Synthesis, Spectroscopic Properties and Redox Behavior Kinetics of Rare-Earth Bistetrakis-4-[3-(3,4-dicyanophenoxy)phenoxy]phthalocyaninato Metal Complexes with Er, Lu and Yb
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of 4,4’-[1,3-Phenylenebis(oxy)]phthalodinitrile
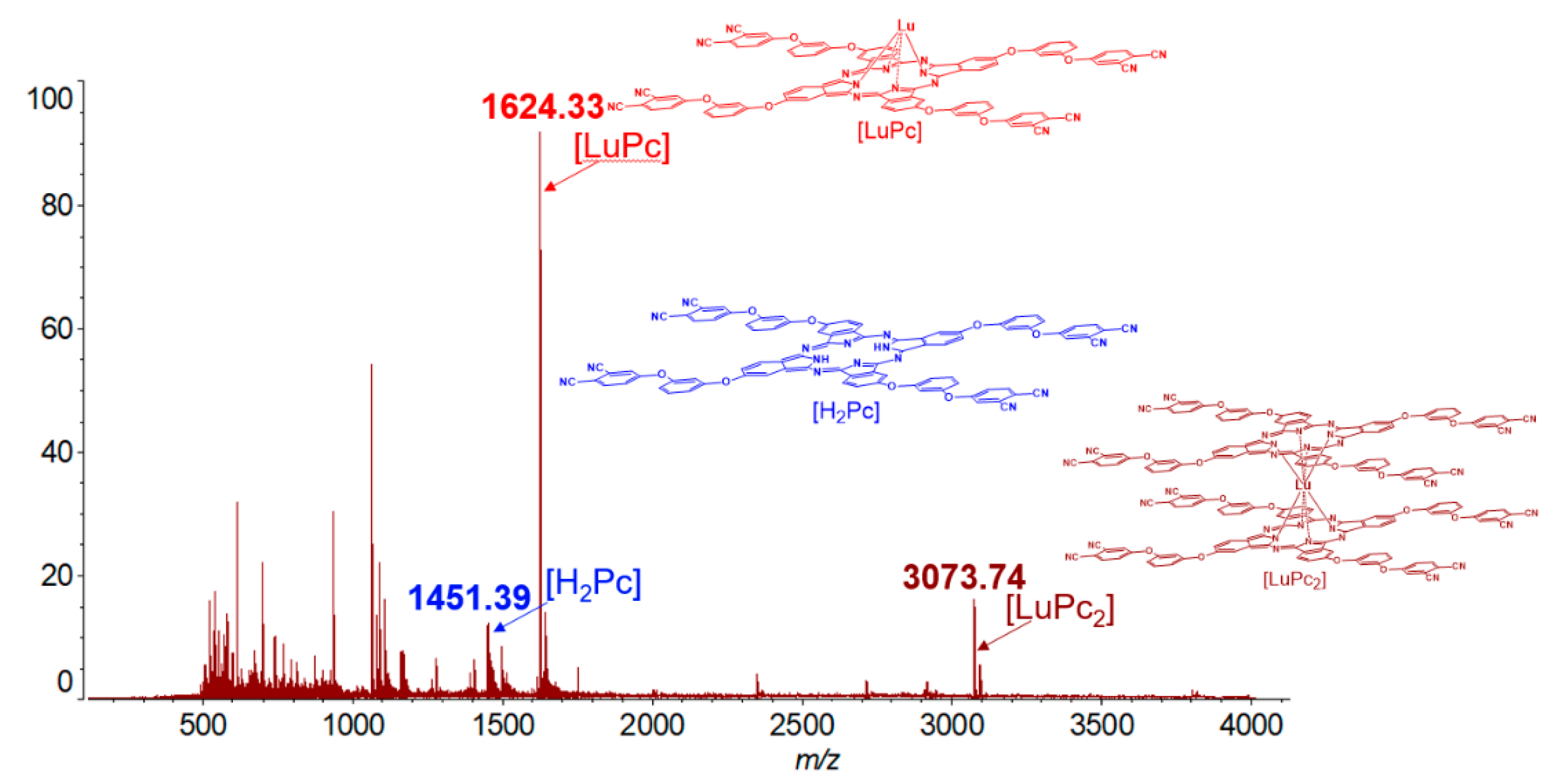
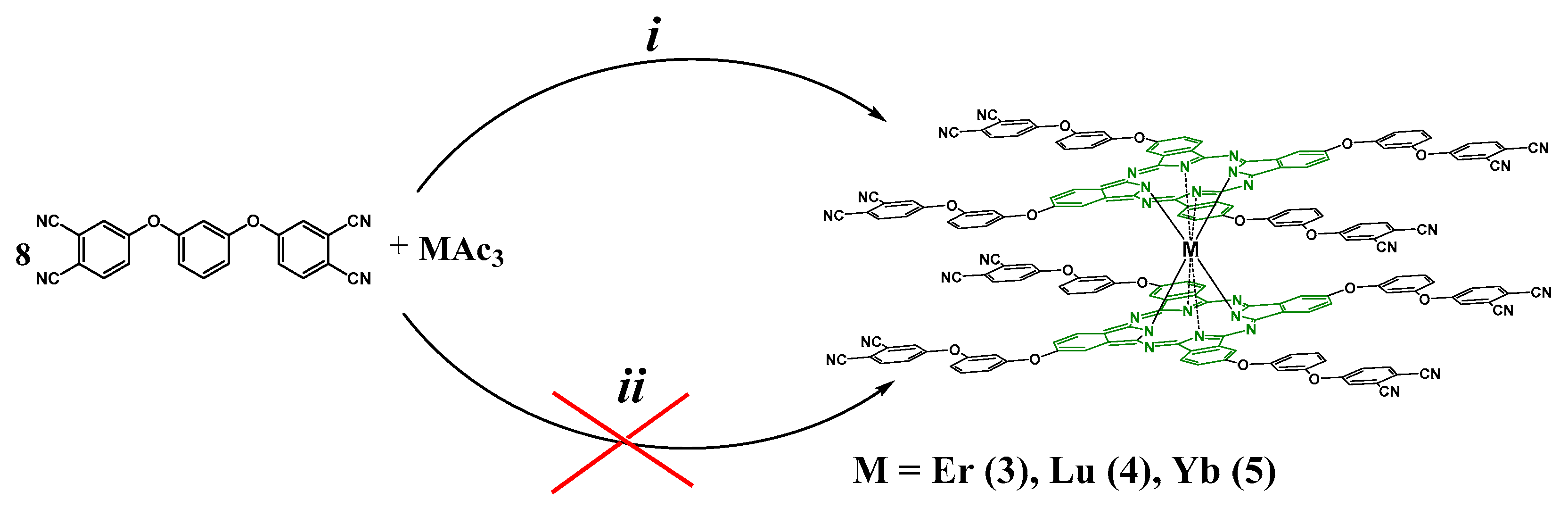
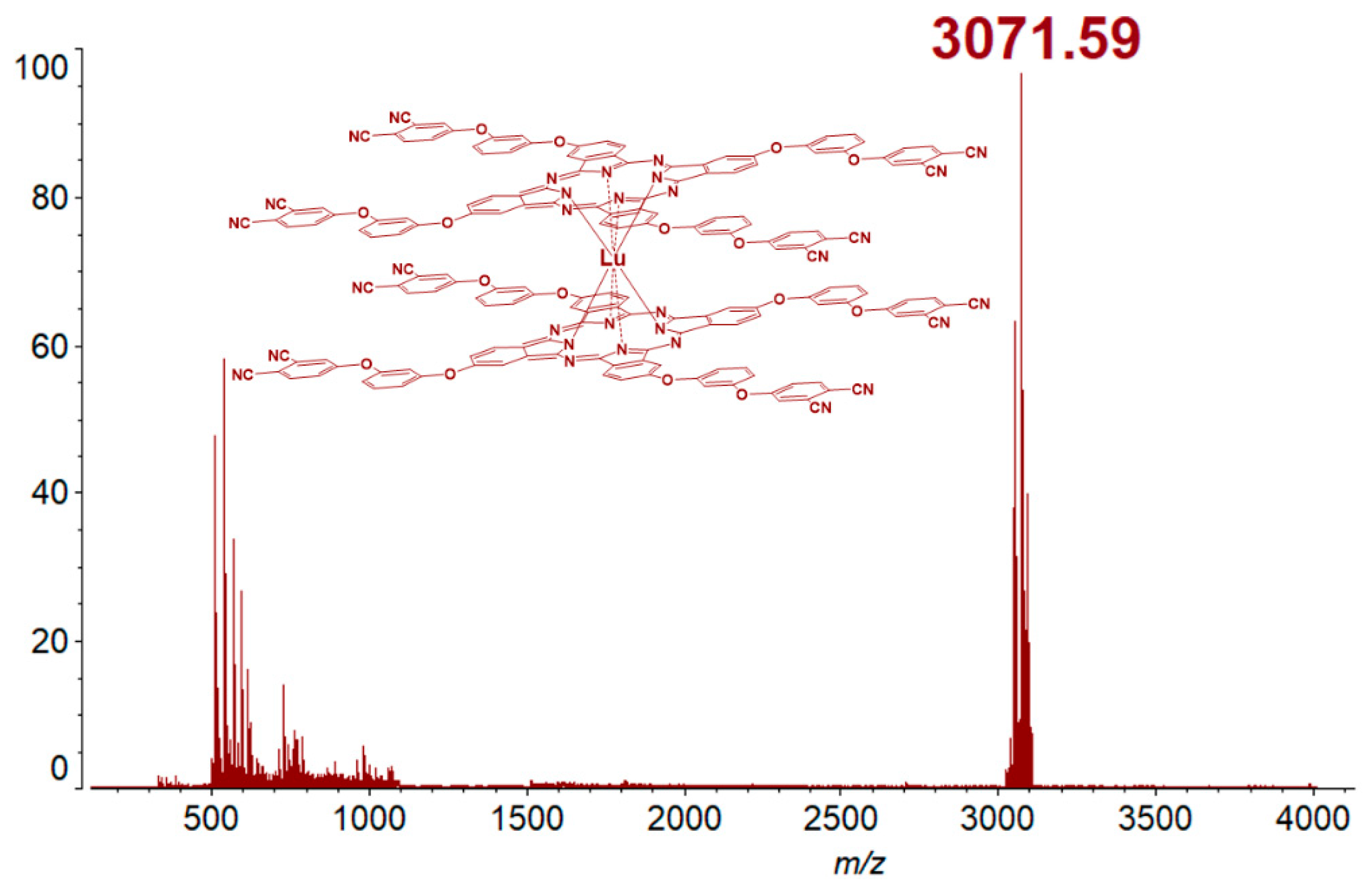
2.2. Synthesis of f-Metal Phthalocyaninates
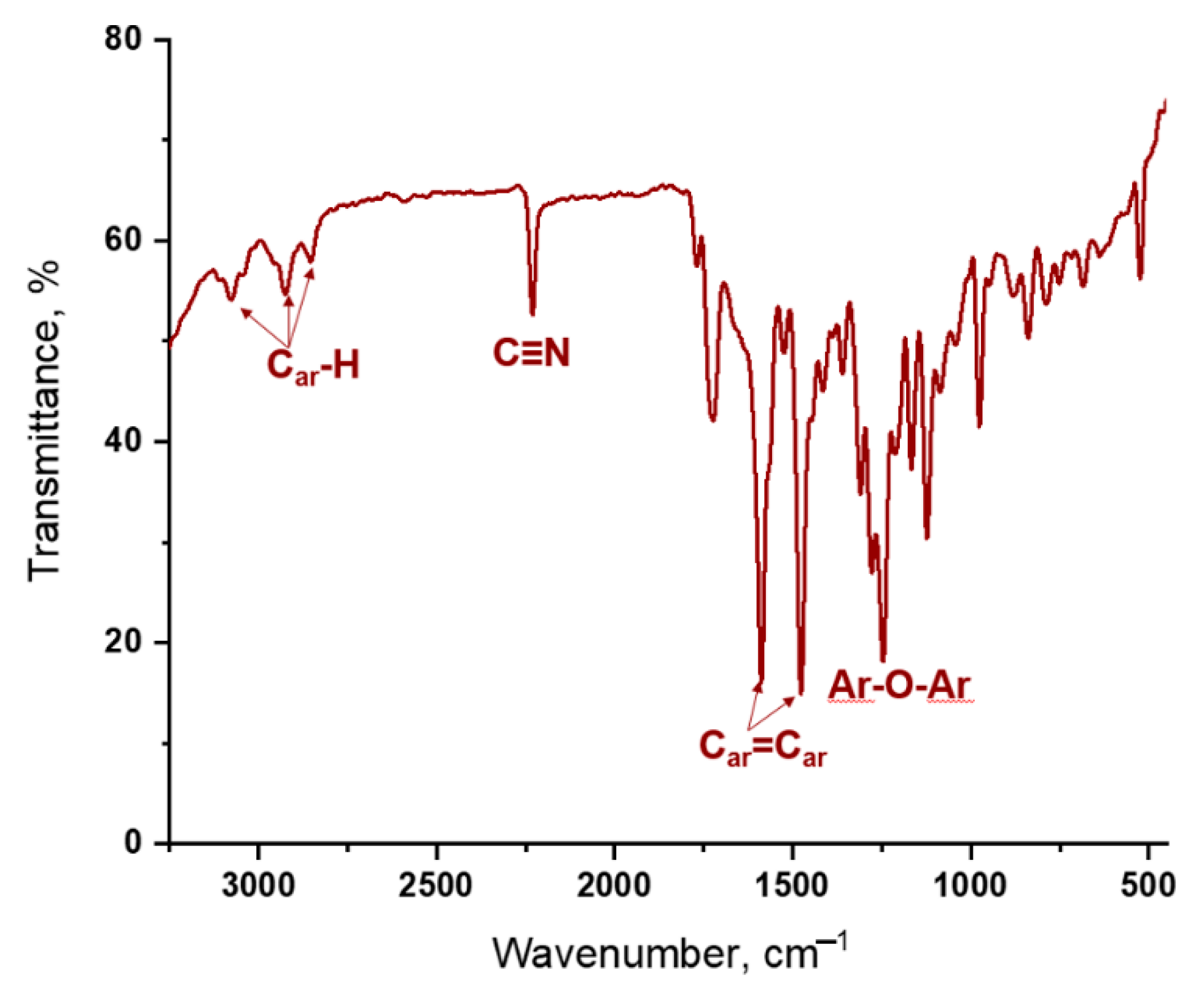
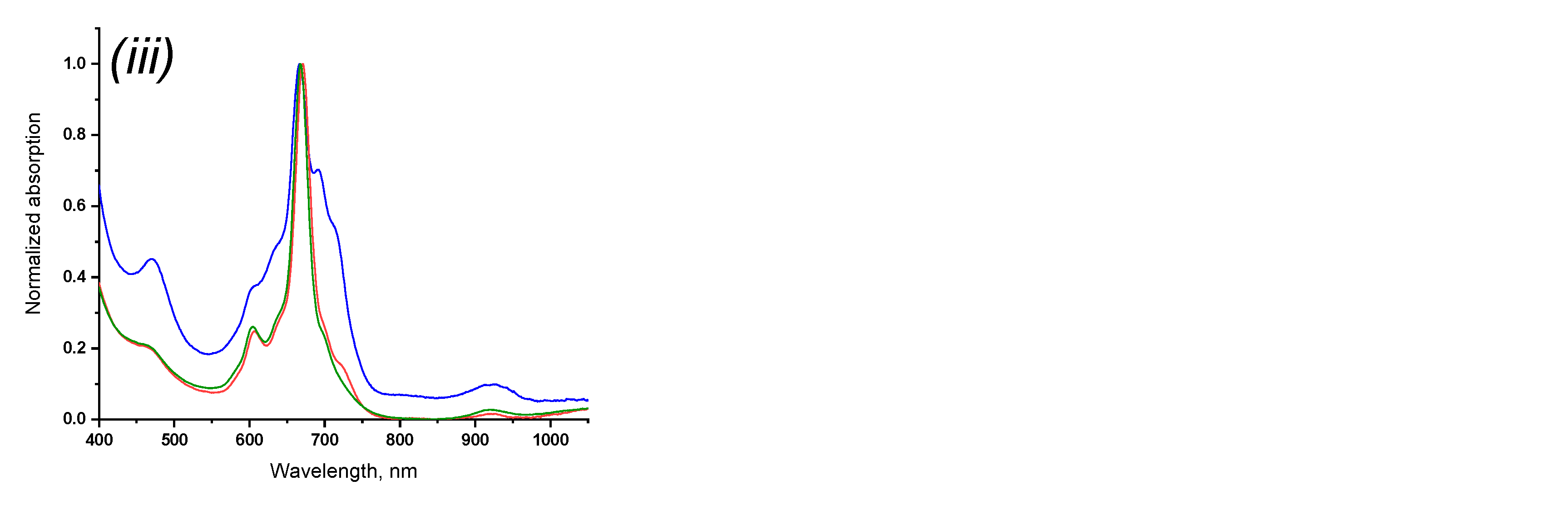
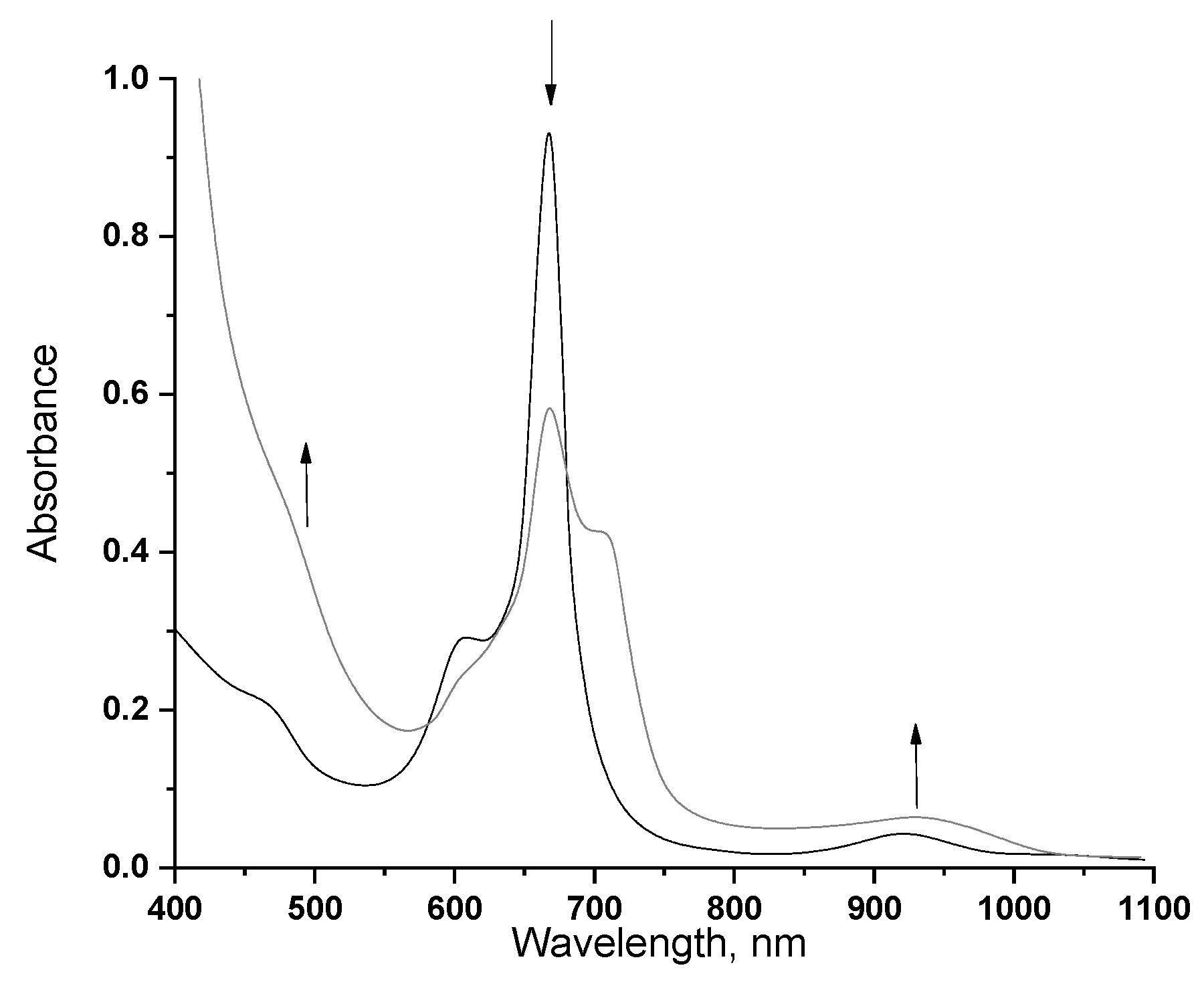
2.3. Spectroscopic Properties of Rare-Earth Metal Phthalocyaninates
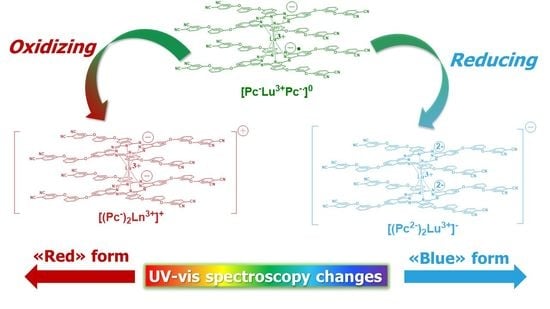
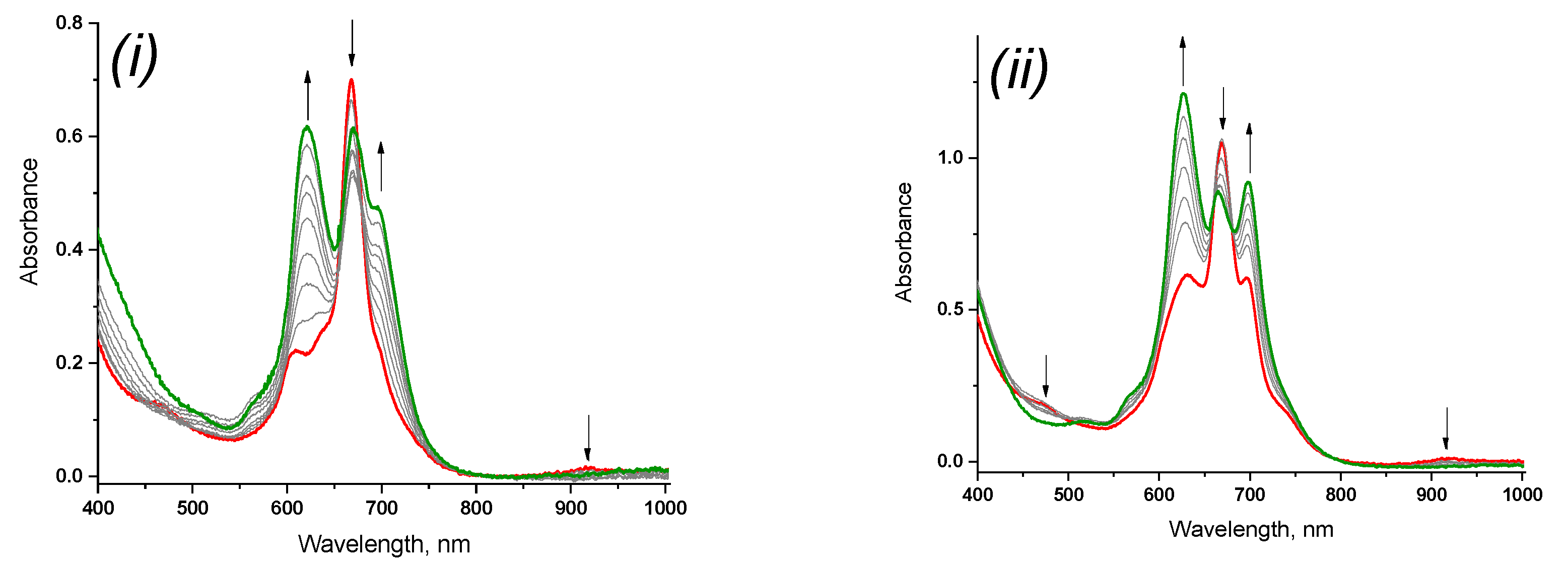
2.4. Redox Equilibriums in the Solutions of Rare-Earth Metal Phthalocyaninates
3. Materials and Methods
3.1. Equipment and Reagent
3.2. The Study of Kinetic Properties
3.3. The Study of Aggregation Properties
3.4. Synthesis
3.4.1. Synthesis of 4,4’-[1,3-Phenylenebis(oxy)]diphthalonitrile
3.4.2. General Route of Sandwich-Type Lanthanide Bisphthalocyaninato Synthesis
3.4.3. Bistetrakis-4-[3-(3,4-Dicyanophenoxy)phenoxy]phthalocyaninato erbium (3)
3.4.4. Bistetrakis-4-[3-(3,4-dicyanophenoxy)phenoxy]phthalocyaninato ytterbium (4)
3.4.5. Bistetrakis-4-[3-(3,4-dicyanophenoxy)phenoxy]phthalocyaninato lutetium (5)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Platonova, Y.B.; Volov, A.N.; Tomilova, L.G. Palladium(II) phthalocyanines efficiently promote phosphine-free Sonogashira cross-coupling reaction at room temperature. J. Catal. 2020, 391, 224–228. [Google Scholar] [CrossRef]
- Guo, J.; Yan, X.; Liu, Q.; Li, Q.; Xu, X.; Kang, L.; Cao, Z.; Chai, G.; Chen, J.; Wang, Y.; et al. The synthesis and synergistic catalysis of iron phthalocyanine and its graphene-based axial complex for enhanced oxygen reduction. Nano Energy 2018, 46, 347–355. [Google Scholar] [CrossRef]
- Hakeim, O.A.; Abdelghaffar, F.; Haroun, A.A. UV-curable hyperbranched polyester acrylate encapsulation of phthalocyanine pigments for high performance synthetic fabrics printing. Dye. Pigment. 2020, 177, 108307. [Google Scholar] [CrossRef]
- Lv, D.; Sung, H.S.; Li, X.; Zhang, X.; Li, Z.; Chen, D. Effects of single layer graphene and graphene oxide modification on the properties of phthalocyanine blue pigments. Dye. Pigment. 2020, 180, 108449. [Google Scholar] [CrossRef]
- Sarı, C.; Nalçaoğlu, A.; Değirmencioğlu, İ.; Celep Eyüpoğlu, F. Tumor-selective new piperazine-fragmented silicon phthalocyanines initiate cell death in breast cancer cell lines. J. Photochem. Photobiol. B Biol. 2021, 216, 112143. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, M.; Köksoy, B.; Yalçın, B.; Taşkın, T.; Selçuki, N.A.; Salan, Ü.; Durmuş, M.; Bulut, M. Novel lutetium(III) phthalocyanine-coumarin dyads; synthesis, characterization, photochemical, theoretical and antioxidant properties. Inorganica Chim. Acta 2020, 517, 120145. [Google Scholar] [CrossRef]
- Wu, Y.; Cao, C.; Xu, J.; Wei, D.; Duan, J.; Wu, J.; Li, L.; Chen, Z. Cobalt phthalocyanine derived bifunctional carbon decorated CoSe with enhanced lithium storage capability. Synth. Met. 2020, 269, 116554. [Google Scholar] [CrossRef]
- Sharma, H.B.; Panigrahi, S.; Sarmah, A.K.; Dubey, B.K. Potato peels biochar composite with Copper phthalocyanine for energy storage application. Sci. Total Environ. 2019, 135907. [Google Scholar] [CrossRef]
- Gounden, D.; Nombona, N.; van Zyl, W.E. Recent advances in phthalocyanines for chemical sensor, non-linear optics (NLO) and energy storage applications. Coord. Chem. Rev. 2020, 420, 213359. [Google Scholar] [CrossRef]
- Majeed, S.A.; Ghazal, B.; Nevonen, D.E.; Nemykin, V.N.; Makhseed, S. Spectroscopic and TDDFT studies on the charge-transfer properties of metallated Octa(carbazolyl)phthalocyanines. Dye. Pigment. 2019, 170, 107593. [Google Scholar] [CrossRef]
- Sabik, A.; Trembułowicz, A.; Antczak, G. Self-organization of CoPc-F16CuPc mixture with non-equal composition on Ag(100): From sub-monolayer to monolayer coverage. Surf. Sci. 2021, 705. [Google Scholar] [CrossRef]
- Vashurin, A.S. Non-covalent associates of metal phthalocyanines: The role of axial ligand and catalytic activity. Russ. Chem. Bull. 2016, 65, 2220–2228. [Google Scholar] [CrossRef]
- Pereira, G.F.M.; Tasso, T.T. From cuvette to cells: How the central metal ion modulates the properties of phthalocyanines and porphyrazines as photosensitizers. Inorganica Chim. Acta 2021, 519, 120271. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Basova, T.V.; Krasnov, P.O.; Sukhikh, A.S. Effect of fluorosubstitution and central metals on the molecular structure and vibrational spectra of metal phthalocyanines. J. Mol. Struct. 2019, 1189, 73–80. [Google Scholar] [CrossRef]
- Vashurin, A.; Maizlish, V.; Kuzmin, I.; Znoyko, S.; Morozova, A.; Razumov, M.; Koifman, O. Symmetrical and difunctional substituted cobalt phthalocyanines with benzoic acids fragments: Synthesis and catalytic activity. J. Porphyr. Phthalocyanines 2017, 21, 37–47. [Google Scholar] [CrossRef]
- Bağda, E.; Yabaş, E.; Bağda, E. Analytical approaches for clarification of DNA-double decker phthalocyanine binding mechanism: As an alternative anticancer chemotherapeutic. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 172, 199–204. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Y.; Wang, H.; Jiang, B.; Xu, X.; Cai, Y. Dissociation and reconstruction of double-decker bis(phthalocyaninato) terbium(III) complex (TbPc2) on Pd(001): A theoretical investigation. Surf. Sci. 2017, 655, 12–16. [Google Scholar] [CrossRef]
- Korostei, Y.S.; Pushkarev, V.E.; Tolbin, A.Y.; Dzuban, A.V.; Chernyak, A.V.; Konev, D.V.; Medvedeva, T.O.; Talantsev, A.D.; Sanina, N.A.; Tomilova, L.G. Sandwich quadruple-decker binuclear lanthanide(III) complexes based on clamshell-type phthalocyanine ligand: Synthesis and physicochemical studies. Dye. Pigment. 2019, 170, 107648. [Google Scholar] [CrossRef]
- Martynov, A.G.; Safonova, E.A.; Tsivadze, A.Y.; Gorbunova, Y.G. Functional molecular switches involving tetrapyrrolic macrocycles. Coord. Chem. Rev. 2019, 387, 325–347. [Google Scholar] [CrossRef]
- Pan, H.; Gong, L.; Liu, W.; Lin, C.; Ma, Q.; Lu, G.; Qi, D.; Wang, K.; Jiang, J. TTF-fused heteroleptic bis(phthalocyaninato) europium double-decker complexes. Synthesis, spectroscopic, and electrochemical properties. Dye. Pigment. 2018, 156, 167–174. [Google Scholar] [CrossRef]
- Sekhosana, K.E.; Nyokong, T. Double- and quintuple-decker phthalocyaninato chelates as optical limiters in solution and thin film. Dye. Pigment. 2020, 172, 107836. [Google Scholar] [CrossRef]
- Kuzmina, E.A.; Dubinina, T.V.; Vasilevsky, P.N.; Saveliev, M.S.; Gerasimenko, A.Y.; Borisova, N.E.; Tomilova, L.G. Novel octabromo-substituted lanthanide(III) phthalocyanines—Prospective compounds for nonlinear optics. Dye. Pigment. 2021, 185, 108871. [Google Scholar] [CrossRef]
- Ren, B.; Sheng, N.; Gu, B.; Wan, Y.; Rui, G.; Lv, C.; Cui, Y. Changing optical nonlinearities of homoleptic bis(phthalocyaninato) rare earth praseodymium double-decker complexes by the redox reaction. Dye. Pigment. 2017, 139, 788–794. [Google Scholar] [CrossRef] [Green Version]
- Shokurov, A.V.; Kutsybala, D.S.; Martynov, A.G.; Raitman, O.A.; Arslanov, V.V.; Gorbunova, Y.G.; Tsivadze, A.Y.; Selektor, S.L. Modulation of transversal conductivity of europium(III) bisphthalocyaninate ultrathin films by peripheral substitution. Thin Solid Films 2019, 692, 137591. [Google Scholar] [CrossRef]
- Wei, J.; Li, X.; Xiao, C.; Lu, F. IR absorption spectroscopic characteristics of peripherally substituted thiophenyl phthalocyanine in sandwich bis(phthalocyaninato) complexes. Vib. Spectrosc. 2017, 92, 105–110. [Google Scholar] [CrossRef]
- Yabaş, E.; Sülü, M.; Dumludağ, F.; Salih, B.; Bekaroğlu, Ö. Imidazole octasubstituted novel mono and double-decker phthalocyanines: Synthesis, characterization, electrical and gas sensing properties. Polyhedron 2018, 153, 51–63. [Google Scholar] [CrossRef]
- Vashurin, A.; Erzunov, D.; Kazaryan, K.; Tonkova, S.; Tikhomirova, T.; Filippova, A.; Koifman, O. Synthesis, catalytic, spectroscopic, fluorescent and coordination properties of dicyanophenoxy-substituted phthalocyaninates of d-metals. Dye. Pigment. 2020, 174, 108018. [Google Scholar] [CrossRef]
- Erzunov, D.A.; Vashurin, A.S.; Koifman, O.I. Synthesis and spectral properties of isomers of cobalt tetrakis(dicyanophenoxy)phthalocyaninate. Russ. Chem. Bull. 2018, 67, 2250–2252. [Google Scholar] [CrossRef]
- Pushkarev, V.E.; Tomilova, L.G.; Nemykin, V.N. Historic overview and new developments in synthetic methods for preparation of the rare-earth tetrapyrrolic complexes. Coord. Chem. Rev. 2016, 319, 110–179. [Google Scholar] [CrossRef] [Green Version]
- Selektor, S.L.; Shokurov, A.V.; Raitman, O.A.; Sheinina, L.S.; Arslanov, V.V.; Birin, K.P.; Gorbunova, Y.G.; Tsivadze, A.Y. Orientation-Induced redox transformations in langmuir monolayers of double-decker cerium bis[tetra-(15-Crown-5)-Phthalocyaninate] and multistability of its Langmuir-Blodgett films. Colloid J. 2012, 74, 334–345. [Google Scholar] [CrossRef]
- Lobanov, A.V.; Gromova, G.A.; Gorbunova, Y.G.; Tsivadze, A.Y. Supramolecular associates of double-decker lanthanide phthalocyanines with macromolecular structures and nanoparticles as the basis of biosensor devices. Prot. Met. Phys. Chem. Surfaces 2014, 50, 570–577. [Google Scholar] [CrossRef]
- Rodriguez-Méndez, M.L.; Gay, M.; De Saja, J.A. New insights into sensors based on radical bisphthalocyanines. J. Porphyr. Phthalocyanines 2009, 13, 1159–1167. [Google Scholar] [CrossRef]
- Önal, E.; Tüncel, Ö.; Albakour, M.; Çelik, G.G.; Gürek, A.G.; Özçelik, S. Synthesizing and evaluating the photodynamic efficacy of asymmetric heteroleptic A7B type novel lanthanide bis-phthalocyanine complexes. RSC Adv. 2021, 11, 6188–6200. [Google Scholar] [CrossRef]



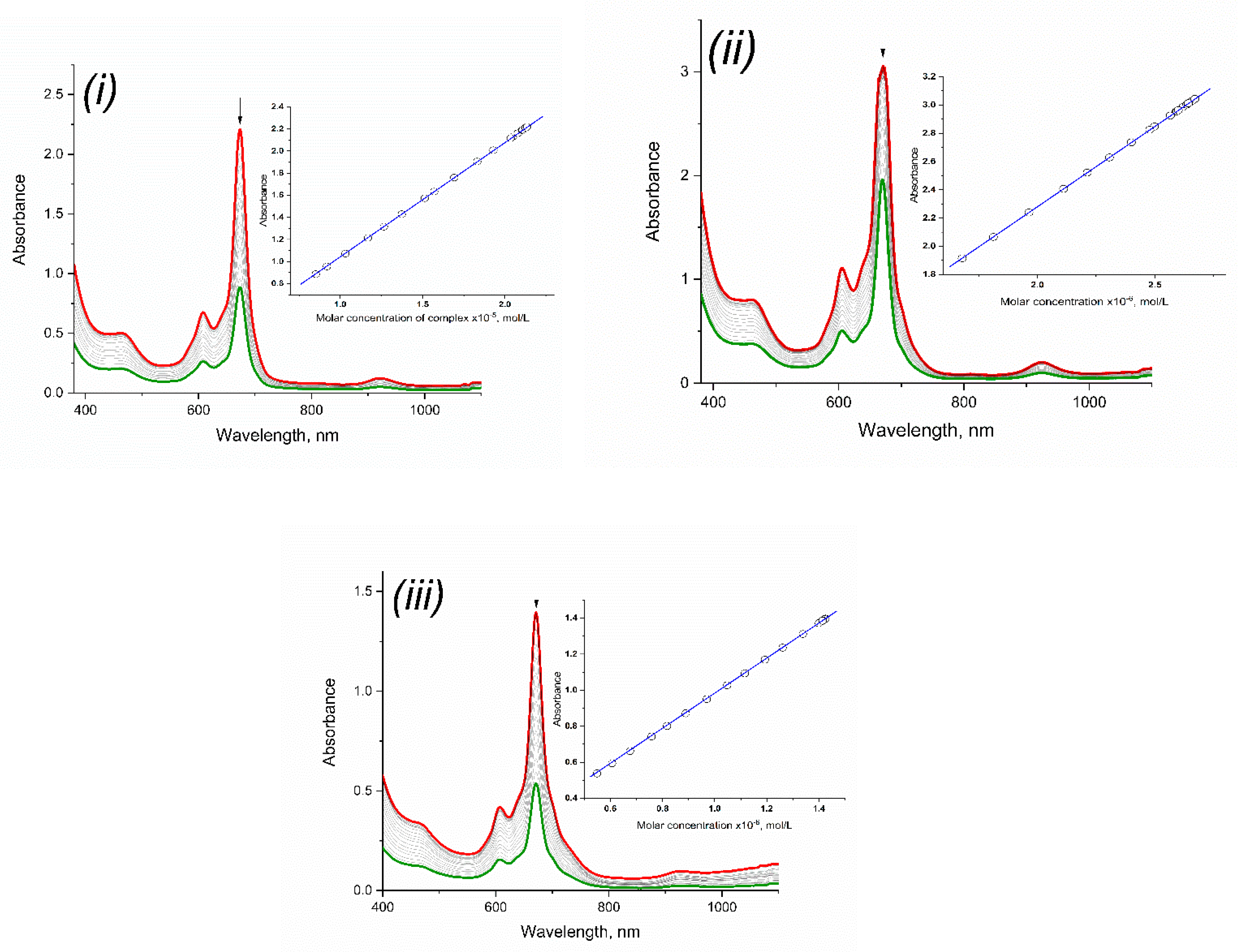

| Compound | Absorption Maxima, nm (lgε) | ||
|---|---|---|---|
| Acetone | CHCl3 | THF | |
| bistetrakis-4-[3-(3,4-dicyanophenoxy)phenoxy]phthalocyaninato erbium (3) | 670 (4.813) | 673 (5.017) | 670 (4.978) |
| bistetrakis-4-[3-(3,4-dicyanophenoxy)phenoxy]phthalocyaninato lutetium (4) | 666 (4.806) | 670 (5.053) | 667 (4.908) |
| bistetrakis-4-[3-(3,4-dicyanophenoxy)phenoxy]phthalocyaninato ytterbium (5) | 667 (4.714) | 671 (4.947) | 668 (4.802) |
| Macrocycle | kobs × 103, s−1 |
|---|---|
| 3 | 2.3 |
| 4 | 3.9 |
| 5 | 3.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erzunov, D.A.; Botnar, A.A.; Domareva, N.P.; Tikhomirova, T.V.; Vashurin, A.S. Synthesis, Spectroscopic Properties and Redox Behavior Kinetics of Rare-Earth Bistetrakis-4-[3-(3,4-dicyanophenoxy)phenoxy]phthalocyaninato Metal Complexes with Er, Lu and Yb. Molecules 2021, 26, 2181. https://doi.org/10.3390/molecules26082181
Erzunov DA, Botnar AA, Domareva NP, Tikhomirova TV, Vashurin AS. Synthesis, Spectroscopic Properties and Redox Behavior Kinetics of Rare-Earth Bistetrakis-4-[3-(3,4-dicyanophenoxy)phenoxy]phthalocyaninato Metal Complexes with Er, Lu and Yb. Molecules. 2021; 26(8):2181. https://doi.org/10.3390/molecules26082181
Chicago/Turabian StyleErzunov, Dmitry A., Anna A. Botnar, Natalia P. Domareva, Tatiana V. Tikhomirova, and Arthur S. Vashurin. 2021. "Synthesis, Spectroscopic Properties and Redox Behavior Kinetics of Rare-Earth Bistetrakis-4-[3-(3,4-dicyanophenoxy)phenoxy]phthalocyaninato Metal Complexes with Er, Lu and Yb" Molecules 26, no. 8: 2181. https://doi.org/10.3390/molecules26082181