | 18.8 | [57] |
| 57 | Clindamycin phosphate | Pale yellow viscous liquid | [59] | |
| 60a | Lactobionic acid | Brown-yellow syrup | [50] | |
| 60b | Lactobionic acid | Light yellow solid | 99.5–103.2 | [50] |
| 60c | Lactobionic acid | Light yellow solid | 129.8–134.5 | [50] |
| 63 | Lactobionic acid | Colorless viscous liquid | [60] | |
| 65 | d-glucosamine | Classified as IL by the authors. No physical state reported. | [61] |
| Sugar Core | Physical State | Melting Point | Ref. | |
|---|---|---|---|---|
| 69a | Isoidide | Yellow oil (Foamy material) | (Not reported) | [65,67] |
| 69b | Isoidide | Solid | 150–152 | [65] |
| 69c | Isoidide | Solid | Not reported | [65] |
| 69d | Isoidide | Solid | 118–122 | [65] |
| 69e | Isoidide | Viscous oil | [65] | |
| 71a | Isosorbide | Not pure | [65] | |
| 71b | Isosorbide | Not pure | [65] | |
| 72a | Isosorbide | Yellow oil | [65] | |
| 72b | Isosorbide | Solid | 130–133 | [65] |
| 69f | Isoidide | Solid | Not reported | [67] |
| 74a | Isosorbide | White solid | 118.5–120.5 | [66] |
| 74b | Isosorbide | Not isolated | [66] | |
| 74c | Isosorbide | Not Isolated | [66] | |
| 74d | Isosorbide | Not Isolated | [66] | |
| 76a | Isoidide | Transparent oil | [66] | |
| 76b | Isoidide | Transparent oil | [66] | |
| 78a | Isomannide | Glassy orange material | [68] | |
| 78b | Isomannide | Transparent viscous material | [68] | |
| 78c | Isomannide | Orange viscous material | [68] | |
| 78d | Isomannide | Orange viscous material | [68] | |
| 80a | Isoidide | Glassy orange material | [68] | |
| 80b | Isoidide | Orange viscous material | [68] | |
| 80c | Isoidide | Orange viscous material | [68] | |
| 80d | Isoidide | Orange viscous material | [68] | |
| 83a | Isomannide | Yellow solid | Degraded before melting | [70] |
| 83b | Isomannide | Orange solid | Degraded before melting | [70] |
| 85a | Isomannide | Yellow orange glassy solid | [70] | |
| 85b | Isomannide | Pale yellow glassy solid | [70] | |
| 84a | Isosorbide | Yellow solid | Degraded before melting | [70] |
| 84b | Isosorbide | Brick red solid | 79.9 | [70] |
| 86a | Isosorbide | Brick red glassy solid | [70] | |
| 86b | Isosorbide | Pale brown glassy solid | [70] |
| Sugar Core | Physical State | Melting Point (°C) | Ref. | |
|---|---|---|---|---|
| 94 | d-glucose | No information | [80] | |
| 95 | d-glucose | Oil | [80] | |
| 96 | d-glucose | Yellow oily solid | [80] | |
| 97 | d-glucose | No information | [80] | |
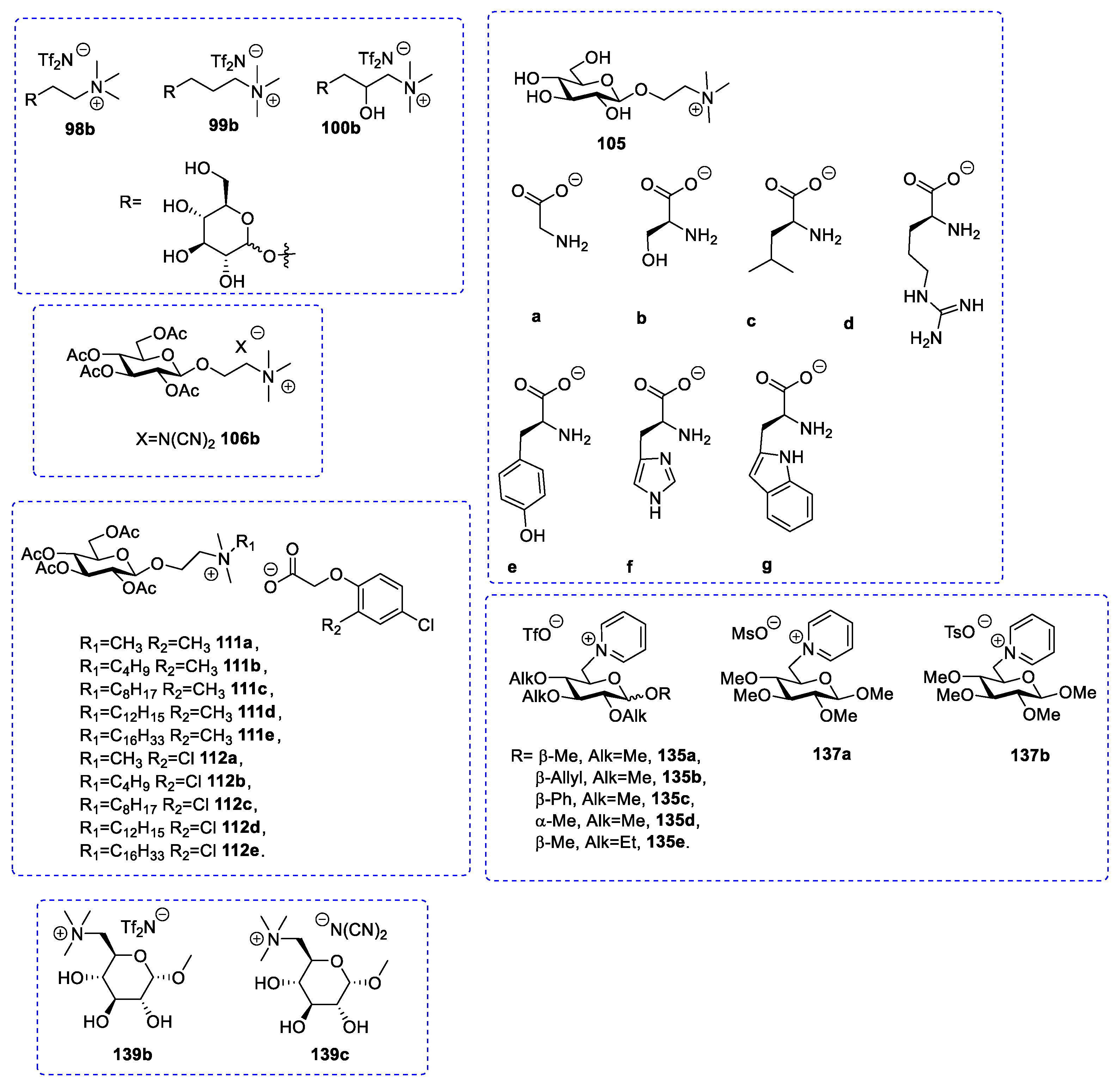
| 98a | d-glucose | White solid | Not reported | [75,76] |
| 98b | d-glucose | Liquid | [75,76] | |
| 99a | d-glucose | White solid | Not reported | [75] |
| 99b | d-glucose | Liquid | [75] | |
| 100a | d-glucose | White solid | Not reported | [75] |
| 100b | d-glucose | Liquid | [75] | |
| 101a | d-glucose | Pale yellow syrup | [78] | |
| 101b | d-glucose | Pale yellow syrup | [78] | |
| 101c | d-glucose | Pale yellow syrup | [78] | |
| 102a | d-glucose | Pale yellow syrup | [78] | |
| 102b | d-glucose | Pale yellow syrup | [78] | |
| 102c | d-glucose | Pale yellow syrup | [78] | |
| 103a | d-glucose | Red brown syrup | [77] | |
| 103b | d-glucose | Red syrup | [77] | |
| 103c | d-glucose | Red syrup | [77] | |
| 104a | d-glucose | Pale yellow syrup | [77] | |
| 104b | d-glucose | Pale yellow syrup | [77] | |
| 104c | d-glucose | Pale yellow syrup | [77] | |
| 105a | d-glucose | Viscous liquid | [81] | |
| 105b | d-glucose | Viscous liquid | [81] | |
| 105c | d-glucose | Viscous liquid | [81] | |
| 105d | d-glucose | Viscous liquid | [81] | |
| 105e | d-glucose | Viscous liquid | [81] | |
| 105f | d-glucose | Viscous liquid | [81] | |
| 105g | d-glucose | Viscous liquid | [81] | |
| 106a | d-glucose | Colorless oil | [32,54,72,74] | |
| 106b | d-glucose | White solid | 87–88 | [54] |
| 106c | d-glucose | Thick syrup | [32,54] | |
| 107a | d-glucose | White solid | 124.2–128.6 | [32] |
| 107c | d-glucose | Thick syrup | [32] | |
| 108a | d-glucose | White powder | [72,74] | |
| 108b | d-glucose | White powder | [72,74] | |
| 108c | d-glucose | White powder | [72,74] | |
| 108d | d-glucose | White powder | [72,74] | |
| 108e | d-glucose | Not reported | [72,74] | |
| 109a | d-glucose | Viscous liquid | [73] | |
| 109b | d-glucose | Viscous liquid | [73] | |
| 109c | d-glucose | Viscous liquid | [73] | |
| 109d | d-glucose | White solid | Not reported | [73] |
| 109e | d-glucose | White solid | Not reported | [73] |
| 109f | d-glucose | White solid | Not reported | [73] |
| 109a | d-glucose | Wax | [74] | |
| 109b | d-glucose | Liquid | [74] | |
| 111c | d-glucose | Wax | [74] | |
| 111d | d-glucose | Liquid | [74] | |
| 111e | d-glucose | Wax | [74] | |
| 112a | d-glucose | Solid | 121 | [74] |
| 112b | d-glucose | Solid | 134 | [74] |
| 112c | d-glucose | Wax | [74] | |
| 112d | d-glucose | Wax | [74] | |
| 112e | d-glucose | Wax | [74] | |
| 116a | d-glucose | Yellow syrup | [79] | |
| 116b | d-glucose | Not pure | [79] | |
| 116c | d-glucose | Yellow syrup | [79] | |
| 116d | d-glucose | Not pure | [79] | |
| 116e | d-glucose | Yellow syrup | [79] | |
| 116f | d-glucose | Yellow syrup | [79] | |
| 117a | d-glucose | Yellow syrup | [79] | |
| 117b | d-glucose | Not pure | [79] | |
| 117c | d-glucose | Yellow syrup | [79] | |
| 117d | d-glucose | Not pure | [79] | |
| 117e | d-glucose | Yellow syrup | [79] | |
| 117f | d-glucose | Yellow syrup | [79] | |
| 118b | d-glucose | Not reported, micellar synthesis | [79] | |
| 122 | d-glucose | No information, they only say IL | [82] | |
| 124a | d-glucose | No information, they only say IL | [82] | |
| 124b | d-glucose | No information, they only say IL | [82] | |
| 125a | d-glucose | Yellow solid | 94.6–98 | [83] |
| 125b | d-glucose | Not isolated | [83] | |
| 126a | d-glucose | Pale yellow viscous compound | [85] | |
| 126b | d-glucose | Pale yellow viscous compound | [85] | |
| 126c | d-glucose | Pale yellow viscous compound | [85] | |
| 126d | d-glucose | Pale yellow viscous compound | [85] | |
| 127a | d-glucose | Colorless viscous compound | [86,87] | |
| 127b | d-glucose | Colorless viscous compound | [86] | |
| 127c | d-glucose | Colorless viscous compound | [86] | |
| 127d | d-glucose | Colorless viscous compound | [86] | |
| 128a | d-glucose | Colorless viscous compound | [84] | |
| 128b | d-glucose | Colorless viscous compound | [84] | |
| 128c | d-glucose | Not reported | [84] | |
| 128d | d-glucose | Not reported | [84] | |
| 128e | d-glucose | Colorless viscous compound | [87] | |
| 128f | d-glucose | Colorless viscous compound | [87] | |
| 128g | d-glucose | Solid | Not reported | [87] |
| 129 | d-glucose | White solid | 229–231 | [88] |
| 130 | d-glucose | Oil | [88] | |
| 135a | d-glucose | Liquid | [99] | |
| 135b | d-glucose | Solid | 66–70 | [99] |
| 135c | d-glucose | Solid | 164–172 | [99] |
| 135d | d-glucose | Liquid | [99] | |
| 135e | d-glucose | Solid | 114–116 | [99] |
| 137a | d-glucose | Solid | 60–63 | [99] |
| 137b | d-glucose | Solid | 135–137 | [99] |
| 139a | d-glucose | Not reported | [54] | |
| 139b | d-glucose | White solid | 124 | [54] |
| 139c | d-glucose | Viscous Liquid | [54] | |
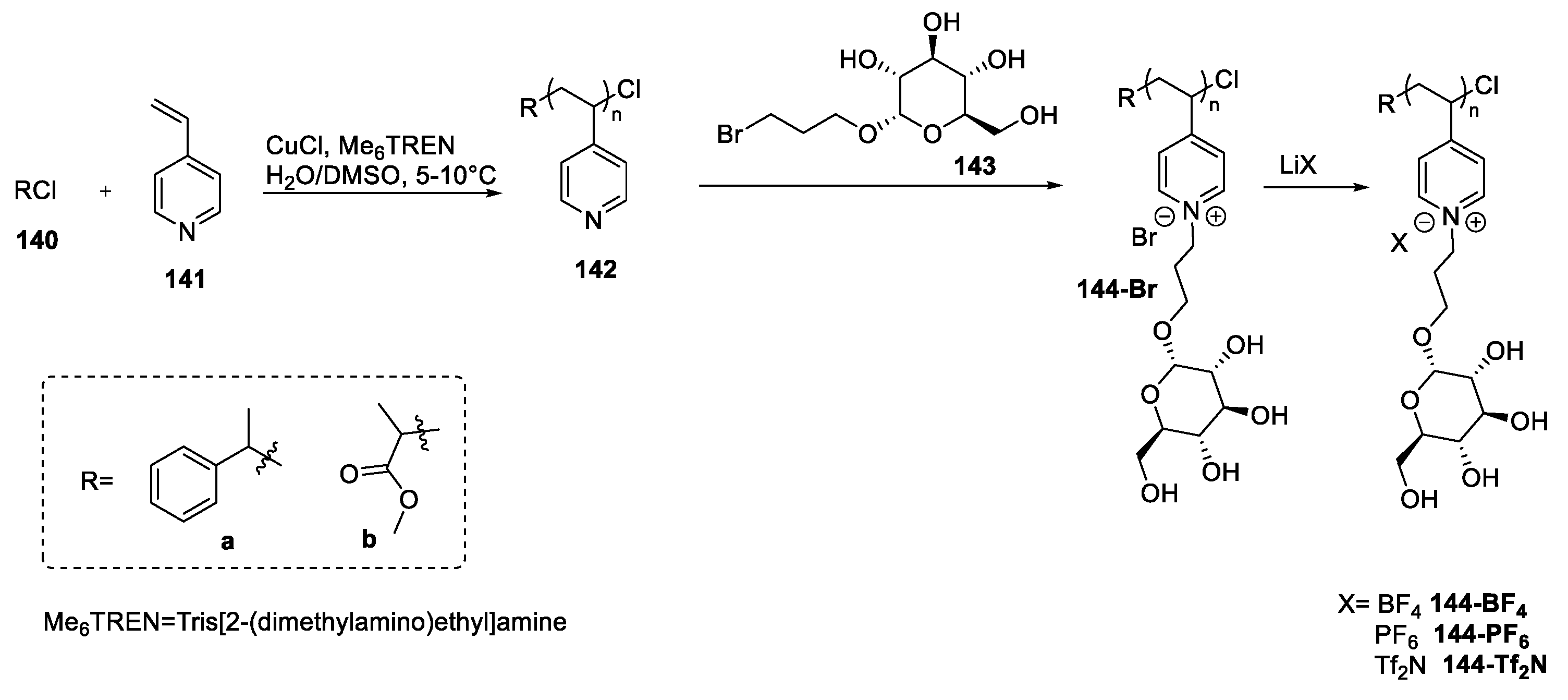
| 144-Br | d-glucose | Not reported (polymer) | [100] | |
| 144-BF4 | d-glucose | Not reported (polymer) | [100] | |
| 144-PF6 | d-glucose | Not reported (polymer) | [100] | |
| 144-Tf2N | d-glucose | Not reported (polymer) | [100] |
| Sugar Core | Physical State | Melting Point (°C) | Ref. | |
|---|---|---|---|---|
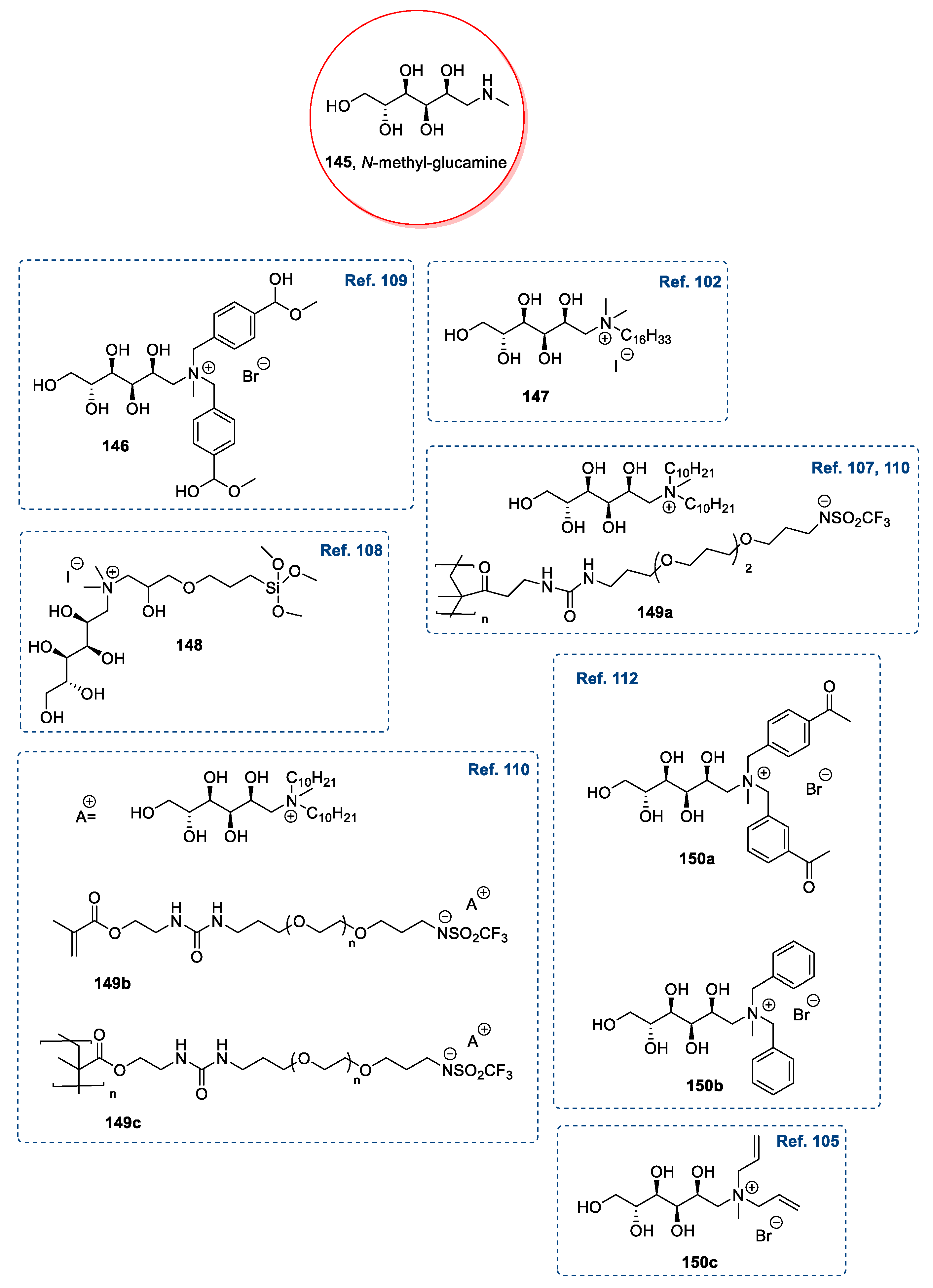
| 146 | N-methyl-d-glucamine | Classified as IL by the authors. No physical state reported. | [109] | |
| 147 | N-methyl-d-glucamine | Solid | 96–98 | [102] |
| 148 | N-methyl-d-glucamine | Not isolated | [108] | |
| 149a | N-methyl-d-glucamine | No information (polymer) | [107] | |
| 149b | N-methyl-d-glucamine | Classified as IL by the authors. No physical state reported. | [107,110] | |
| 149c | N-methyl-d-glucamine | No information (polymer) | [107,110] | |
| 150a | N-methyl-d-glucamine | Classified as IL by the authors. No physical state reported. | [112] | |
| 150b | N-methyl-d-glucamine | Classified as IL by the authors. No physical state reported. | [112] | |
| 149d | N-methyl-d-glucamine | Liquid (the authors reported the melting point for solid compounds) | [103,104] | |
| 152a | N-methyl-d-glucamine | Liquid (the authors reported the melting point for solid compounds) | [103] | |
| 152b | N-methyl-d-glucamine | Liquid (the authors reported the melting point for solid compounds) | [103] | |
| 152b | N-methyl-d-glucamine | Liquid (the authors reported the melting point for solid compounds) | [103] | |
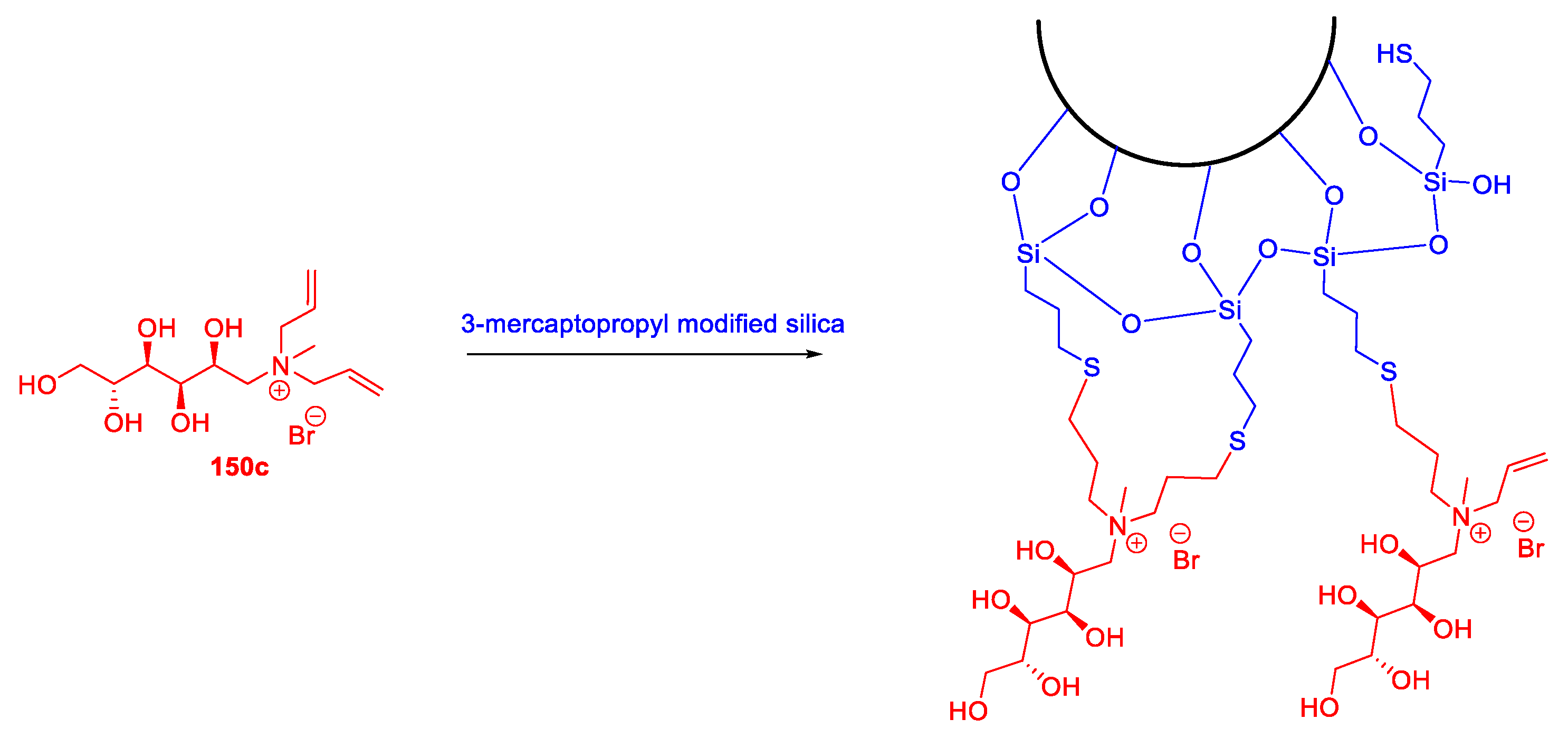
| 150c | N-methyl-d-glucamine | Classified as IL by the authors. No physical state reported. | [105] |
| Sugar Core | Physical state | Melting Point (°C) | Ref. | |
|---|---|---|---|---|
| 153a | d-mannose | No information | [80] | |
| 154a | d-mannose | No information | [80] | |
| 154b | d-mannose | Oil | [80] | |
| 153b | d-mannose | Yellow oily solid | [80] | |
| 156a | d-mannose | Oil | [32] | |
| 156b | d-mannose | Thick oil | [32] | |
| 157a | d-mannose | Oil (In “Experimental section” the authors reported “Solid, mp 225.1–260.3 °C”) | [32] | |
| 158b | d-mannose | Yellow oil | [32] | |
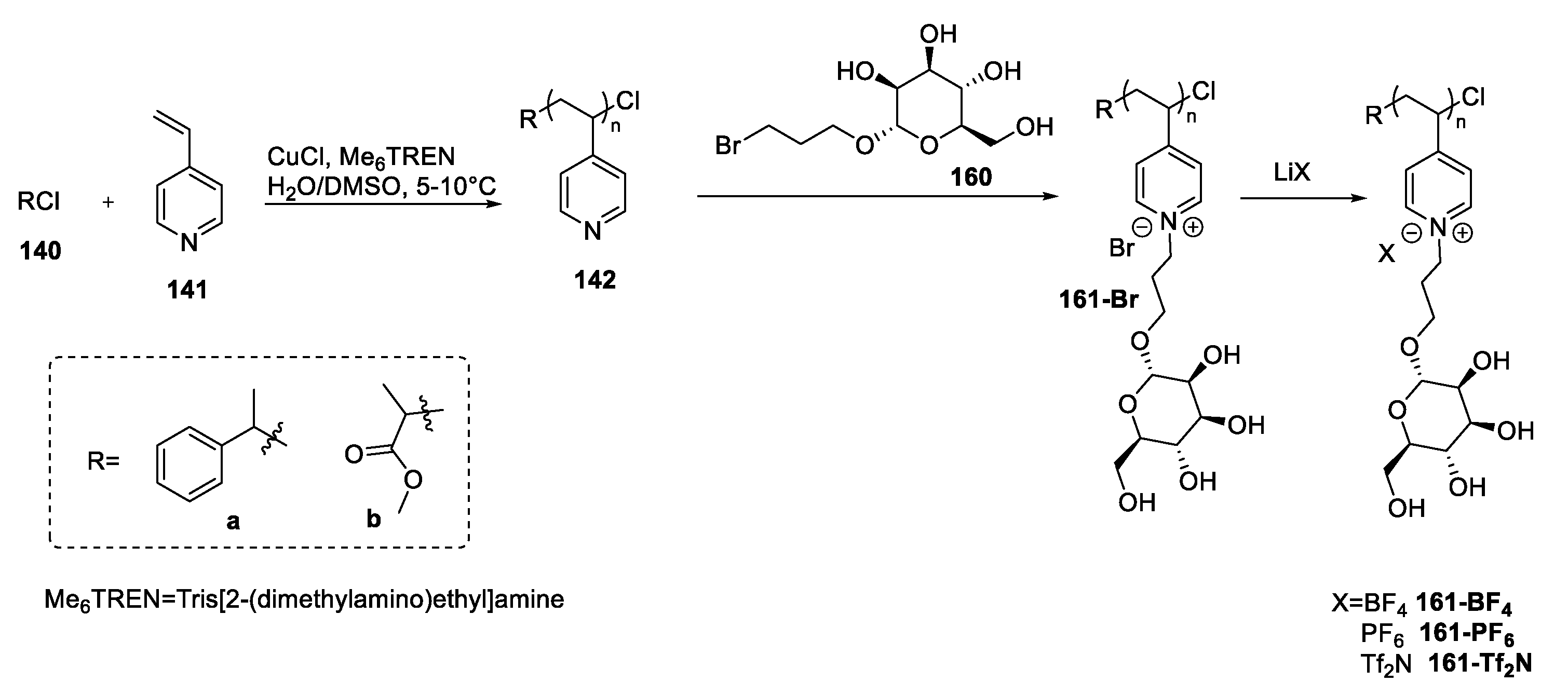
| 161-Br | d-mannose | Not reported (polymer) | [100] | |
| 161-BF4 | d-mannose | Not reported (polymer) | [100] | |
| 161-PF6 | d-mannose | Not reported (polymer) | [100] | |
| 161-Tf2N | d-mannose | Not reported (polymer) | [100] | |
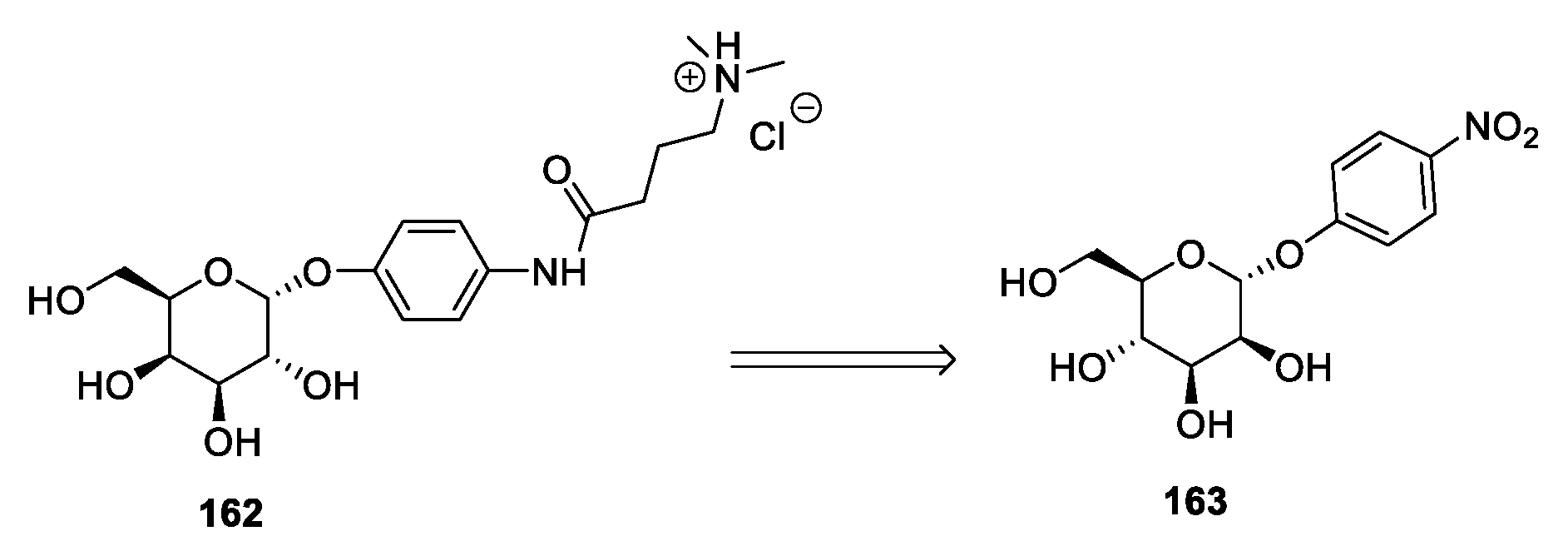
| 162 | d-mannose | White solid glassy material | 49–50 | [33] |
| Sugar Core | Physical State | Melting Point (°C) | Ref. | |
|---|---|---|---|---|
| 167a | d-ribose | Light brown color solid | 157–160 | [21] |
| 167b | d-ribose | Yellow color solid | 124–126 | [21] |
| 167c | d-ribose | Oily liquid | [21] | |
| 167d | d-ribose | Oily liquid | [21] | |
| 167e | d-ribose | Colorless solid | 96–98 | [21] |
| 167f | d-ribose | Oily liquid | [21] | |
| 168 | d-ribose | Solid | 75–78 | [115] |
| 177a | 1-deoxyribose | Solid | 48–51 | [99] |
| 178 | 1-deoxylyxose | Liquid | [99] | |
| 179 | 1-deoxyxylose | Solid | 32–36 | [99] |
| 180 | 1-deoxyarabinose | Liquid | [99] | |
| 177b | 1-deoxyribose | Liquid | [99] | |
| 177c | 1-deoxyribose | Liquid | [99] | |
| 177d | 1-deoxyribose | Liquid | [99] | |
| 182 | 1-deoxyribose | Liquid | [99] | |
| 183 | 1-deoxyribose | Solid | 92–94 | [99] |
| Sugar Core | Physical State | Melting Point (°C) | Ref. | |
|---|---|---|---|---|
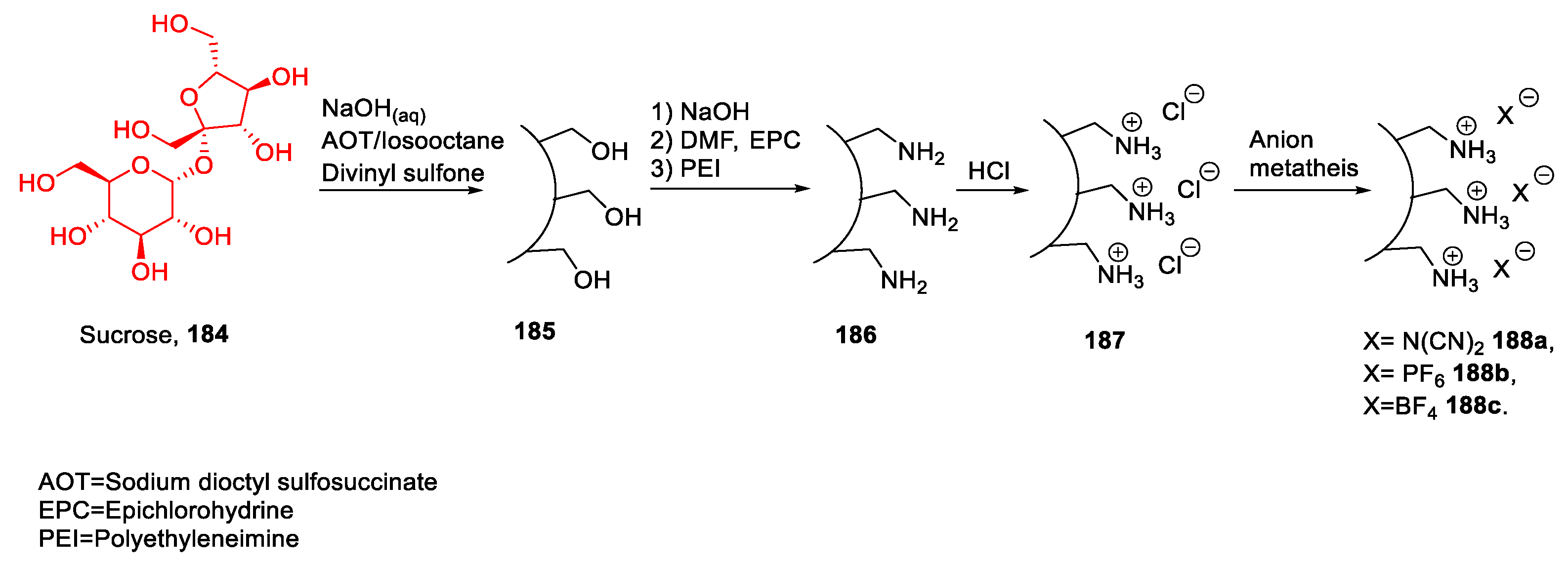
| 187 | Sucrose | Microgel | [117] | |
| 188a | Sucrose | Microgel | [117] | |
| 188b | Sucrose | Microgel | [117] | |
| 188c | Sucrose | Microgel | [117] | |
| 192a | Lactose | Red syrup | [77] | |
| 192b | Lactose | Red syrup | [77] | |
| 192c | Lactose | Red syrup | [77] | |
| 193a | Lactose | Red syrup | [77] | |
| 193b | Lactose | Red syrup | [77] | |
| 193c | Lactose | Red syrup | [77] | |
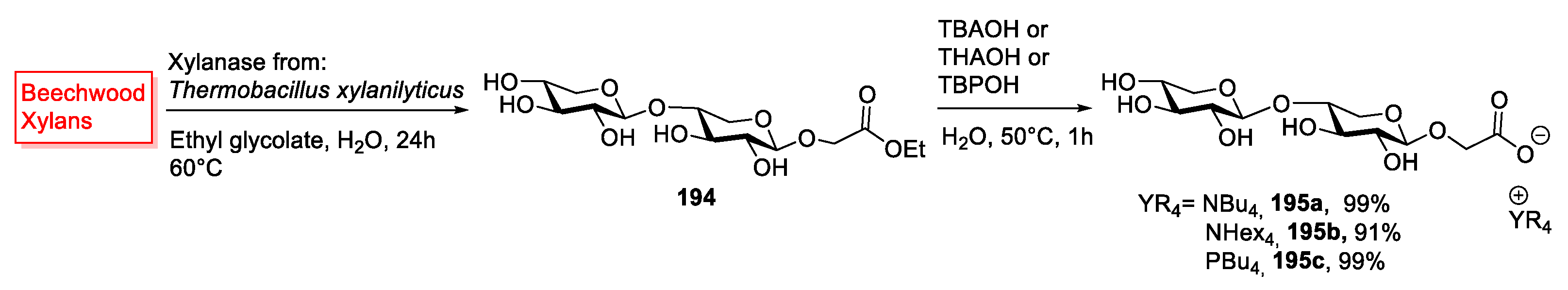
| 195a | Xyloside | White solid | Not reported | [38] |
| 195b | Xyloside | White solid | 93 | [38] |
| 195c | Xyloside | Oily | [38] |
References
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008. [Google Scholar] [CrossRef]
- Rádai, Z.; Kiss, N.Z.; Keglevich, G. An overview of the applications of ionic liquids as catalysts and additives in organic chemical reactions. Curr. Org. Chem. 2018, 1111, 533–556. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H. Ionic liquid-based electrolytes for energy storage devices: A brief review on their limits and applications. Polymers 2020, 12, 918. [Google Scholar]
- Morais, E.S.; Costa, M.; Freire, M.G.; Freire, C.S.R.; Coutinho, A.P.; Silvestre, A.J.D. Use of ionic liquids and deep eutectic solvents in polysaccharides dissolution and extraction processes towards sustainable biomass valorization. Molecules 2020, 25, 3652. [Google Scholar]
- Egorova, K.S.; Ananikov, V.P. Fundamental importance of ionic interactions in the liquid phase: A review of recent studies of ionic liquids in biomedical and pharmaceutical applications. J. Mol. Liq. 2018, 272, 271–300. [Google Scholar] [CrossRef]
- Oskarsson, A.; Wright, M.C. Ionic Liquids: New Emerging Pollutants, Similarities with Perfluorinated Alkyl Substances (PFASs). Environ. Sci. Technol. 2019, 10539–10541. [Google Scholar] [CrossRef]
- Jordan, A.; Gathergood, N. Biodegradation of ionic liquids—A critical review. Chem. Soc. Rev. 2015, 22, 8200–8237. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, M. Ionic liquids toxicity—Benefits and threats. Int. J. Mol. Sci. 2020, 21, 6267. [Google Scholar] [CrossRef]
- Hulsbosch, J.; De Vos, D.E.; Binnemans, K.; Ameloot, R.; De Vos, D.E.; Binnemans, K.; Ameloot, R. Biobased ionic liquids: Solvents for a green processing industry? ACS Sustain. Chem. Eng. 2016, 4, 2917–2931. [Google Scholar] [CrossRef]
- Mezzetta, A.; Łuczak, J.; Woch, J.; Chiappe, C.; Nowicki, J.; Guazzelli, L.; Justyna, Ł.; Woch, J.; Chiappe, C.; Nowicki, J.; et al. Surface active fatty acid ILs: Influence of the hydrophobic tail and/or the imidazolium hydroxyl functionalization on aggregates formation. J. Mol. Liq. 2019, 289, 111155. [Google Scholar] [CrossRef]
- Tampucci, S.; Guazzelli, L.; Burgalassi, S.; Carpi, S.; Chetoni, P.; Mezzetta, A.; Nieri, P.; Polini, B.; Pomelli, C.S.; Terreni, E.; et al. pH-Responsive nanostructures based on surface active fatty acid-protic ionic liquids for imiquimod delivery in skin cancer topical therapy. Pharmaceutics 2020, 12, 1078. [Google Scholar] [CrossRef]
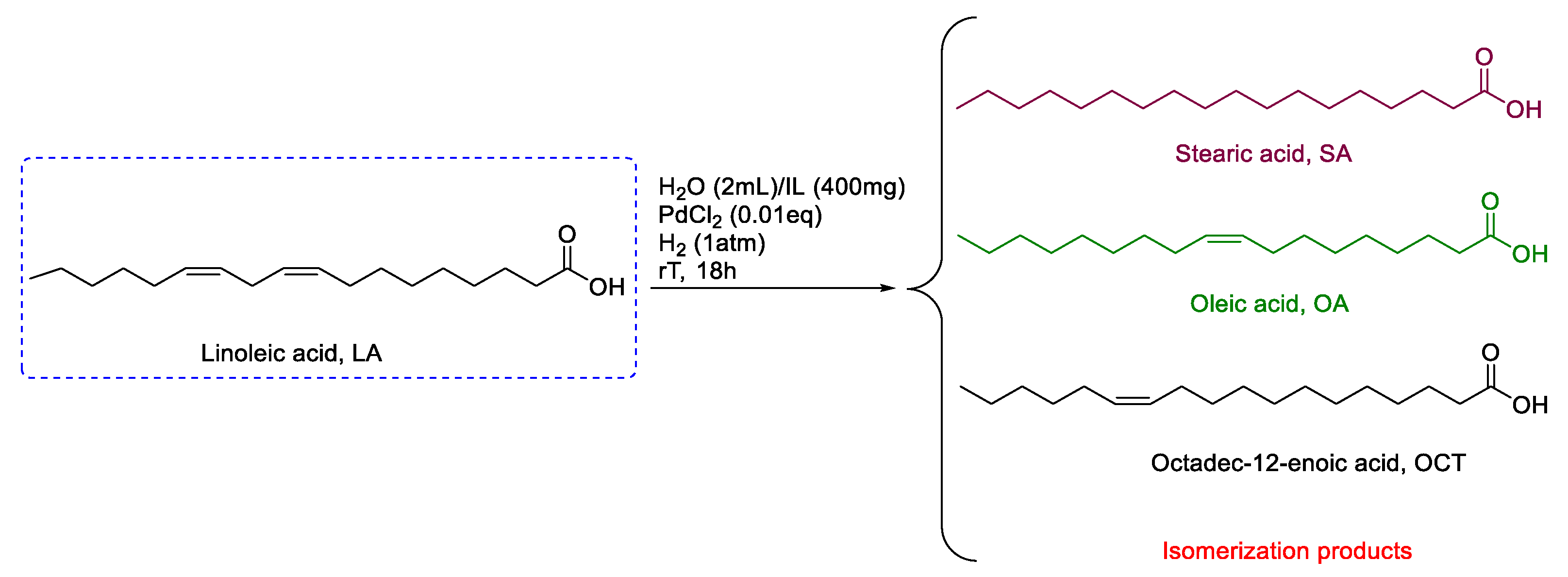
- Mezzetta, A.; Guazzelli, L.; Seggiani, M.; Pomelli, C.S.; Puccini, M.; Chiappe, C. A general environmentally friendly access to long chain fatty acid ionic liquids (LCFA-ILs). Green Chem. 2017, 19, 3103–3111. [Google Scholar] [CrossRef] [Green Version]
- Becherini, S.; Mezzetta, A.; Chiappe, C.; Guazzelli, L. Levulinate amidinium protic ionic liquids (PILs) as suitable media for the dissolution and levulination of cellulose. New J. Chem. 2019, 43, 4554–4561. [Google Scholar] [CrossRef]
- Mezzetta, A.; Becherini, S.; Pretti, C.; Monni, G.; Casu, V.; Chiappe, C.; Guazzelli, L. Insights into the levulinate-based ionic liquid class: Synthesis, cellulose dissolution evaluation and ecotoxicity assessment. New J. Chem. 2019, 43, 13010–13019. [Google Scholar] [CrossRef]
- Kirchhecker, S.; Bake, S.; Antonietti, M.; Taubert, A.; Esposito, D.; Tröger-Müller, S.; Bake, S.; Antonietti, M.; Taubert, A.; Esposito, D. Renewable pyridinium ionic liquids from the continuous hydrothermal decarboxylation of furfural-amino acid derived pyridinium zwitterions. Green Chem. 2015, 17, 4151–4156. [Google Scholar] [CrossRef] [Green Version]
- Billeci, F.; D’anna, F.; Feroci, M.; Cancemi, P.; Feo, S.; Forlino, A.; Tonnelli, F.; Seddon, K.R.; Gunaratne, H.Q.N.N.; Plechkova, N.V.; et al. When functionalization becomes useful: Ionic liquids with a “Sweet” appended moiety demonstrate drastically reduced toxicological effects. ACS Sustain. Chem. Eng. 2020, 8, 926–938. [Google Scholar] [CrossRef]
- Marra, A.; Chiappe, C.; Mele, A. Sugar-Derived ionic liquids. Chimia 2011, 65, 76–80. [Google Scholar] [CrossRef]
- Tran, A.T.; Burden, R.; Racys, D.T.; Galan, M.C. Ionic catch and release oligosaccharide synthesis (ICROS). Chem. Commun. 2011, 47, 4526–4528. [Google Scholar] [CrossRef]
- Yang, S.T.; Silva, E.M. Novel products and new technologies for use of a familiar carbohydrate, milk lactose. J. Dairy Sci. 1995, 78, 2541–2562. [Google Scholar] [CrossRef]
- Jayachandra, R.; Reddy, S.R. Synthesis of d-ribose and d-galactose derived chiral ionic liquids as recyclable chiral solvent for michael addition reaction. Trends Carbohydr. Res. 2015, 7, 60–67. [Google Scholar]
- Jayachandra, R.; Reddy, S.R.; Lakshmipathy, R. d-Galactose based hydrophobic ionic liquid: A new adsorbent for the removal of Cd 2+ ions from aqueous solution. Environ. Prog. Sustain. Energy 2019, 38, S139–S145. [Google Scholar] [CrossRef]
- Jayachandra, R.; Lakshmipathy, R.; Reddy, S.R. Hydrophobic d-galactose based ionic liquid for the sequestration of Pb2 + ions from aqueous solution. J. Mol. Liq. 2016, 219, 1172–1178. [Google Scholar] [CrossRef]
- Kaur, N.; Chopra, H.K. Synthesis and applications of carbohydrate based chiral ionic liquids as chiral recognition agents and organocatalysts. J. Mol. Liq. 2020, 298, 111994. [Google Scholar] [CrossRef]
- Pétursson, S. Protecting groups in carbohydrate chemistry. J. Chem. Educ. 1997, 74, 1297–1303. [Google Scholar] [CrossRef]
- McDonnell, C.; Cronin, L.; O’Brie, J.L.; Murphy, P.V. A general synthesis of iminosugars. J. Org. Chem. 2004, 69, 3565–3568. [Google Scholar] [CrossRef] [PubMed]
- Garegg, P.J.; Samuelsson, B. Novel reagent system for converting a hydroxy-group into an iodo-group in carbohydrates with inversion of configuration. Part 2. J. Chem. Soc. Perkin Trans. 1 1980, 2866. [Google Scholar] [CrossRef]
- Qiao, W.; Zhou, M.; Luo, L. Synthesis and characterization of novel cationic lipids derived from thio galactose. J. Surfactants Deterg. 2014, 17, 261–268. [Google Scholar] [CrossRef]
- Yang, Q.R.; Qiao, W.H.; Zhang, S.M.; Liu, D.L. Synthesis and characterization of a new cationic galactolipid with carbamate for gene delivery. Tenside Surfactants Deterg. 2010, 47, 294–299. [Google Scholar] [CrossRef]
- Doyle, L.M.; O’Sullivan, S.; Di Salvo, C.; McKinney, M.; McArdle, P.; Murphy, P.V. Stereoselective epimerizations of glycosyl thiols. Org. Lett. 2017, 19, 5802–5805. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.M.; Qiao, W.H.; Chen, Y.X.; Liu, D.L. Synthesis of cationic surfactant intermediates 3-(dimethylamino)-propane-1, 2-di-alkylcarbamate. Tenside Surfactants Deterg. 2009, 46, 228–231. [Google Scholar] [CrossRef]
- Dmochowska, B.; Piosik, J.; Woziwodzka, A.; Sikora, K.; Wiśniewski, A.; Wegrzyn, G. Mutagenicity of quaternary ammonium salts containing carbohydrate moieties. J. Hazard. Mater. 2011, 193, 272–278. [Google Scholar] [CrossRef]
- Ahmad, M.U.; Ali, S.M.; Ahmad, A.; Sheikh, S.; Chen, P.; Ahmad, I. Carbohydrate mediated drug delivery: Synthesis and characterization of new lipid-conjugates. Chem. Phys. Lipids 2015, 186, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Ferlin, N.; Gatard, S.; Van Nhien, A.N.; Courty, M.; Bouquillon, S.; Van Nhien, A.N.; Courty, M.; Bouquillon, S. Click reactions as a key step for an efficient and selective synthesis of d-xylose-based ILs. Molecules 2013, 18, 11512–11525. [Google Scholar] [CrossRef] [Green Version]
- Brusa, C.; Muzard, M.; Rémond, C.; Plantier-Royon, R. β-Xylopyranosides: Synthesis and applications. RSC Adv. 2015, 5, 91026–91055. [Google Scholar] [CrossRef]
- Mereyala, H.B.; Gurrala, S.R. A highly diastereoselective, practical synthesis of allyl, propargyl 2,3,4,6-tetra-O-acetyl-β-d-gluco, β-d-galactopyranosides and allyl, propargyl heptaacetyl-β-d-lactosides. Carbohydr. Res. 1998, 307, 351–354. [Google Scholar] [CrossRef]
- Mikata, Y.; Shinohara, Y.; Yoneda, K.; Nakamura, Y.; Esaki, K.; Tanahashi, M.; Brudzin, I.; Hirohara, S.; Yokoyama, M.; Mogami, K.; et al. Sugar-Pendant diamines. J. Org. Chem. 2001, 66, 3783–3789. [Google Scholar] [CrossRef]
- Gatard, S.; Plantier-Royon, R.; Rémond, C.; Muzard, M.; Kowandy, C.; Bouquillon, S. Preparation of new β-d-xyloside- and β-d-xylobioside-based ionic liquids through chemical and/or enzymatic reactions. Carbohydr. Res. 2017, 451, 72–80. [Google Scholar] [CrossRef]
- Jayachandra, R.; Reddy, S.R. A remarkable chiral recognition of racemic Mosher’s acid salt by naturally derived chiral ionic liquids using 19F NMR spectroscopy. RSC Adv. 2016, 6, 39758–39761. [Google Scholar] [CrossRef]
- Jayachandra, R.; Reddy, S.R. Balakrishna natural sugars derived chiral ionic liquids for asymmetric michael addition reaction. ChemistrySelect 2016, 1, 2341–2343. [Google Scholar] [CrossRef]
- Sanarico, D.; Motta, S.; Bertolini, L.; Antonelli, A. HPLC determination of organic acids in traditional balsamic vinegar of Reggio Emilia. J. Liq. Chromatogr. Relat. Technol. 2003, 26, 2177–2187. [Google Scholar] [CrossRef]
- Mato, I.; Huidobro, J.F.; Sánchez, M.P.; Muniategui, S.; Fernández-Muiño, M.A.; Sancho, M.T. Enzymatic determination of total d-gluconic acid in honey. J. Agric. Food Chem. 1997, 45, 3550–3553. [Google Scholar] [CrossRef]
- Costa, A.; Forte, A.; Zalewska, K.; Tiago, G.; Petrovski, Z.; Branco, L.C. Novel biocompatible ionic liquids based on gluconate anion. Green Chem. Lett. Rev. 2015, 8, 8–12. [Google Scholar] [CrossRef]
- Billeci, F.; D’Anna, F.; Gunaratne, H.Q.N.; Plechkova, N.V.; Seddon, K.R. “Sweet” ionic liquid gels: Materials for sweetening of fuels. Green Chem. 2018, 20, 4260–4276. [Google Scholar] [CrossRef] [Green Version]
- Javed, F.; Ullah, F.; Akil, H.M. Synthesis, characterization and cellulose dissolution capabilities of ammonium-based room temperature ionic liquids (RTILs). Pure Appl. Chem. 2018, 90, 1019–1034. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, Q.; Chen, J.; Bai, G.; Zhuo, K. Interaction of gluconate-based ionic liquids with common solvents: A study of volumetric, viscosity and conductivity properties. J. Mol. Liq. 2016, 223, 1013–1020. [Google Scholar] [CrossRef]
- Zhi, L.; Li, Q.; Sun, Y.; Yao, S. Mixed stability and antimicrobial properties of gluconamide-type cationic surfactants. J. Surfactants Deterg. 2016, 19, 337–342. [Google Scholar] [CrossRef]
- Billeci, F.; Gunaratne, H.Q.N.N.; D’Anna, F.; Morgan, G.G.; Seddon, K.R.; Plechkova, N.V.; Anna, F.D.; Morgan, G.G.; Kenneth, R. A magnetic self-contained thermochromic system with convenient temperature range. Green Chem. 2019, 21, 1412–1416. [Google Scholar] [CrossRef] [Green Version]
- Zhi, L.; Li, Q.; Li, Y.; Song, Y. Adsorption and aggregation properties of novel star-shaped gluconamide-type cationic surfactants in aqueous solution. Colloid Polym. Sci. 2014, 292, 1041–1050. [Google Scholar] [CrossRef]
- Zhi, L.; Li, Q.; Li, Y.; Song, Y. Colloids and Surfaces A: Physicochemical and Engineering Aspects Synthesis, adsorption and aggregation properties of new saccharide-cationic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 684–692. [Google Scholar] [CrossRef]
- Zhi, L.; Li, Q.; Li, Y.; Sun, Y. Colloids and Surfaces A: Physicochemical and Engineering Aspects Self-aggregation and antimicrobial activity of saccharide-cationic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2014, 456, 231–237. [Google Scholar] [CrossRef]
- Mehltretter, C.L. The chemical synthesis of d-glucuronic acid. Adv. Carbohydr. Chem. 1953, 1673, 231–249. [Google Scholar]
- Pińkowska, H.; Krzywonos, M.; Wolak, P.; Złocińska, A. Production of uronic acids by hydrothermolysis of pectin as a model substance for plant biomass waste. Green Process. Synth. 2019, 8, 683–690. [Google Scholar] [CrossRef]
- Brzeczek-Szafran, A.; Erfurt, K.; Blacha-Grzechnik, A.; Krzywiecki, M.; Boncel, S.; Chrobok, A. Carbohydrate ionic liquids and salts as all-in-one precursors for n-doped carbon. ACS Sustain. Chem. Eng. 2019, 7, 19880–19888. [Google Scholar] [CrossRef]
- Ferlin, N.; Courty, M.; Gatard, S.; Spulak, M.; Quilty, B.; Beadham, I.; Ghavre, M.; Haiß, A.; Kümmerer, K.; Gathergood, N.; et al. Biomass derived ionic liquids: Synthesis from natural organic acids, characterization, toxicity, biodegradation and use as solvents for catalytic hydrogenation processes. Tetrahedron 2013, 69, 6150–6161. [Google Scholar] [CrossRef]
- Hayouni, S.; Robert, A.; Ferlin, N.; Amri, H.; Bouquillon, S. New biobased tetrabutylphosphonium ionic liquids: Synthesis, characterization and use as a solvent or co-solvent for mild and greener Pd-catalyzed hydrogenation processes. RSC Adv. 2016, 6, 113583–113595. [Google Scholar] [CrossRef]
- Mondal, D.; Sharma, M.; Quental, M.V.; Tavares, A.P.M.; Prasad, K.; Freire, M.G. Suitability of bio-based ionic liquids for the extraction and purification of IgG antibodies. Green Chem. 2016, 18, 6071–6081. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Du, Y.; Feng, Z.; Sun, X.; Liu, J. Synthesis of a chiral ionic liquid, cholinium-clindamycin phosphate, as sole chiral selector in capillary electrophoresis. J. Chromatogr. A 2020, 1615, 460721. [Google Scholar] [CrossRef]
- Sarenkova, I.; Ciprovica, I. The current status and future perspectives of lactobionic acid production: A review. Res. Rural Dev. 2018, 1, 233–239. [Google Scholar] [CrossRef]
- Xu, H.; Feng, Z.; Du, Y. Synthesis, application and molecular modeling study of ionic liquid functionalized lactobionic acid, 3-methyl-1-(3-sulfopropyl)-1: H -imidazol-3-ium lactobionate, as a chiral selector in capillary electrophoresis. Analyst 2020, 145, 1025–1032. [Google Scholar] [CrossRef]
- Iranpour, P.; Ajamian, M.; Safavi, A.; Iranpoor, N.; Abbaspour, A.; Javanmardi, S. Synthesis of highly stable and biocompatible gold nanoparticles for use as a new X-ray contrast agent. J. Mater. Sci. Mater. Med. 2018, 29. [Google Scholar] [CrossRef]
- Sitanggang, A.B.; Wu, H.S.; Wang, S.S.; Ho, Y.C. Effect of pellet size and stimulating factor on the glucosamine production using Aspergillus sp. BCRC 31742. Bioresour. Technol. 2010, 101, 3595–3601. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Shin, H.D.; Chen, R.; Li, J.; Du, G.; Chen, J. Microbial production of glucosamine and N-acetylglucosamine: Advances and perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 6149–6158. [Google Scholar] [CrossRef] [PubMed]
- Koert, U. Isomannide and isosorbide. e-EROS Encycl. Reagents Org. Synth. 2012, 17, 1–5. [Google Scholar] [CrossRef]
- Gomes Da Silva, M.D.R.; Pereira, M.M.A. New chiral imidazolium ionic liquids from isomannide. Carbohydr. Res. 2011, 346, 197–202. [Google Scholar] [CrossRef]
- Sikora, K.; Nowacki, A.; Liberek, B.; Dmochowska, B. Methyl transfer in quaternary alkylammonium salts, derivatives of 1,4:3,6-dianhydrohexitols. J. Mol. Struct. 2020, 1206, 127701. [Google Scholar] [CrossRef]
- Carcedo, C.; Knight, J.C.; Pope, S.J.A.; Fallis, I.A.; Dervisi, A. Chiral silver and gold rings: Synthesis and structural, spectroscopic, and photophysical properties of Ag and Au metallamacrocycles of bridging NHC ligands. Organometallics 2011, 30, 2553–2562. [Google Scholar] [CrossRef]
- Malek, M.; Obadia, M.M.; Medimagh, R.; Serghei, A.; Zina, M.S.; Drockenmuller, E.; M’Sahel, M.; Obadia, M.M.; Medimagh, R.; Serghei, A.; et al. Biosourced 1,2,3-triazolium ionic liquids derived from isosorbide. New J. Chem. 2016, 40, 740–747. [Google Scholar] [CrossRef]
- Lemieux, R.U.; McInnes, A.G. The preferential tosylation of the endo -5-hydroxyl group of 1,4;3,6-dianhydro- d -glucitol. Can. J. Chem. 1960, 38, 136–140. [Google Scholar] [CrossRef]
- Zullo, V.; Górecki, M.; Guazzelli, L.; Mezzetta, A.; Pescitelli, G.; Iuliano, A. Exploiting isohexide scaffolds for the preparation of chiral ionic liquids tweezers. J. Mol. Liq. 2020, 114528. [Google Scholar] [CrossRef]
- Fellows, P.J. Food Processing Technology: Principles and Practice, 4th ed.; Woodhead Publishing: Witney, Oxford, UK, 2016; ISBN 9780081019078. [Google Scholar]
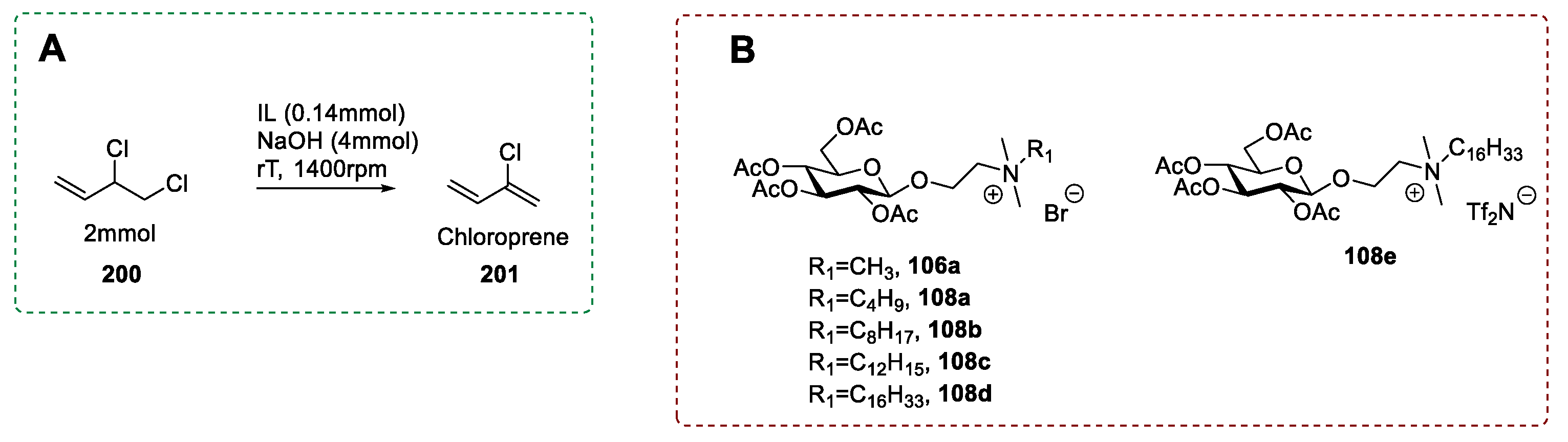
- Erfurt, K.; Markiewicz, M.; Siewniak, A.; Lisicki, D.; Zalewski, M.; Stolte, S.; Chrobok, A. Biodegradable surface active d-glucose based quaternary ammonium ionic liquids in the solventless synthesis of chloroprene. ACS Sustain. Chem. Eng. 2020, 8, 10911–10919. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, J.; Wu, Y.; Yuan, Y.; Kong, L.; Xue, J.; Huang, Z. Glucopyranoside-substituted imidazolium-based chiral ionic liquids for Pd-catalyzed homo-coupling of arylboronic acids in water. J. Carbohydr. Chem. 2020, 39, 288–299. [Google Scholar] [CrossRef]
- Pernak, J.; Czerniak, K.; Biedziak, A.; Marcinkowska, K.; Praczyk, T.; Erfurt, K.; Chrobok, A. Herbicidal ionic liquids derived from renewable sources. RSC Adv. 2016, 6, 52781–52789. [Google Scholar] [CrossRef]
- Erfurt, K.; Wandzik, I.; Walczak, K.; Matuszek, K.; Chrobok, A. Hydrogen-bond-rich ionic liquids as effective organocatalysts for Diels-Alder reactions. Green Chem. 2014, 16, 3508–3514. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Kobus, D.; Turczyn, R.; Erfurt, K.; Chrobok, A.; Biggs, M.J.P. Low resistance, highly corrugated structures based on poly(3,4-ethylenedioxythiophene) doped with a d-glucopyranoside-derived ionic liquid. Electrochem. Commun. 2020, 110, 106616. [Google Scholar] [CrossRef]
- Salman, A.A.; Goh, E.W.; Heidelberg, T.; Hussen, R.S.D.; Ali, H.M. Bis-(alkylimidazolium)-glycosides—Promising materials for easy vesicle preparation. J. Mol. Liq. 2016, 222, 609–613. [Google Scholar] [CrossRef]
- Salman, A.A.; Tabandeh, M.; Heidelberg, T.; Hussen, R.S.D.; Ali, H.M. Alkyl-imidazolium glycosides: Non-ionic—Cationic hybrid surfactants from renewable resources. Carbohydr. Res. 2015, 412, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.W.; Heidelberg, T.; Duali Hussen, R.S.; Salman, A.A. Imidazolium-linked azido-functionalized guerbet glycosides: Multifunctional surfactants for biofunctionalization of vesicles. ACS Omega 2019, 4, 17039–17047. [Google Scholar] [CrossRef]
- Cuthbert, T.J.; Hisey, B.; Harrison, T.D.; Trant, J.F.; Gillies, E.R.; Ragogna, P.J. Surprising antibacterial activity and selectivity of hydrophilic polyphosphoniums featuring sugar and hydroxy substituents. Angew. Chem. Int. Ed. 2018, 57, 12707–12710. [Google Scholar] [CrossRef]
- Brzęczek-Szafran, A.; Więcek, P.; Guzik, M.; Chrobok, A. Combining amino acids and carbohydrates into readily biodegradable, task specific ionic liquids. RSC Adv. 2020, 10, 18355–18359. [Google Scholar] [CrossRef]
- Jha, A.K.; Jain, N. Synthesis of glucose-tagged triazolium ionic liquids and their application as solvent and ligand for copper(I) catalyzed amination. Tetrahedron Lett. 2013, 54, 4738–4741. [Google Scholar] [CrossRef]
- Yuan, R.; Wang, Y.J.; Fang, Y.; Ge, W.H.; Lin, W.; Li, M.Q.; Xu, J.B.; Wan, Y.; Liu, Y.; Wu, H. The first direct synthesis of chiral TrÖger’s bases catalyzed by chiral glucose-containing pyridinium ionic liquids. Chem. Eng. J. 2017, 316, 1026–1034. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, Y.; Xie, Y.; Li, M. Green, efficient and reusable bis(imidazolium) ionic liquids promoted pd-catalyzed aqueous suzuki reaction for organic functional materials. Catal. Lett. 2018, 148, 2696–2702. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, Y.; Zhen, H.; Lin, Z.; Ling, Q. Poly(ethylene glycol)- and glucopyranoside-substituted N-heterocyclic carbene precursors for the synthesis of arylfluorene derivatives using efficient palladium-catalyzed aqueous Suzuki reaction. Appl. Organomet. Chem. 2016, 30, 924–931. [Google Scholar] [CrossRef]
- Zhou, Z.; Qiu, J.; Xie, L.; Du, F.; Xu, G.; Xie, Y.; Ling, Q. Synthesis of chiral imidazolium salts from a carbohydrate and their application in Pd-catalyzed Suzuki-Miyaura reaction. Catal. Lett. 2014, 144, 1911–1918. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, M.; Liu, G.; Xu, G.; Xue, J. Ultra-Small sugar-substituted N-heterocyclic carbene-protected palladium nanoparticles and catalytic activity. Appl. Organomet. Chem. 2019, 33, 1–8. [Google Scholar] [CrossRef]
- Zhou, Q.J.; Wan, Y.; Zhang, X.X.; Zhang, L.Z.; Zou, H.; Cui, H.; Zhou, S.L.; Wang, H.Y.; Wu, H. The first example of glucose-containing carbene Brønsted acid synthesis and catalysis: Efficient synthesis of five substituted tetrahydropyridines. Tetrahedron 2015, 71, 7070–7072. [Google Scholar] [CrossRef]
- Kopitzki, S.; Jensen, K.J.; Thiem, J. Synthesis of benzaldehyde-functionalized glycans: A novel approach towards glyco-SAMs as a tool for surface plasmon resonance studies. Chem. A Eur. J. 2010, 16, 7017–7029. [Google Scholar] [CrossRef]
- Wessel, H.P. Use Of trifluoromethanesulfonic acid in fischer glycosylations. J. Carbohydr. Chem. 1988, 7, 263–269. [Google Scholar] [CrossRef]
- Saada, M.C.; Ombouma, J.; Montero, J.L.; Supuran, C.T.; Winum, J.Y. Thiol–ene click chemistry for the synthesis of highly effective glycosyl sulfonamide carbonic anhydrase inhibitors. Chem. Commun. 2013, 49, 5699–5701. [Google Scholar] [CrossRef] [Green Version]
- Dahmén, J.; Frejd, T.; Grönberg, G.; Lave, T.; Magnusson, G.; Noori, G. 2-Bromoethyl glycosides: Synthesis and characterisation. Carbohydr. Res. 1983, 116, 303–307. [Google Scholar] [CrossRef]
- Hashim, R.; Hassan, H.; Hamzah, A.S.; Vill, V.; Wulf, M. Synthesis of branched-chain alkyl glucosides and their liquid crystal behaviour. Liq. Cryst. Commun. 2003, 1–17. [Google Scholar]
- Hashim, R.; Hashim, H.H.A.; Rodzi, N.Z.M.; Hussen, R.S.D.; Heidelberg, T. Branched chain glycosides: Enhanced diversity for phase behavior of easily accessible synthetic glycolipids. Thin Solid Films 2006, 509, 27–35. [Google Scholar] [CrossRef]
- Hanessian, S.; Ponpipom, M.M.; Lavallee, P. Procedures for the direct replacement of primary hydroxyl groups in carbohydrates by halogen. Carbohydr. Res. 1972, 24, 45–56. [Google Scholar] [CrossRef]
- Tewes, F.; Schlecker, A.; Harms, K.; Glorius, F. Carbohydrate-containing N-heterocyclic carbene complexes. J. Organomet. Chem. 2007, 692, 4593–4602. [Google Scholar] [CrossRef]
- Bay, S.; Huteau, V.; Zarantonelli, M.L.; Pires, R.; Ughetto-Monfrin, J.; Taha, M.K.; England, P.; Lafaye, P. Phosphorylcholine-carbohydrate-protein conjugates efficiently induce hapten-specific antibodies which recognize both Streptococcus pneumoniae and Neisseria meningitidis: A potential multitarget vaccine against respiratory infections. J. Med. Chem. 2004, 47, 3916–3919. [Google Scholar] [CrossRef]
- Bárczai-Martos, M.; Kőrösy, F. Preparation of acetobrome-sugars. Nature 1950, 165, 369. [Google Scholar] [CrossRef]
- Reiß, M.; Brietzke, A.; Eickner, T.; Stein, F.; Villinger, A.; Vogel, C.; Kragl, U.; Jopp, S. Synthesis of novel carbohydrate based pyridinium ionic liquids and cytotoxicity of ionic liquids for mammalian cells. RSC Adv. 2020, 10, 14299–14304. [Google Scholar] [CrossRef]
- Chen, J.; Li, D.; Bao, C.; Zhang, Q. Controlled synthesis of sugar-containing poly(ionic liquid)s. Chem. Commun. 2020, 56, 3665–3668. [Google Scholar] [CrossRef]
- Dunn, P.J. The importance of green chemistry in process research and development. Chem. Soc. Rev. 2012, 41, 1452–1461. [Google Scholar] [CrossRef]
- Gabdrakhmanov, D.R.; Valeeva, F.G.; Syakaev, V.V.; Lukashenko, S.S.; Zakharov, S.V.; Kuryashov, D.A.; Bashkirtseva, N.Y.; Zakharova, L.Y.; Latypov, S.K.; Sinyashin, O.G. Novel supramolecular system based on a cationic amphiphile bearing glucamine fragment: Structural behavior and hydrophobic probe binding. Mendeleev Commun. 2015, 25, 174–176. [Google Scholar] [CrossRef]
- Joshi, M.D.; Chalumot, G.; Kim, Y.W.; Anderson, J.L. Synthesis of glucaminium-based ionic liquids and their application in the removal of boron from water. Chem. Commun. 2012, 48, 1410–1412. [Google Scholar] [CrossRef]
- Li, T.; Joshi, M.D.; Ronning, D.R.; Anderson, J.L. Ionic liquids as solvents for in situ dispersive liquid-liquid microextraction of DNA. J. Chromatogr. A 2013, 1272, 8–14. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, S.; Li, H.; Shan, Y.; Dou, A.; Shi, X.; Xu, G. A novel surface-confined glucaminium-based ionic liquid stationary phase for hydrophilic interaction/anion-exchange mixed-mode chromatography. J. Chromatogr. A 2014, 1360, 240–247. [Google Scholar] [CrossRef]
- Joshi, M.D.; Steyer, D.J.; Anderson, J.L. Evaluating the complexation behavior and regeneration of boron selective glucaminium-based ionic liquids when used as extraction solvents. Anal. Chim. Acta 2012, 740, 66–73. [Google Scholar] [CrossRef]
- Gionfriddo, E.; Souza-Silva, É.A.; Ho, T.D.; Anderson, J.L.; Pawliszyn, J. Exploiting the tunable selectivity features of polymeric ionic liquid-based SPME sorbents in food analysis. Talanta 2018, 188, 522–530. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, M.; Wang, X.; Guo, Y.; Qiu, H.; Zhang, S. Glucaminium ionic liquid-functionalized stationary phase for the separation of nucleosides in hydrophilic interaction chromatography. Anal. Bioanal. Chem. 2015, 407, 7667–7672. [Google Scholar] [CrossRef]
- Zhao, M.; Li, T.; Jia, L.; Li, H.; Yuan, W.; Li, C.M. Pristine-graphene-supported nitrogen-doped carbon self-assembled from glucaminium-based ionic liquids as metal-free catalyst for oxygen evolution. ChemSusChem 2019, 12, 5041–5050. [Google Scholar] [CrossRef]
- Ho, T.D.; Joshi, M.D.; Silver, M.A.; Anderson, J.L. Selective extraction of genotoxic impurities and structurally alerting compounds using polymeric ionic liquid sorbent coatings in solid-phase microextraction: Alkyl halides and aromatics. J. Chromatogr. A 2012, 1240, 29–44. [Google Scholar] [CrossRef]
- Meng, F.; Miao, H.; Shi, J.; Hu, Z.; Li, G.; Ding, Y. The synthesis of carbon/cerium oxide composites clusters with the assistance of the glucaminium-based surfactant and their electrochemical performance in the glucose monitoring. J. Alloys Compd. 2017, 713, 125–131. [Google Scholar] [CrossRef]
- Joshi, M.D.; Li, T.; Zhong, Q.; Anderson, J.L. Using glucaminium-based ionic liquids for improving the separation of 2-aminopyrimidine-5-ylboronic acid and its pinacol ester by high performance liquid chromatography. J. Chromatogr. A 2013, 1308, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shi, Y.; Zhang, P.; Miao, M.; Zhang, T.; Jiang, B. d-Mannose: Properties, production, and applications: An overview. Compr. Rev. Food Sci. Food Saf. 2016, 15, 773–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wulf, P.; Vandamme, E.J. Production of d-ribose by fermentation. Appl. Microbiol. Biotechnol. 1997, 48, 141–148. [Google Scholar] [CrossRef]
- Dmochowska, B.; Sikora, K.; Chojnacki, J.; Wojnowski, W.; Wiśniewski, A. N,N,N-Trimethyl-N-(methyl 5-deoxy-2,3-O-isopropylidene-β-d- ribofuranosid-5-yl)ammonium 4-methylbenzenesulfonate sesquihydrate. Acta Crystallogr. Sect. E Struct. Rep. Online 2013, 69. [Google Scholar] [CrossRef]
- Sairam, P.; Puranik, R.; Sreenivasa Rao, B.; Veerabhadra Swamy, P.; Chandra, S. Synthesis of 1,2,3-tri-O-acetyl-5-deoxy-d-ribofuranose from d-ribose. Carbohydr. Res. 2003, 338, 303–306. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S. Sucrose based ionic liquid colloidal microgels in separation of biomacromolecules. Sep. Purif. Technol. 2018, 196, 191–199. [Google Scholar] [CrossRef]
- Tsunashima, K.; Niwa, E.; Kodama, S.; Sugiya, M.; Ono, Y. Thermal and transport properties of ionic liquids based on benzyl-substituted phosphonium cations. J. Phys. Chem. B 2009, 113, 15870–15874. [Google Scholar] [CrossRef]
- Carvalho, P.J.; Ventura, S.P.M.; Batista, M.L.S.; Schröder, B.; Gonçalves, F.; Esperança, J.; Mutelet, F.; Coutinho, J.A.P. Understanding the impact of the central atom on the ionic liquid behavior: Phosphonium vs ammonium cations. J. Chem. Phys. 2014, 140. [Google Scholar] [CrossRef]
- Kumari, K.; Singh, P.; Mehrotra, G.K. Ionic liquid: Best alternate to organic solvent to carry out organic synthesis. Int. J. Green Nanotechnol. Biomed. 2012, 4, 262–276. [Google Scholar] [CrossRef]
- Endres, F.; Zein El Abedin, S. Air and water stable ionic liquids in physical chemistry. Phys. Chem. Chem. Phys. 2006, 8, 2101–2116. [Google Scholar] [CrossRef]
- Tindale, J.J.; Na, C.; Jennings, M.C.; Ragogna, P.J. Synthesis and characterization of fluorinated phosphonium ionic liquids. Can. J. Chem. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Anderson, J.L.; Armstrong, D.W. High-Stability ionic liquids. A new class of stationary phases for gas chromatography. Anal. Chem. 2003, 75, 4851–4858. [Google Scholar] [CrossRef] [Green Version]
- Baranyai, K.J.; Deacon, G.B.; MacFarlane, D.R.; Pringle, J.M.; Scott, J.L. Thermal degradation of ionic liquids at elevated temperatures. Aust. J. Chem. 2004, 57, 145–147. [Google Scholar] [CrossRef]
- Kroon, M.C.; Buijs, W.; Peters, C.J.; Witkamp, G.J. Quantum chemical aided prediction of the thermal decomposition mechanisms and temperatures of ionic liquids. Thermochim. Acta 2007, 465, 40–47. [Google Scholar] [CrossRef]
- Kulkarni, P.S.; Branco, L.C.; Crespo, J.G.; Nunes, M.C.; Raymundo, A.; Afonso, C.A.M. Comparison of physicochemical properties of new ionic liquids based on imidazolium, quaternary ammonium, and guanidinium cations. Chem. A. Eur. J. 2007, 13, 8478–8488. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Tokuda, H.; Ishii, K.; Susan, M.A.B.H.; Tsuzuki, S.; Hayamizu, K.; Watanabe, M. Physicochemical properties and structures of room-temperature ionic liquids. 3. Variation of cationic structures. J. Phys. Chem. B 2006, 110, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, Y.; Gilbert, B.; Guibard, I. Catalytic dimerization of alkenes by nickel complexes in organochloroaluminate molten salts. J. Chem. Soc. Chem. Commun. 1990, 1715–1716. [Google Scholar] [CrossRef]
- Guerrero-Sanchez, C.; Lara-Ceniceros, T.; Jimenez-Regalado, E.; Raşa, M.; Schubert, U.S. Magnetorheological fluids based on ionic liquids. Adv. Mater. 2007, 19, 1740–1747. [Google Scholar] [CrossRef]
- Martin, D.F.; Shamma, M.; Fernelius, W.C. Bis-(β-Diketones). I. synthesis of compounds of the type RCOCH2CO-Y-COCH2COR. J. Am. Chem. Soc. 1958, 80, 4891–4895. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.-J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Q.; Zhu, L.; Zhang, S.; Li, J.; Zhang, X.; Deng, Y. Novel ionic liquid crystals based on N-alkylcaprolactam as cations. Chem. Mater. 2007, 19, 2544–2550. [Google Scholar] [CrossRef]
- Cui, F.M.; Zhang, X.Y.; Shang, L.M. Thermogravimetric analysis of glucose-based and fructose-based carbohydrates. Adv. Mater. Res. 2013, 805–806, 265–268. [Google Scholar] [CrossRef]
- Perlin, A.S. Thermal decarboxylation of uronic acids. Can. J. Chem. 1952, 30, 278–290. [Google Scholar] [CrossRef]
- Zhang, S.; Dokko, K.; Watanabe, M. Carbon materialization of ionic liquids: From solvents to materials. Mater. Horizons 2015, 2, 168–197. [Google Scholar] [CrossRef]
- Bowers, J.; Butts, C.P.; Martin, P.J.; Vergara-Gutierrez, M.C.; Heenan, R.K. Aggregation behavior of aqueous solutions of ionic liquids. Langmuir 2004, 20, 2191–2198. [Google Scholar] [CrossRef]
- Cojocaru, O.A.; Shamshina, J.L.; Gurau, G.; Syguda, A.; Praczyk, T.; Pernak, J.; Rogers, R.D. Ionic liquid forms of the herbicide dicamba with increased efficacy and reduced volatility. Green Chem. 2013, 15, 2110–2120. [Google Scholar] [CrossRef]
- Fukumoto, K.; Yoshizawa, M.; Ohno, H. Room temperature ionic liquids from 20 natural amino acids. J. Am. Chem. Soc. 2005, 127, 2398–2399. [Google Scholar] [CrossRef]
- Liu, Q.P.; Hou, X.D.; Li, N.; Zong, M.H. Ionic liquids from renewable biomaterials: Synthesis, characterization and application in the pretreatment of biomass. Green Chem. 2012, 14, 304–307. [Google Scholar] [CrossRef]
- Tao, D.J.; Cheng, Z.; Chen, F.F.; Li, Z.M.; Hu, N.; Chen, X.S. Synthesis and thermophysical properties of biocompatible cholinium-based amino acid ionic liquids. J. Chem. Eng. Data 2013, 58, 1542–1548. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Thermal stability of ionic liquids. In Encyclopedia of Ionic Liquids; Fehrmann, R., Santini, C., Eds.; Springer: Singapore, 2020; pp. 1–13. [Google Scholar]
- Kuhn, P.; Forget, A.; Su, D.; Thomas, A.; Antonietti, M. From microporous regular frameworks to mesoporous materials with ultrahigh surface area: Dynamic reorganization of porous polymer networks. J. Am. Chem. Soc. 2008, 130, 13333–13337. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Pereira, C.S. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Cevasco, G.; Chiappe, C. Are ionic liquids a proper solution to current environmental challenges? Green Chem. 2014, 16, 2375–2385. [Google Scholar] [CrossRef]
- Weber, C.C.; Masters, A.F.; Maschmeyer, T. Structural features of ionic liquids: Consequences for material preparation and organic reactivity. Green Chem. 2013, 15, 2655–2679. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Solvent nanostructure, the solvophobic effect and amphiphile self-assembly in ionic liquids. Chem. Soc. Rev. 2013, 42, 1096–1120. [Google Scholar] [CrossRef]
- Hubbard, C.D.; Illner, P.; Van Eldik, R. Understanding chemical reaction mechanisms in ionic liquids: Successes and challenges. Chem. Soc. Rev. 2011, 40, 272–290. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Shi, F.; Liu, S.; He, Y.; Lu, L.; Ma, X.; Deng, Y. Quaternary ammonium ionic liquids as Bi-functional catalysts for one-step synthesis of dimethyl carbonate from ethylene oxide, carbon dioxide and methanol. Catal. Lett. 2011, 141, 339–346. [Google Scholar] [CrossRef]
- Zhao, H.; Jones, C.L.; Baker, G.A.; Xia, S.; Olubajo, O.; Person, V.N. Regenerating cellulose from ionic liquids for an accelerated enzymatic hydrolysis. J. Biotechnol. 2009, 139, 47–54. [Google Scholar] [CrossRef]
- Labbé, N.; Kline, L.M.; Moens, L.; Kim, K.; Kim, P.C.; Hayes, D.G. Activation of lignocellulosic biomass by ionic liquid for biorefinery fractionation. Bioresour. Technol. 2012, 104, 701–707. [Google Scholar] [CrossRef]
- Huang, Y.B.; Xin, P.P.; Li, J.X.; Shao, Y.Y.; Huang, C.B.; Pan, H. Room-Temperature dissolution and mechanistic investigation of cellulose in a tetra-butylammonium acetate/dimethyl sulfoxide system. ACS Sustain. Chem. Eng. 2016, 4, 2286–2294. [Google Scholar] [CrossRef]
- Li, C.; Knierim, B.; Manisseri, C.; Arora, R.; Scheller, H.V.; Auer, M.; Vogel, K.P.; Simmons, B.A.; Singh, S. Comparison of dilute acid and ionic liquid pretreatment of switchgrass: Biomass recalcitrance, delignification and enzymatic saccharification. Bioresour. Technol. 2010, 101, 4900–4906. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.A.P.; Frizzo, C.P.; Moreira, D.N.; Zanatta, N.; Bonacorso, H.G. Ionic liquids in heterocyclic synthesis. Chem. Rev. 2008, 108, 2015–2050. [Google Scholar] [CrossRef]
- Eijsbouts, S. Hydrotreating catalysts. Synth. Solid Catal. 2009, 301–328. [Google Scholar] [CrossRef]
- Dupont, J.; Suarez, P.A.Z.; Umpierre, A.; De Souza, R.F. Pd(II)-Dissolved in ionic liquids: A recyclable catalytic system for the selective biphasic hydrogenation of dienes to monoenes. J. Braz. Chem. Soc. 2000, 11, 293–297. [Google Scholar] [CrossRef]
- Umpierre, A.P.; Machado, G.; Fecher, G.H.; Morais, J.; Dupont, J. Selective hydrogenation of 1,3-butadiene to 1-butene by Pd(0) nanoparticles embedded in imidazolium ionic liquids. Adv. Synth. Catal. 2005, 347, 1404–1412. [Google Scholar] [CrossRef]
- Bouquillon, S.; Courant, T.; Dean, D.; Gathergood, N.; Morrissey, S.; Pegot, B.; Scammells, P.J.; Singer, R.D. Biodegradable ionic liquids: Selected synthetic applications. Aust. J. Chem. 2007, 60, 843–847. [Google Scholar] [CrossRef]
- Couling, D.J.; Bernot, R.J.; Docherty, K.M.; Dixon, J.N.K.; Maginn, E.J. Assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure–property relationship modeling. Green Chem. 2006, 8, 82–90. [Google Scholar] [CrossRef]
- Anthony, J.L.; Maginn, E.J.; Brennecke, J.F. Solution thermodynamics of imidazolium-based ionic liquids and water. J. Phys. Chem. B 2001, 105, 10942–10949. [Google Scholar] [CrossRef]
- She, J.; Ye, L.; Zhu, J.; Yuan, Y. Catalytic performance of chiral rhodium complex with water-soluble sulfonated (R)-2,2′-bis(diphenylphosphino)-1,1′-binaphthyl for enantioseletive hydrogenation in ionic liquid biphasic system. Catal. Lett. 2007, 116, 70–75. [Google Scholar] [CrossRef] [Green Version]
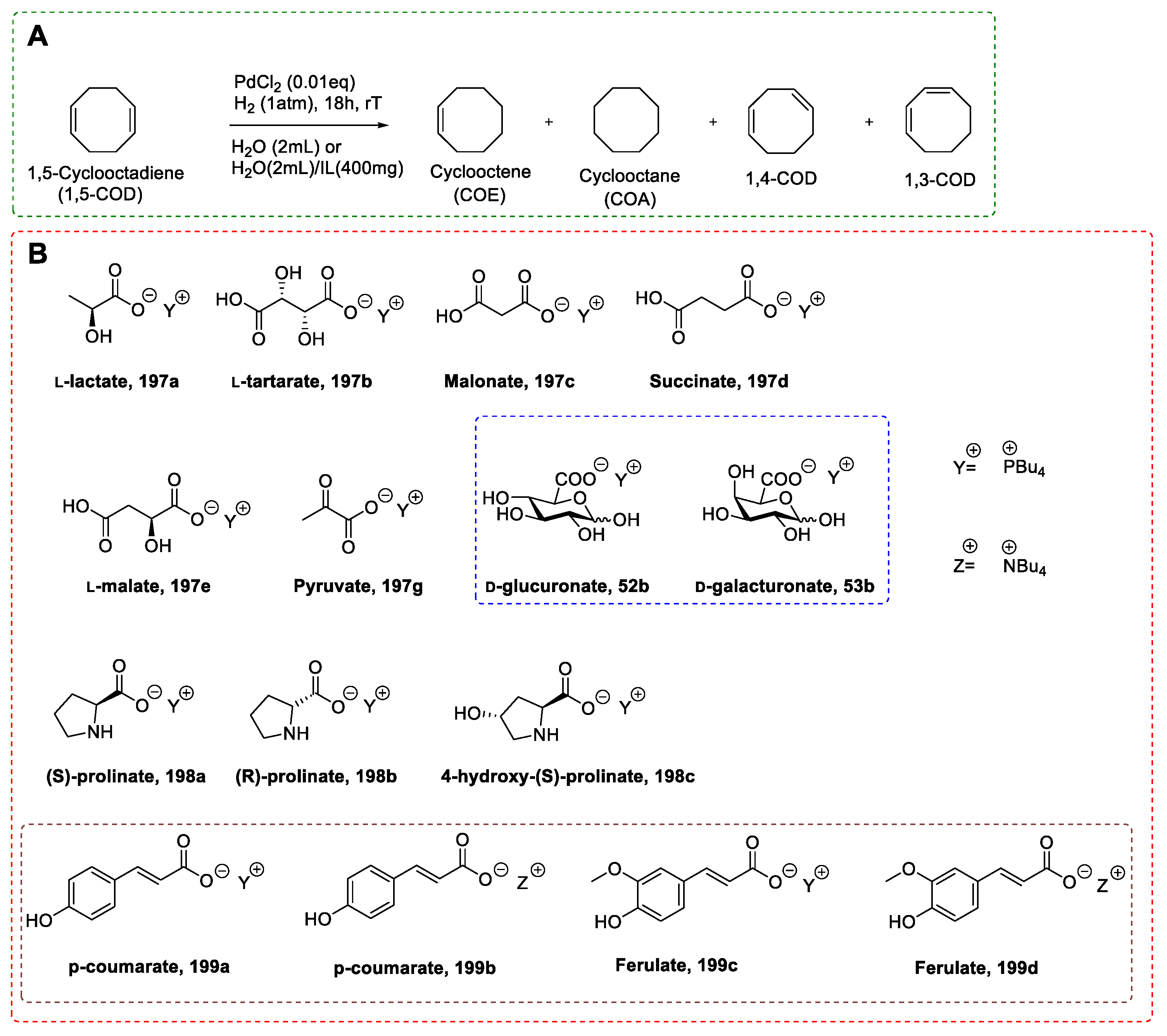
- Schmidt, A.; Schomäcker, R. Kinetics of 1,5-cyclooctadiene hydrogenation on Pd/α-Al 2O3. Ind. Eng. Chem. Res. 2007, 46, 1677–1681. [Google Scholar] [CrossRef]
- Aguilera-herrador, E.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Sample treatments based on ionic liquids. Ion. Liq. Appl. Perspect. 2008. [Google Scholar]
- Hilal, N.; Kim, G.J.; Somerfield, C. Boron removal from saline water: A comprehensive review. Desalination 2011, 273, 23–35. [Google Scholar] [CrossRef]
- Bryjak, M.; Wolska, J.; Kabay, N. Removal of boron from seawater by adsorption-membrane hybrid process: Implementation and challenges. Desalination 2008, 223, 57–62. [Google Scholar] [CrossRef]
- Hubbard, S.A. Comparative toxicology of borates. Biol. Trace Elem. Res. 1998, 66, 343–357. [Google Scholar] [CrossRef]
- Simonnot, M.O.; Castel, C.; Nicolaï, M.; Rosin, C.; Sardin, M.; Jauffret, H. Boron removal from drinking water with a boron selective resin: Is the treatment really selective? Water Res. 2000, 34, 109–116. [Google Scholar] [CrossRef]
- Kaftan, Ö.; Açikel, M.; Eroǧlu, A.E.; Shahwan, T.; Artok, L.; Ni, C. Synthesis, characterization and application of a novel sorbent, glucamine-modified MCM-41, for the removal/preconcentration of boron from waters. Anal. Chim. Acta 2005, 547, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Anderson, J.L. Dispersive liquid-liquid microextraction using an in situ metathesis reaction to form an ionic liquid extraction phase for the preconcentration of aromatic compounds from water. Anal. Bioanal. Chem. 2009, 395, 1491–1502. [Google Scholar] [CrossRef]
- Ho, T.D.; Canestraro, A.J.; Anderson, J.L. Ionic liquids in solid-phase microextraction: A review. Anal. Chim. Acta 2011, 695, 18–43. [Google Scholar] [CrossRef]
- Yu, H.; Ho, T.D.; Anderson, J.L. Ionic liquid and polymeric ionic liquid coatings in solid-phase microextraction. TrAC Trends Anal. Chem. 2013, 45, 219–232. [Google Scholar] [CrossRef]
- Mecerreyes, D. Applications of Ionic Liquids in Polymer Science and Technology; Springer: Berlin, Germany, 2015; ISBN 9783662449035. [Google Scholar]
- Karimi, B.; Tavakolian, M.; Akbari, M.; Mansouri, F. Ionic liquids in asymmetric synthesis: An overall view from reaction media to supported ionic liquid catalysis. ChemCatChem 2018, 10, 3173–3205. [Google Scholar] [CrossRef]
- Santiago, C.C.; Lafuente, L.; Bravo, R.; Díaz, G.; Ponzinibbio, A. Ionic liquids as phase transfer catalysts: Enhancing the biphasic extractive epoxidation reaction for the selective synthesis of β-O-glycosides. Tetrahedron Lett. 2017, 58, 3739–3742. [Google Scholar] [CrossRef]
- Kryshtal, G.V.; Zhdankina, G.M.; Zlotin, S.G. Tetraalkylammonium and 1,3-dialkylimidazolium salts with fluorinated anions as recoverable phase-transfer catalysts in solid base-promoted cross-aldol condensations. Eur. J. Org. Chem. 2005, 2822–2827. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, S.; Duan, H. An ionic liquid based on a cyclic guanidinium cation is an efficient medium for the selective oxidation of benzyl alcohols. Tetrahedron Lett. 2004, 45, 2013–2015. [Google Scholar] [CrossRef]
- Muthusamy, S.; Gnanaprakasam, B. Imidazolium salts as phase transfer catalysts for the dialkylation and cycloalkylation of active methylene compounds. Tetrahedron Lett. 2005, 46, 635–638. [Google Scholar] [CrossRef]
- Lourenço, N.M.T.; Afonso, C.A.M. Ionic liquid as an efficient promoting medium for two-phase nucleophilic displacement reactions. Tetrahedron 2003, 59, 789–794. [Google Scholar] [CrossRef]
- Baj, S.; Chrobok, A.; Derfla, S. A new method for dialkyl peroxides synthesis in ionic liquids as solvents. Green Chem. 2006, 8, 292–295. [Google Scholar] [CrossRef]
- Pullan, N.; Liu, M.; Topham, P.D. Reversible addition-fragmentation chain transfer polymerization of 2-chloro-1,3-butadiene. Polym. Chem. 2013, 4, 2272–2277. [Google Scholar] [CrossRef] [Green Version]
- Wells, A.S.; Coombe, V.T. On the freshwater ecotoxicity and biodegradation properties of some common ionic liquids. Org. Process Res. Dev. 2006, 10, 794–798. [Google Scholar] [CrossRef]
- Wasserscheid, P. Transition metal catalysis in ionic liquids. Handb. Green Chem. 2010, 6, 65–91. [Google Scholar]
- Shaterian, H.R.; Kangani, M. Mild Brønsted basic ionic liquids catalyzed three component synthesis of pyrazolo[1,2-a][1,2,4]triazole-1,3-dione and 2-amino-3-cyano-5,10- dioxo-4-phenyl-5,10-dihydro-4H-benzo[g]chromene derivatives. Sci. Iran. 2013, 20, 571–579. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Artacho, J.; Wärnmark, K. The 125th anniversary of the Tröger’s base molecule: Synthesis and applications of Tröger’s base analogues. Eur. J. Org. Chem. 2012, 7015–7041. [Google Scholar] [CrossRef]
- Dolenský, B.; Elguero, J.; Král, V.; Pardo, C.; Valík, M. Current Tröger’s base chemistry. Adv. Heterocycl. Chem. 2007, 93, 1–56. [Google Scholar] [CrossRef]
- Michon, C.; Sharma, A.; Bernardinelli, G.; Francotte, E.; Lacour, J. Stereoselective synthesis of configurationally stable functionalized ethano-bridged Tröger bases. Chem. Commun. 2010, 46, 2206–2208. [Google Scholar] [CrossRef]
- Harmata, M.; Rayanil, K.O.; Barnes, C.L. Sequential alkylation of Tröger’s base. An approach to new chiral ligands. Supramol. Chem. 2006, 18, 581–586. [Google Scholar] [CrossRef]
- Benkhauser-Schunk, C.; Wezisla, B.; Urbahn, K.; Kiehne, U.; Daniels, J.; Schnakenburg, G.; Neese, F.; Lützen, A. Synthesis, chiral resolution, and absolute configuration of functionalized tröger’s base derivatives: Part II. Chempluschem 2012, 77, 396–403. [Google Scholar] [CrossRef]
- Kamiyama, T.; Özer, M.S.; Otth, E.; Deska, J.; Cvengroš, J. Modular synthesis of optically active tröger’s base analogues. Chempluschem 2013, 78, 1510–1516. [Google Scholar] [CrossRef]
- Dolenský, B.; Kessler, J.; Jakubek, M.; Havlík, M.; Čejka, J.; Novotná, J.; Král, V. Synthesis and characterisation of a new naphthalene tris-Tröger’s base derivative—A chiral molecular clip. Tetrahedron Lett. 2013, 54, 308–311. [Google Scholar] [CrossRef]
- Yasukawa, T.; Miyamura, H.; Kobayashi, S. Cellulose-supported chiral rhodium nanoparticles as sustainable heterogeneous catalysts for asymmetric carbon-carbon bond-forming reactions. Chem. Sci. 2015, 6, 6224–6229. [Google Scholar] [CrossRef] [Green Version]
- Lokman, I.M.; Rashid, U.; Taufiq-Yap, Y.H.; Yunus, R. Methyl ester production from palm fatty acid distillate using sulfonated glucose-derived acid catalyst. Renew. Energy 2015, 81, 347–354. [Google Scholar] [CrossRef]
- Anand, N.; Chanda, T.; Koley, S.; Chowdhury, S.; Singh, M.S. CuSO 4—d -glucose, an inexpensive and eco-efficient catalytic system: Direct access to diverse quinolines through modified Friedländer approach involving S N Ar/reduction/annulation cascade in one pot. RSC Adv. 2015, 5, 7654–7660. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, P.; Shen, Y.; Zhang, F.R.; Wan, Y.; Shi, D.Q. Convenient syntheses of Tröger’s base derivatives in ionic liquids. Synlett 2007, 90, 336–338. [Google Scholar] [CrossRef]
- Komorek, U.; Wilk, K.A. Surface and micellar properties of new nonionic gemini aldonamide-type surfactants. J. Colloid Interface Sci. 2004, 271, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Cameotra, S.S. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 2004, 22, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Bazito, R.C.; El Seoud, O.A. Sugar-Based surfactants: Adsorption and micelle formation of sodium methyl 2-acylamido-2-deoxy-6-O-sulfo-d-glucopyranosides. Langmuir 2002, 18, 4362–4366. [Google Scholar] [CrossRef]
- Zielińska, K.; Wilk, K.A.; Jezierski, A.; Jesionowski, T. Microstructure and structural transition in microemulsions stabilized by aldonamide-type surfactants. J. Colloid Interface Sci. 2008, 321, 408–417. [Google Scholar] [CrossRef]
- Soussan, E.; Pasc-Banu, A.; Consola, S.; Labrot, T.; Perez, E.; Blanzat, M.; Oda, R.; Vidal, C.; Rico-Lattes, I. New catanionic triblock amphiphiles: Supramolecular organization of a sugar-derived bolaamphiphile associated with dicarboxylates. ChemPhysChem 2005, 6, 2492–2494. [Google Scholar] [CrossRef]
- Viscardi, G.; Quagliotto, P.; Barolo, C.; Savarino, P.; Barni, E.; Fisicaro, E. Synthesis and surface and antimicrobial properties of novel cationic surfactants. J. Org. Chem. 2000, 65, 8197–8203. [Google Scholar] [CrossRef]
- Fisicaro, E.; Biemmi, M.; Compari, C.; Duce, E.; Peroni, M.; Viscardi, G.; Quagliotto, P. Thermodynamic properties of aqueous micellar solutions of some new acetylated gluco-cationic surfactants. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 301, 129–136. [Google Scholar] [CrossRef]
- Negm, N.A.; Mohamed, A.S. Synthesis, characterization and biological activity of sugar-based gemini cationic amphiphiles. J. Surfactants Deterg. 2008, 11, 215–221. [Google Scholar] [CrossRef]
- Rózycka-Roszak, B.; Misiak, P.; Woźniak, E.; Zaczyńska, E.; Czarny, A.; Wilk, K.A. Effect of biocompatible gluconamide-type cationic surfactants on thermotropic phase behavior of phosphatidylcholine/cholesterol bilayers. Thermochim. Acta 2014, 590, 219–225. [Google Scholar] [CrossRef]
- Chen, B.; He, M.; Mao, X.; Cui, R.; Pang, D.; Hu, B. Ionic liquids improved reversed-phase HPLC on-line coupled with ICP-MS for selenium speciation. Talanta 2011, 83, 724–731. [Google Scholar] [CrossRef]
- Tang, Y.; Sun, A.; Liu, R.; Zhang, Y. Simultaneous determination of fangchinoline and tetrandrine in stephania tetrandra s. Moore by using 1-alkyl-3-methylimidazolium-based ionic liquids as the RP-HPLC mobile phase additives. Anal. Chim. Acta 2013, 767, 148–154. [Google Scholar] [CrossRef]
- Fernández-Navarro, J.J.; García-Álvarez-Coque, M.C.; Ruiz-Ángel, M.J. The role of the dual nature of ionic liquids in the reversed-phase liquid chromatographic separation of basic drugs. J. Chromatogr. A 2011, 1218, 398–407. [Google Scholar] [CrossRef]
- Kapnissi-Christodoulou, C.P.; Stavrou, I.J.; Mavroudi, M.C. Chiral ionic liquids in chromatographic and electrophoretic separations. J. Chromatogr. A 2014, 1363, 2–10. [Google Scholar] [CrossRef]
- Marszall, M.P.; Kaliszan, R. Application of ionic liquids in liquid chromatography. Crit. Rev. Anal. Chem. 2007, 37, 127–140. [Google Scholar] [CrossRef]
- Pino, V.; Afonso, A.M. Surface-bonded ionic liquid stationary phases in high-performance liquid chromatography-A review. Anal. Chim. Acta 2012, 714, 20–37. [Google Scholar] [CrossRef]
- Vidal, L.; Riekkola, M.L.; Canals, A. Ionic liquid-modified materials for solid-phase extraction and separation: A review. Anal. Chim. Acta 2012, 715, 19–41. [Google Scholar] [CrossRef]
- Ho, T.D.; Zhang, C.; Hantao, L.W.; Anderson, J.L. Ionic liquids in analytical chemistry: Fundamentals, advances, and perspectives. Anal. Chem. 2014, 86, 262–285. [Google Scholar] [CrossRef]
- Fields, P.R.; Sun, Y.; Stalcup, A.M. Application of a modified linear solvation energy relationship (LSER) model to retention on a butylimidazolium-based column for high performance liquid chromatography. J. Chromatogr. A 2011, 1218, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Lancioni, C.; Keunchkarian, S.; Castells, C.B.; Gagliardi, L.G. Enantiomeric separations by capillary electrophoresis: Theoretical method to determine optimum chiral selector concentration. J. Chromatogr. A 2018, 1539, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Beutner, A.; Herl, T.; Matysik, F.M. Selectivity enhancement in capillary electrophoresis by means of two-dimensional separation or dual detection concepts. Anal. Chim. Acta 2019, 1057, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Kahle, J.; Zagst, H.; Wiesner, R.; Wätzig, H. Comparative charge-based separation study with various capillary electrophoresis (CE) modes and cation exchange chromatography (CEX) for the analysis of monoclonal antibodies. J. Pharm. Biomed. Anal. 2019, 174, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Ha, P.T.T.; Hoogmartens, J.; Van Schepdael, A. Recent advances in pharmaceutical applications of chiral capillary electrophoresis. J. Pharm. Biomed. Anal. 2006, 41, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alhusban, A.A.; Breadmore, M.C.; Gueven, N.; Guijt, R.M. Capillary electrophoresis for automated on-line monitoring of suspension cultures: Correlating cell density, nutrients and metabolites in near real-time. Anal. Chim. Acta 2016, 920, 94–101. [Google Scholar] [CrossRef]
- Matarashvili, I.; Kobidze, G.; Chelidze, A.; Dolidze, G.; Beridze, N.; Jibuti, G.; Farkas, T.; Chankvetadze, B. The effect of temperature on the separation of enantiomers with coated and covalently immobilized polysaccharide-based chiral stationary phases. J. Chromatogr. A 2019, 1599, 172–179. [Google Scholar] [CrossRef]
- Gyeong, M.; Duk, M.; Hag, J. Doxycycline as a new chiral selector in capillary electrophoresis. J. Chromatogr. A 2017, 1508, 176–181. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, M.; Li, F.; Breadmore, M.C. β-Cyclodextrin-copper (II) complex as chiral selector in capillary electrophoresis for the enantioseparation of β-blockers. J. Chromatogr. A 2019, 1596, 233–240. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Sun, X. Investigation of maltodextrin-based synergistic system with amino acid chiral ionic liquid as additive for enantioseparation in capillary electrophoresis. Chirality 2017, 29, 824–835. [Google Scholar] [CrossRef]
- Seitkalieva, M.M.; Vavina, A.V.; Posvyatenko, A.V.; Egorova, K.S.; Kashin, A.S.; Gordeev, E.G.; Strukova, E.N.; Romashov, L.V.; Ananikov, V.P. Biomass-Derived ionic liquids based on a 5-hmf platform chemical: Synthesis, characterization, biological activity, and tunable interactions at the molecular level. ACS Sustain. Chem. Eng. 2021, 9, 3552–3570. [Google Scholar] [CrossRef]























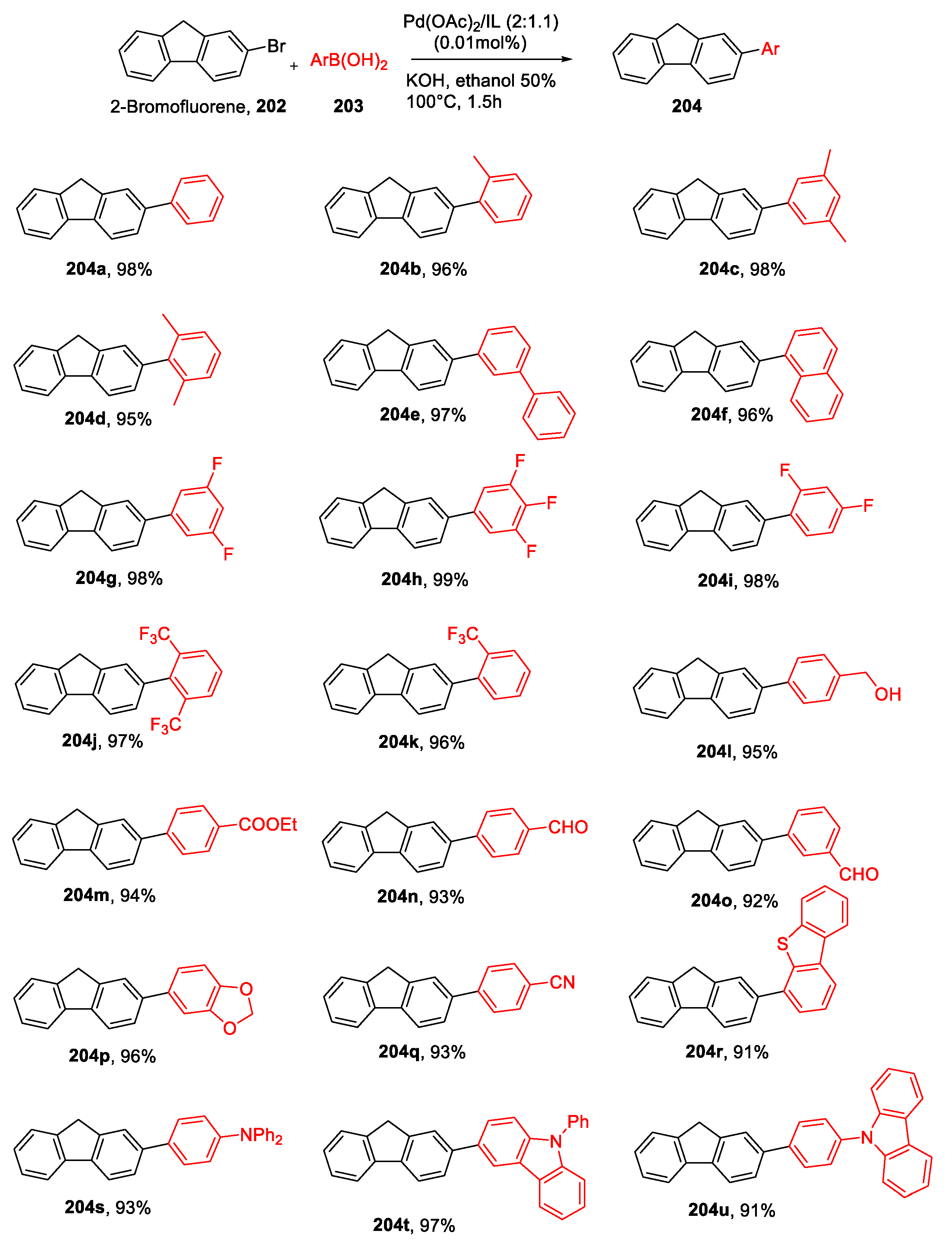

| Physical State | Tm(°C) | Td(°C) | Ref. | |
|---|---|---|---|---|
| 4 | Colorless solid | 196–198 1 (202.9) 2 | 261.0 2,3,4 | [23] |
| 5a | Colorless solid | 142–145 1 (136.5) 2 | 266.7 2,3,4 | [23] |
| 6 | White solid | not reported | 189–192 1 | [23] |
| 7a | Off-white solid | not reported | 222–224 1 | [24] |
| 7b | White solid | not reported | 249–252 1 | [24] |
| 7c | Light green solid | not reported | 198–200 1 | [24] |
| 7d | Off-white solid | not reported | 178–182 1 | [24] |
| 7e | White solid | not reported | 202–206 1 | [24] |
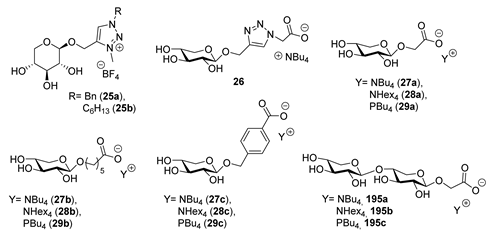
| Physical State | Tm(°C) | Tg(°C) | Td(°C) | Ref. | |
|---|---|---|---|---|---|
| 25a | White wax | --- | 4 1,2,3 | 150 4 | [34] |
| 25b | White wax | --- | 2.7 1,2,3 | 120 4 | [34] |
| 26 | Oil | --- | −26 5 | ≈140–260 6,7 | [38] |
| 27a | Solid | 59 8 (58) 5 | --- | ≈140–260 6,7 | [38] |
| 27b | Oil | --- | −35 5 | ≈140–260 6,7 | [38] |
| 27c | Solid | 98 8 (77) 5 | −32 5 | ≈140–260 6,7 | [38] |
| 28a | Solid | 74 8,9 | −4 5 | ≈140–260 6,7 | [38] |
| 28b | Oil | --- | −30 5 | ≈140–260 6,7 | [38] |
| 28c | Solid | 112 5,9 | −4 5 | ≈140–260 6,7 | [38] |
| 29a | Oil | --- | --- | ≈140–260 6,7 | [38] |
| 29b | Oil | --- | 0 5 | ≈140–260 6,7 | [38] |
| 29c | Oil | --- | −19 5 | ≈140–260 6,7 | [38] |
| 195a | Solid | 9 | −32 5 | ≈140–260 6,7 | [38] |
| 195b | Solid | 93 5,9 | 16 5 | ≈140–260 6,7 | [38] |
| 195c | Oil | --- | 2 5 | ≈140–260 6,7 | [38] |
| Physical State | Tm (°C) | Tc (°C) | Tg (°C) | Td (°C) | Ref. | |
|---|---|---|---|---|---|---|
| 39a | Yellow viscous liquid | --- | --- | --- | >250 °C 1,2,3,4 | [43] |
| 39b | Pale yellow gel | --- | --- | --- | >250 °C 1,2,3,4 | [43] |
| 39c | Yellow gel | --- | --- | --- | >230 °C 1,2,3,4 | [43] |
| 39d | Yellow viscous liquid | --- | --- | --- | >230 °C 1,2,3,4 | [43] |
| 40a | White solid | 81.5 5 | --- | −21.8 6; 80.2 6 | 155.0 7,8 | [44] |
| 40b | White solid | 35.6 5 | 73.4 5 | 34.0 6 | 161.8 7,8 | [44] |
| 40c | White solid | 120 5 | --- | 0.4 6 | 172.5 7,8 | [44] |
| 43 | Yellow viscous oil | --- | --- | −24.1 9 | 103.1 7,8 | [17] |
| 44a | Light-yellow oil | --- | --- | −0.1 9 | 195.1 7,8 | [17] |
| 44b | Light yellow oil | --- | --- | −19.4 9 | 191.7 7,8 | [17] |
| 44c | Light yellow oil | --- | --- | −7.9 9 | 210.3 7,8 | [17] |
| 44d | Light yellow oil | --- | --- | −46.6 9 | 127.8 7,8 | [17] |
| 44e | Light yellow oil | --- | --- | −55.5 9 | 200.6 7,8 | [17] |
| 44f | Light yellow oil | --- | --- | −36.1 9 | 200.6 7,8 | [17] |
| 44g | Light yellow oil | 87.1 8 | --- | −22.8 9 | 121.8 7,8 | [17] |
| 44h | Yellow oil | --- | --- | −16.2 9 | 188.5 7,8 | [17] |
| 45 | Light yellow oil | --- | --- | −3.7 9 | 197.3 7,8 | [17] |
| 46a | Light yellow oil | --- | --- | −16.6 10 | 168.4 7,8 | [48] |
| 46b | Yellow oil | --- | --- | −15.9 10 | 191.1 7,8 | [48] |

| Physical State | Tm(°C) | Tg(°C) | Td(°C) | Ref. | |
|---|---|---|---|---|---|
| 52a | Solid | 142–143 1 | 2,3 (200–300) 4 | [54] | |
| 52a | Solid | 95 °C 1 | −20.5 5 | 136 6,7 | [55] |
| 52b | Oil | --- | −8.7 5 | 130 7 | [56] |
| 52c | Liquid | --- | --- | 2,3 (200–300, higher than 52a) 4 | [54] |
| 53a | Solid | 98 °C 1 | 18.3 5 | n.d.7 | [55] |
| 53b | Wax | --- | −22.5 5 | 142 7 | [56] |
| 53c | Solid | 50.1 8 | −63.3 8 | 245 9,10 | [57] |
| 56 | Liquid | 18.8 8 | −76.3 8 | 330 9,10 | [57] |
| Physical State | Tg(°C) | Tonset(°C) | Td5%(°C) | Td10%(°C) | Tpeak(°C) | Ref. | |
|---|---|---|---|---|---|---|---|
| 78a | Glass | −21 1,2 | --- | --- | 231 3 | --- | [68] |
| 78b | Viscous material | −68 1,2 | --- | --- | 232 3 | --- | [68] |
| 78c | Viscous material | −51 1,2 | --- | --- | 229 3 | --- | [68] |
| 78d | Viscous material | −60 1,2 | --- | --- | 245 3 | --- | [68] |
| 80a | Glass | −8 1,2 | --- | --- | 238 3 | --- | [68] |
| 80b | Viscous material | −27 1,2 | --- | --- | 250 3 | --- | [68] |
| 80c | Viscous material | −37 1,2 | --- | --- | 251 3 | --- | [68] |
| 80d | Viscous material | −58 1,2 | --- | --- | 269 3 | --- | [68] |
| 83a | Yellow solid | --- | 219.6 4,5 336.5 4,6 | 218.3 4 | 226.9 4 | 238.6 4,5 363.5 4,6 | [70] |
| 83b | Orange solid | --- | 222.3 4,5 | 223.0 4 | 228.7 4 | 277.1 4,5 349.5 4,6 | [70] |
| 84a | Yellow solid | --- | 181.8 4,5 227.4 4,6 | 192.2 4 | 228.4 4 | 192.2 4,5 246.1 4,6 353.1 4,7 | [70] |
| 84b | Brick red solid | 79.9 8 | 233.7 4 | 216.1 4 | 228.9 4 | 288.8 4 | [70] |
| 85a | Yellow orange glassy solid | 49.3 8 | 370.7 4 | 358.3 4 | 370.8 4 | 391.7 4 | [70] |
| 85b | Pale yellow glassy solid | 31.4 8 | 354.3 4 | 347.8 4 | 357.03 4 | 366.4 4,5 382.4 4,6 | [70] |
| 86a | Brick red glassy solid | 48.1 8 | 370.1 4 | 362.5 4 | 372.2 4 | 394.8 4 | [70] |
| 86b | Pale brown glassy solid | 28.4 8 | 370.5 4 | 356.7 4 | 376.6 4 | 387.5 4 | [70] |
| Physical State | Tm (°C) | Tg (°C) | Td (°C) | Ref. | |
|---|---|---|---|---|---|
| 98b | Liquid | --- | −30 1 | 433 2,3(454) 3,4[≈400–500] 5,6,7 | [75] |
| 99b | Liquid | --- | −20 1 | 435 2,3(456) 3,4 | [75] |
| 100b | Liquid | --- | −14 1 | 446 2,3(460) 3,4 | [75] |
| 105a | Viscous liquid | --- | −19 8 | 198 6,9(≈300) 6.7 | [81] |
| 105b | Viscous liquid | --- | −18 8 | 198 6,9(≈300) 6.7 | [81] |
| 105c | Viscous liquid | --- | 4 8 | 208 6,9(≈300) 6.7 | [81] |
| 105d | Viscous liquid | --- | −15 8 | 107 6,9(≈200–300) 6.7 | [81] |
| 105e | Viscous liquid | --- | 6 8 | 209 6,9(≈300) 6.7 | [81] |
| 105f | Viscous liquid | --- | −9 8 | 207 6,9(≈300) 6.7 | [81] |
| 105g | Viscous liquid | --- | 0 8 | 211 6,9(≈300) 6.7 | [81] |
| 106b | Solid | 87–88 10 | --- | 6,11 (≈300) 6,7 | [54] |
| 111a | Wax | --- | 12 12 | 164 13,14(235) 2,13 | [74] |
| 111b | Liquid | --- | 20 12 | 208 13,14(285) 2,13 | [74] |
| 111c | Wax | --- | 10 12 | 191 13,14(259) 2,13 | [74] |
| 111d | Liquid | --- | 13 12 | 224 13,14(327) 2,13 | [74] |
| 111e | Wax | --- | 8 12 | 191 13,14(287) 2,13 | [74] |
| 112a | Solid | 121 12 | 21 12 | 178 13,14(251) 2,13 | [74] |
| 112b | Solid | 134 12 | --- | 202 13,14(247) 2,13 | [74] |
| 112c | Wax | --- | 22 12 | 201 13,14(279) 2,13 | [74] |
| 112d | Wax | --- | 1212 | 221 13,14(340) 2,13 | [74] |
| 112e | Wax | --- | 1512 | 204 13,14(296) 2,13 | [74] |
| 139b | Solid | 124 10 | --- | 6,11 (≈400–500, higher than 98b) 6,7 | [54] |
| 139c | Liquid | --- | --- | 6,11 (≈300, lower than 106b) 6,7 | [54] |
| 135a | Liquid | --- | --- | 225 11,15 | [99] |
| 135b | Solid | 66–70 10 | --- | 205 11,15 | [99] |
| 135c | Solid | 164–168 10 | --- | 225 11,15 | [99] |
| 135d | Solid | 95–100 10 | --- | 242 11,15 | [99] |
| 135e | Solid | 118–120 10 | --- | 215 11,15 | [99] |
| 137a | Solid | 60–63 10 | --- | 250 11,15 | [99] |
| 137b | Solid | 135–138 10 | --- | 242 11,15 | [99] |
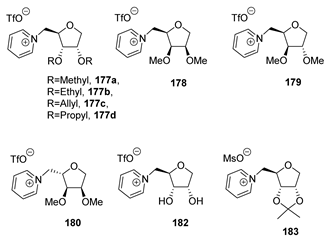
| Physical State | Tm (°C) | Tg (°C) | Td (°C) | Ref. | |
|---|---|---|---|---|---|
| 177a | Solid | 48–51 1 | −18 2 | 345 2,3 | [99] |
| 177b | Liquid | −26 2 | 316 2,3 | [99] | |
| 177c | Liquid | 301 2,3 | [99] | ||
| 177d | Liquid | −27 2 | 235 2,3 | [99] | |
| 178 | Liquid | −41 2 | 325 2,3 | [99] | |
| 179 | Solid | 32–36 1 | −28 2 | 345 2,3 | [99] |
| 180 | Liquid | −38 2 | 340 2,3 | [99] | |
| 182 | Liquid | −30 2 | 297 2,3 | [99] | |
| 183 | Solid | 92–94 1 | 296 2,3 | [99] |
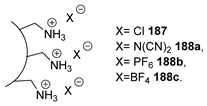
| Sugar Core | Td(°C) | Ref. | |
|---|---|---|---|
| 187 | Sucrose microgel | --- | [117] |
| 188a | Sucrose microgel | ≈200 1 (638) 2,3 | [117] |
| 188b | Sucrose microgel | ≈200 1 (790) 2,3 | [117] |
| 188c | Sucrose microgel | ≈200 1 (840) 2,3 | [117] |
| Ionic Liquid | Cellulose Recovery |
|---|---|
| 41-As | 48% |
| 41-Ph | 65% |
| 41-Ac | 62% |
| 41-Fo | 58% |
| 41-Ci | 53% |
| 41 | 52% |
| Compound | Escherichia coli | Bacillus subtilis | Pseudomonas fluorescens | Pseudomonas putida (CP1) | Pseudomonas putida (KT2440) | Biodegradation 1 |
|---|---|---|---|---|---|---|
| TBABr | 03.13–06.25 | 25.00–50.00 | 06.25–12.50 | 25.00–50.00 | 12.50–25.00 | 7.9 |
| TBAOH·30H2O | 06.25–12.50 | 12.50–25.00 | 12.50–25.00 | 12.50–25.00 | 12.50–25.00 | 3.8 |
| TEABr | --- | --- | --- | --- | --- | 0 |
| TMABr | --- | --- | --- | --- | --- | 2.6 |
| 196a | 12.50–25.00 | 06.25–12.50 | 12.50–25.00 | 06.25–12.50 | 12.50–25.00 | 15.5 |
| 196b | 06.25–12.50 | 03.13–06.25 | 03.13–06.25 | 03.13–06.25 | 12.50–25.00 | 15.5 |
| 196c | 12.50–25.00 | 06.25–12.50 | 06.25–12.50 | 06.25–12.50 | 12.50–25.00 | 9.9 |
| 196d | 12.50–25.00 | 03.13–06.25 | 12.50–25.00 | 06.25–12.50 | 12.50–25.00 | Nd 2 |
| 196e | 06.25–12.50 | 03.13–06.25 | 03.13–06.25 | 03.13–06.25 | 03.13–06.25 | 14.2 |
| 196f | 12.50–25.00 | 06.25–12.50 | 06.25–12.50 | 06.25–12.50 | 12.50–25.00 | 12.2 |
| 52a | 06.25–12.50 | 25.00–50.00 | 25.00–50.00 | 25.00–50.00 | 25.00–50.00 | 18.6 |
| 53a | 12.50–25.00 | 25.00–50.00 | 25.00–50.00 | 25.00–50.00 | 25.00–50.00 | 22.8 |
| IL | EC50 (μmol/L) 1 | EC50 (mg/L) 1 | Biodegradability 2 |
|---|---|---|---|
| 106a | >584 | >202 | 74–83% 3 |
| 108c | 85.1 (66.1–97.7) 4 | 42.6 (33.1–48.9) 4 | 57% 5 |
| 108d | 9.5 (8.9–10.2) 4 | 5.3 (5.0–5.7) 4 | 50–60% 6 |
| 108e | 13.5 (12.3–14.5) 4 | 10.2 (9.3–10.9) 4 | Not tested 7 |
| Carbendazim8 | 8.1 (7.4–8.7) 4 | 1.6 (1.4–1.7) 4 | --- |

| IL | T (°C) | t (h) | Yield (%) | ee (%) |
|---|---|---|---|---|
| 35a | rT | 7 | 81 | 14 |
| 35a | 0 to −5 | 12 | 73 | 33 |
| 35b | rT | 7 | 84 | 2 |
| 35b | 0 to −5 | 12 | 71 | 8 |
| 35c | rT | 8 | 76 | 3 |
| 35c | 0 to −5 | 13 | 69 | 9 |
| 35d | rT | 8 | 79 | Racemic |
| 35d | 0 to −5 | 12 | 70 | 4 |
| 35e | rT | 8 | 78 | Racemic |
| 35e | 0 to −5 | 13 | 72 | 4 |
| Entry | R | X | t (h) | Yield (%) 1 | ee (%) 2 |
|---|---|---|---|---|---|
| 1 | CH3 | NPh | 5 | 83 | 98 |
| 2 | p-MePh | NPh | 7 | 76 | >99 |
| 3 | p-OMePh | NPh | 7 | 73 | >99 |
| 4 | CH 3 | p-MePhN | 6 | 71 | 90 |
| 5 | Ph | NPh | 7.5 | 70 | 84 |
| 6 | H | NPh | 9 | 79 | 44 3 |
| 7 | p-ClPh | NPh | 9 | 65 | Racemic |
| 8 | CH3 | O | 10 | 51 | Racemic |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zullo, V.; Iuliano, A.; Guazzelli, L. Sugar-Based Ionic Liquids: Multifaceted Challenges and Intriguing Potential. Molecules 2021, 26, 2052. https://doi.org/10.3390/molecules26072052
Zullo V, Iuliano A, Guazzelli L. Sugar-Based Ionic Liquids: Multifaceted Challenges and Intriguing Potential. Molecules. 2021; 26(7):2052. https://doi.org/10.3390/molecules26072052
Chicago/Turabian StyleZullo, Valerio, Anna Iuliano, and Lorenzo Guazzelli. 2021. "Sugar-Based Ionic Liquids: Multifaceted Challenges and Intriguing Potential" Molecules 26, no. 7: 2052. https://doi.org/10.3390/molecules26072052











