Phytochemical Differentiation of Saffron (Crocus sativus L.) by High Resolution Mass Spectrometry Metabolomic Studies
Abstract
:1. Introduction
2. Results and Discussion
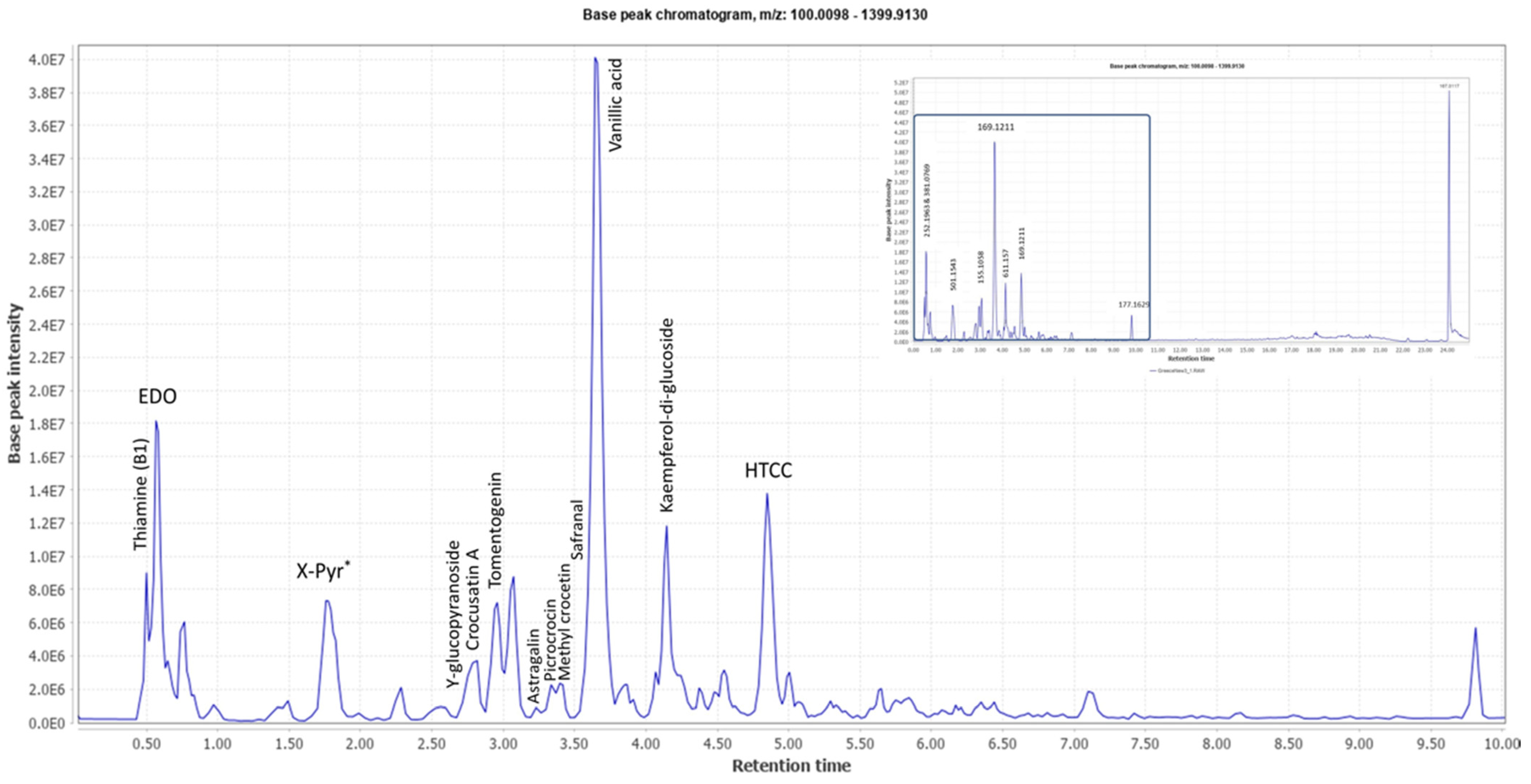
2.1. UPLC-MS Analysis
2.2. Multivariate Statistical Analysis
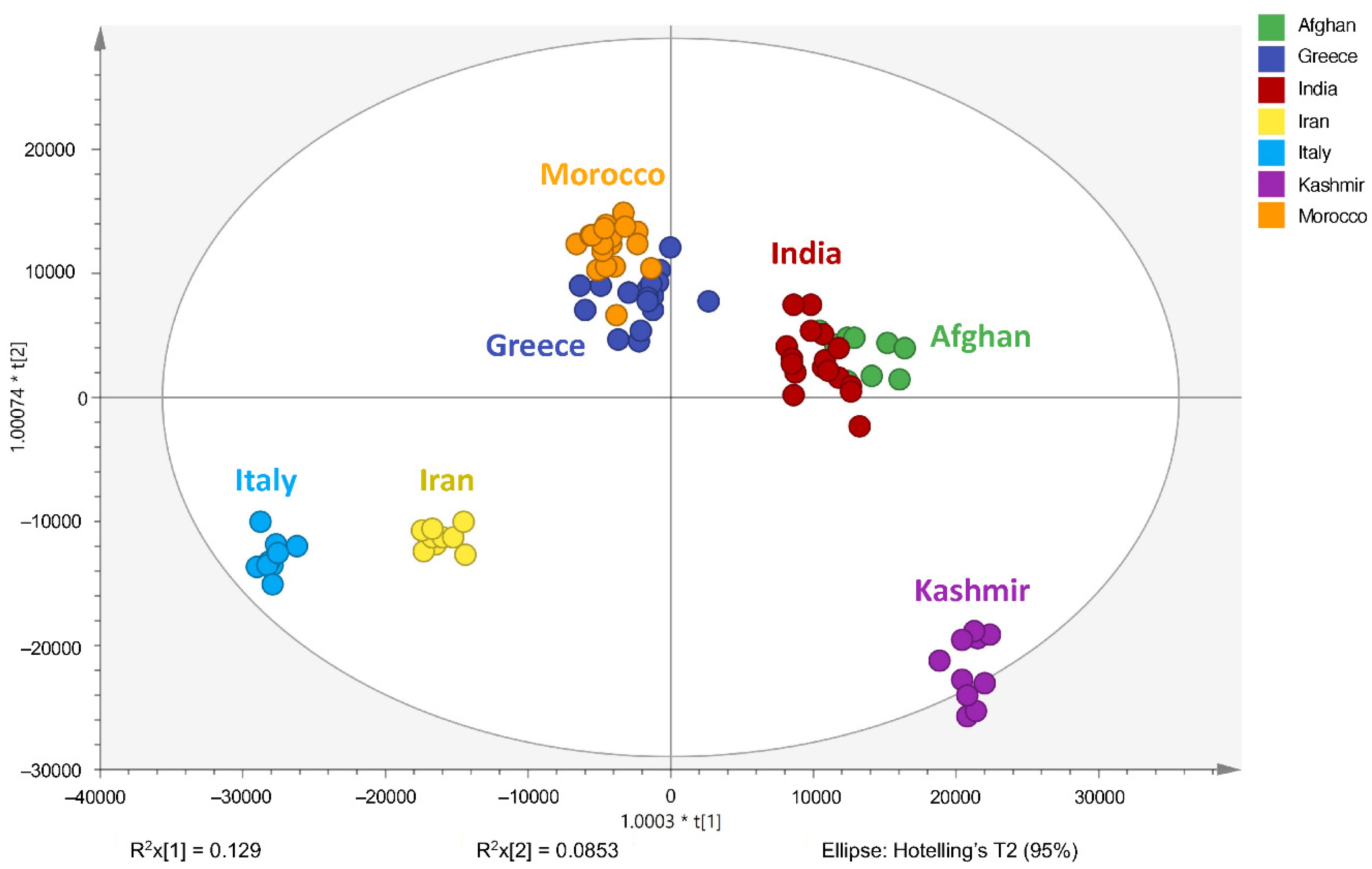
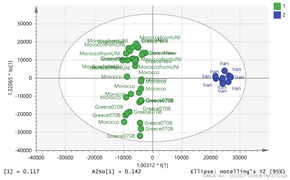
2.3. Geographical Region Differentiation
2.4. Saffron Components in the Saffron Samples from Various Regions
2.5. Marker Discriminating Power
3. Materials and Methods
3.1. Chemical Reagents and Standards
3.2. Sample Collection
3.3. Sample Preparation
3.4. UPLC—HR MS Metabolomics Analysis
3.5. Statistical Analysis
3.6. Feature Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Serrano-Díaz, J.; Sánchez, A.M.; Martínez-Tomé, M.; Winterhalter, P.; Alonso, G.L. A contribution to nutritional studies on Crocus sativus flowers and their value as food. J. Food Compos. Anal. 2013, 31, 101–108. [Google Scholar] [CrossRef]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Day, J. Crocuses in Context: A Diachronic Survey of the Crocus Motif in the Aegean Bronze Age. Hesperia 2011, 80, 337–379. [Google Scholar] [CrossRef]
- Ferrence, S.C.; Bendersky, G. Therapy with saffron and the goddess at Thera. Perspect. Biol. Med. 2004, 47, 199–226. [Google Scholar] [CrossRef]
- Mathew, B. The Crocus: A revision of the genus Crocus (Iridaceae); BT Batsford Ltd.: London, UK, 1982; Volume 127, p. 96. [Google Scholar]
- Zubor, A.; Suranyi, G.; Györi, Z.; Borbely, G.; Prokisch, J. Molecular biological approach of the systematics of Crocus sativus L. and its allies. Acta Hortic. 2004, 650, 85–93. [Google Scholar] [CrossRef]
- Brandizzi, F.; Caiola, M.G. Flow cytometric analysis of nuclear DNA inCrocus sativus and allies (Iridaceae). Plant Syst. Evol. 1998, 211, 149–154. [Google Scholar] [CrossRef]
- Agayev, Y.; Zarifi, E.; Fernandez, J. Study of karyotypes in the Crocus sativus L. aggregate and origin of cultivated saffron. Acta Hortic. 2010, 850, 47–54. [Google Scholar] [CrossRef]
- Caiola, M.G.; Caputo, P.; Zanier, R. RAPD Analysis in Crocus sativus L. Accessions and Related Crocus Species. Biol. Plant. 2004, 48, 375–380. [Google Scholar] [CrossRef]
- Winterhalter, P.; Straubinger, M. Saffron—Renewed Interest in an Ancient Spice. Food Rev. Int. 2000, 16, 39–59. [Google Scholar] [CrossRef]
- Moradzadeh, M.; Kalani, M.R.; Avan, A. The antileukemic effects of saffron (Crocus sativus L.) and its related molecular targets: A mini review. J. Cell Biochem. 2019, 120, 4732–4738. [Google Scholar] [CrossRef]
- Tarantilis, P.A.; Polissiou, M.G. Isolation and identification of the aroma components from saffron (Crocus sativus). J. Agric. Food Chem. 1997, 45, 459–462. [Google Scholar] [CrossRef]
- Carmona, M.; Zalacain, A.; Salinas, M.R.; Alonso, G.L.; Delgado, M.C. A New Approach to Saffron Aroma. Crit. Rev. Food Sci. Nutr. 2007, 47, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Anastasaki, E.; Kanakis, C.; Pappas, C.; Maggi, L.; del Campo, C.P.; Carmona, M.; Alonso, G.L.; Polissiou, M.G. Geographical differentiation of saffron by GC–MS/FID and chemometrics. Eur. Food. Res. Technol. 2009, 229, 899–905. [Google Scholar] [CrossRef]
- Xu, S.; Ge, X.; Li, S.; Guo, X.; Dai, D.; Yang, T. Discrimination of Different Parts of Saffron by Metabolomic-Based Ultra-Performance Liquid Chromatography Coupled with High-Definition Mass Spectrometry. Chem. Biodivers. 2019, 16, e1900363. [Google Scholar] [CrossRef]
- Sunanda, B.P.V.; Rammohan, B.; Kumar, A. The effective study of aqueous extract of Crocus sativus Linn. (Saffron) in depressed mice. Int. J. PharmTech Res. 2014, 6, 1143–1152. [Google Scholar]
- Poma, A.; Fontecchio, G.; Carlucci, G.; Chichiriccò, G. Anti-inflammatory properties of drugs from saffron crocus. Antiinflamm. Antiallergy. Agents Med. Chem. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacogn. Res. 2014, 6, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Papandreou, M.A.; Kanakis, C.D.; Polissiou, M.G.; Efthimiopoulos, S.; Cordopatis, P.; Margarity, M.; Lamari, F.N. Inhibitory Activity on Amyloid-β Aggregation and Antioxidant Properties of Crocus sativus Stigmas Extract and Its Crocin Constituents. J. Agric. Food Chem. 2006, 54, 8762–8768. [Google Scholar] [CrossRef]
- Sheng, L.; Qian, Z.; Zheng, S.; Xi, L. Mechanism of hypolipidemic effect of crocin in rats: Crocin inhibits pancreatic lipase. Eur. J. Pharmacol. 2006, 543, 116–122. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D. Saffron (Crocus sativus) for depression: A systematic review of clinical studies and examination of underlying antidepressant mechanisms of action. Human Psychopharmacol. Clin. Exp. 2014, 29, 517–527. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Karimi, G.; Niapoor, M. Antidepressant effect of Crocus sativus L. stigma extracts and their constituents, crocin and saffranal, in mice. Acta Hortic. 2004, 650, 435–445. [Google Scholar] [CrossRef]
- Pitsikas, N.; Zisopoulou, S.; Tarantilis, P.A.; Kanakis, C.D.; Polissiou, M.G.; Sakellaridis, N. Effects of the active constituents of Crocus sativus L., crocins on recognition and spatial rats’ memory. Behav. Brain Res. 2007, 183, 141–146. [Google Scholar] [CrossRef]
- Finley, J.W.; Gao, S. A Perspective on Crocus sativus L. (Saffron) Constituent Crocin: A Potent Water-Soluble Antioxidant and Potential Therapy for Alzheimer’s Disease. J. Agric. Food Chem. 2017, 65, 1005–1020. [Google Scholar] [CrossRef]
- Koulakiotis, N.S.; Pittenauer, E.; Halabalaki, M.; Tsarbopoulos, A.; Allmaier, G. Comparison of different tandem mass spectrometric techniques (ESI-IT, ESI- and IP-MALDI-QRTOF and vMALDI-TOF/RTOF) for the analysis of crocins and picrocrocin from the stigmas of Crocus sativus L. Rapid Commun. Mass Spectrom. 2012, 26, 670–678. [Google Scholar] [CrossRef]
- Srivastava, R.; Ahmed, H.; Dixit, R.; Dharamveer; Saraf, S. Crocus sativus L.: A comprehensive review. Pharmacogn. Rev. 2010, 4, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Pitsikas, N.; Boultadakis, A.; Georgiadou, G.; Tarantilis, P.; Sakellaridis, N. Effects of the active constituents of Crocus sativus L., crocins, in an animal model of anxiety. Phytomedicine 2008, 15, 1135–1139. [Google Scholar] [CrossRef]
- Maggi, M.A.; Bisti, S.; Picco, C. Saffron: Chemical Composition and Neuroprotective Activity. Molecules 2020, 25, 5618. [Google Scholar] [CrossRef]
- Koulakiotis, N.S.; Purhonen, P.; Gikas, E.; Hebert, H.; Tsarbopoulos, A. Crocus-derived compounds alter the aggregation pathway of Alzheimer’s Disease—Associated beta amyloid protein. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Pavarini, D.P.; Pavarini, S.P.; Niehues, M.; Lopes, N.P. Exogenous influences on plant secondary metabolite levels. Anim. Feed. Sci. Technol. 2012, 176, 5–16. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [Green Version]
- Cirak, C.; Radusiene, J.; Ivanauskas, L.; Jakstas, V.; Camas, N. Changes in the content of bioactive substances among Hypericum montbretii populations from Turkey. Rev. Bras. Farm. 2014, 24, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Tarantilis, P.A.; Tsoupras, G.; Polissiou, M. Determination of Saffron (Crocus-Sativus L) Components in Crude Plant-Extract Using High-Performance Liquid-Chromatography Uv-Visible Photodiode-Array Detection-Mass Spectrometry. J. Chromatogr. A 1995, 699, 107–118. [Google Scholar] [CrossRef]
- Carmona, M.; Sanchez, A.M.; Ferreres, F.; Zalacain, A.; Tomas-Barberan, F.; Alonso, G.L. Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: Comparative study of samples from different geographical origins. Food Chem. 2007, 100, 445–450. [Google Scholar] [CrossRef]
- Koulakiotis, N.S.; Gikas, E.; Iatrou, G.; Lamari, F.N.; Tsarbopoulos, A. Quantitation of Crocins and Picrocrocin in Saffron by HPLC: Application to Quality Control and Phytochemical Differentiation from Other Crocus Taxa. Planta Medica 2015, 81, 606–612. [Google Scholar] [CrossRef]
- Pittenauer, E.; Koulakiotis, N.S.; Tsarbopoulos, A.; Allmaier, G. In-Chain neutral hydrocarbon loss from crocin apocarotenoid ester glycosides and the crocetin aglycon (Crocus sativus L.) by ESI-MSn. J. Mass Spectrom. 2013, 48, 1299–1307. [Google Scholar]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Myers, O.D.; Sumner, S.J.; Li, S.; Barnes, S.; Du, X. One Step Forward for Reducing False Positive and False Negative Compound Identifications from Mass Spectrometry Metabolomics Data: New Algorithms for Constructing Extracted Ion Chromatograms and Detecting Chromatographic Peaks. Anal. Chem. 2017, 89, 8696–8703. [Google Scholar] [CrossRef]
- Storey, J.D.; Bass, A.J.; Dabney, A.; Robinson, D. Qvalue: Q-value Estimation for False Discovery Rate Control. R Package Version 2.22.0. Available online: http://github.com/jdstorey/qvalue (accessed on 7 April 2021).
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for significance testing of PLS and OPLS® models. J. Chemom. 2008, 22, 594–600. [Google Scholar] [CrossRef]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Cheminform. 2016, 8, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| A. Scores Plot and S-Plot | B. Features (Accurate MH+_Retention Time) | C. Putative Metabolite (Calculated Mass) * {Fragment} 1 |
|---|---|---|
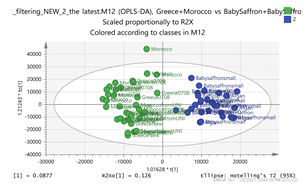 Greece + Morocco vs. India + Afghan Par 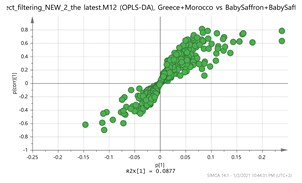 | 307.1003_4.95 365.1196_3.36 369.15_2.95 383.1298_2.85 472.1734_3.24 vs. 252.1061_0.59 367.135_3.42 369.1508_1.49 305.0824_0.71 167.1057_1.49 | EGC: C15H14O7 (307.0818) {263, 139} Methyl crocetin: C21H26O4 (MNa+ = 365.1729) {118} Tomentogenin: C21H36O5 (369.2641) {232} Astragalin: C21H20O11 (MNa+ = 472.0982) {287, 145} EDO: C15H22O2 (NH4+: 252.1963) {140} Bornyl ferulate: C20H26O4 (MK+ = 369.1470) {266, 239} |
 Iran vs. Italy par 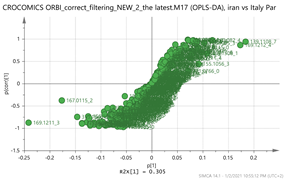 | 139.1108_7.13 169.1212_4.39 369.1508_1.49203.082_4.75 171.1005_3.58 vs. 169.1211_3.66 177.1629_9.84 353.1548_3.66 167.0115_24.08 265.1216_0.58 | HTCC isomer: C10H16O2 (169.1228) Bornyl ferulate: C20H26O4 (MK+ = 369.1470) {266, 239} Vanillic acid: C8H8O4 (169.0601) {151, 105} Picrocrocin: C21H26O4 (MNa+ = 353.1577) {185, 151} Thiamine (B1): C12H17N4OS (265.1123)2 {144, 122} |
 Greece + Morocco vs. Iran Par 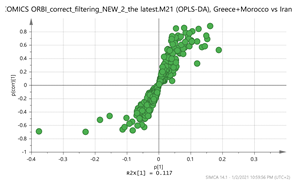 | 155.1057_2.77 169.1212_4.39 294.1526_0.79 317.1579_2.81 265.1216_0.58 vs. 151.1105_3.67 169.1211_3.66 169.1211_4.86 369.15_2.95 472.1734_3.24 | Crocusatin A: C9H14O2 (155.1073) {159} HTCC isomer: C10H16O2 (MH+ 169.1228) Y-glucopyranoside: C15H24O7 (317.1600) {383, 261, 196} Thiamine (B1): C12H17N4OS (265.1123)2 {144, 122} Safranal: C10H14O (151.1123) {123} Vanillic acid: C8H8O4 (169.0601) {151, 105} Hydroxy-β-cyclocitral (HTCC): C10H16O2 {169.1228} Tomentogenin: C21H36O5 (369.2641) {232} Astragalin: C21H20O11 (MNa+ = 472.0982) {287, 145} |
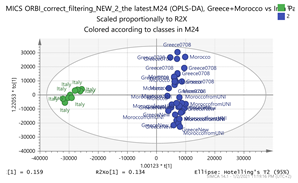 Greece + Morocco vs. Italy Par 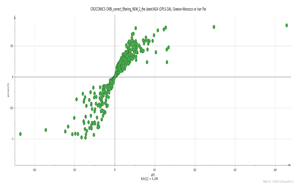 | 123.1156_3.66 151.1105_3.67 169.1211_4.86 348.1994_3.66 369.15_2.95 vs. 139.1108_7.13 169.1212_4.39 185.1159_3.69 203.082_4.75 171.1005_3.58 | Safranal: C10H14O (151,1123) {123} Hydroxy-β-cyclocitral (HTCC): C10H16O2 (169.1228) Tomentogenin: C21H36O5 (369.2641) {232} HTCC isomer: C10H16O2 (169.1228) |
 India + Afghan vs. Iran Par 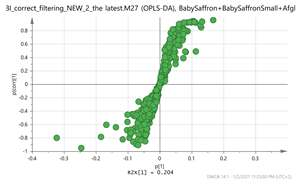 | 252.1061_0.59 294.1526_0.79 280.1373_0.73 265.1216_0.58 606.229_4.15 vs. 151.1105_3.67 169.1211_3.66 169.1211_4.86369.15_2.95 472.1734_3.24 | EDO: C15H22O2 (NH4+: 252.1963) {140} Thiamine (B1): C12H17N4OS (265.1123)2 {144, 122} Safranal: C10H14O (151.1123) Vanillic acid: C8H8O4 (169.0601) {151, 105} Hydroxy-β-cyclocitral (HTCC): C10H16O2 (169.1228) Tomentogenin: C21H36O5 (369.2641) {232} Astragalin: C21H20O11 (MNa+ = 472.0982) {287, 145} |
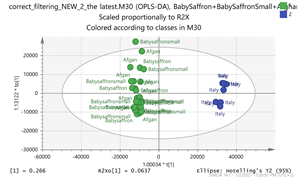 India + Afghan vs. Italy Par 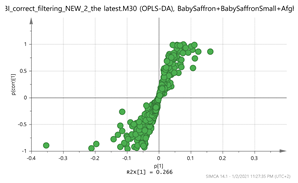 | 139.1108_7.13 169.1212_4.39 252.1061_0.59 369.1508_1.49 203.082_4.75 vs. 151.1105_3.67 169.1211_3.66 353.1548_3.66 365.1196_3.36 369.15_2.95 | HTCC isomer: C10H16O2 (169.1228) EDO: C15H22O2 (MNH4+ = 252.1963) {140} Bornyl ferulate: C20H26O4 (MK+ = 369.1470) {266, 239} Safranal: C10H14O (151.1123) Vanillic acid: C8H8O4 (169.0601) {151, 105} Picrocrocin: C21H26O4 (MNa+ = 353.1577) {185, 151} Methyl crocetin: C21H26O4 (MNa+ = 365.1729) {118} Tomentogenin: C21H36O5 (369.2641) {232} |
 Afghan + India vs. Kashmir Par  | 155.1057_2.77 307.1003_4.95 317.1579_2.81 331.173_3.66 364.1944_2.96 vs. 151.1105_3.67 151.1105_4.86 169.1211_3.66 169.1211_4.86 611.157_4.14 | Crocusatin A: C9H14O2 (155.1073) EGC: C15H14O7 (307.0818) {263, 139} Y-glucopyranoside: C15H24O7 (317.1600) Picrocrocin: C16H26O7 (331.1757) {185, 151} Methyl crocetin-C21H26O4 (MNa+ = 365.1729) {118} Safranal: C10H14O (151.1123) {123} Vanillic acid: C8H8O4 (169.0601) {151, 105} Hydroxy-β-cyclocitral (HTCC): C10H16O2 (169.1228) Kaempferol-di-glucoside: C27H30O16 (611.1611) {287} |
 Greece + Morocco vs. Kashmir Par 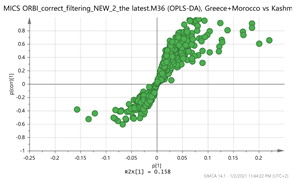 | 155.1057_2.77 183.1005_3.33 307.1003_4.95 317.1579_2.81 331.173_3.66 vs. 151.1105_3.67 151.1105_4.86 169.1211_3.66 169.1211_4.86 611.157_4.14 | Crocusatin A: C9H14O2 (155.1073) EGC: C15H14O7 (307.0818) {263, 139} Y-glucopyranoside: C15H24O7 (317.1600) Picrocrocin: C16H26O7 (331.1757) {185, 151} Safranal: C10H14O (151.1123) {123} Vanillic acid: C8H8O4 (169.0601) {151, 105} Hydroxy-β-cyclocitral (HTCC): C10H16O2 (169.1228) Kaempferol-di-glucoside: C27H30O16 (611.1611) {287} |
 Italy vs. Kashmir Par 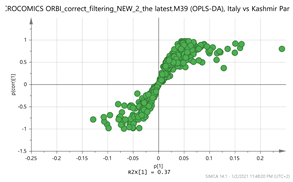 | 169.1211_3.66 307.1003_4.95 331.173_3.66 353.1548_3.66 369.15_2.95 vs. 169.1212_4.39 252.1061_0.59 369.1508_1.49 203.082_4.75 611.157_4.14 | Vanillic acid: C8H8O4 (169.0601) {151, 105} EGC: C15H14O7 (307.0818) {263, 139} Picrocrocin: C16H26O7 (331.1757) {185, 151} Picrocrocin: C16H26O7 (MNa+ = 353.1577) {185, 151} Tomentogenin: C21H36O5 (369.2641) {232} HTCC isomer: C10H16O2 (169.1228) EDO: C15H22O2 (MNH4+ = 252.1963) {140} Bornyl ferulate: C20H26O4 (MK+ = 369.1470) {266, 239} Kaempferol-di-glucoside: C27H30O16 (611.1611) {287} |
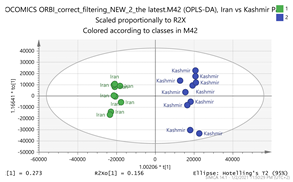 Iran vs. Kashmir Par  | 169.1211_3.66 307.1003_4.95 331.173_3.66 369.15_2.95 511.1749_4.94 vs. 252.1061_0.59 294.1526_0.79 280.1373_0.73 265.1216_0.58 611.157_4.14 | Vanillic acid: C8H8O4 (169.0601) {151, 105} EGC: C15H14O7 (307. 0818) {263, 139} Picrocrocin: C16H26O7 (331.1757) {185, 151} Tomentogenin: C21H36O5 (369.2641) {232} EDO: C15H22O2 (MNH4+ = 252.1963) {140} Thiamine (B1): C12H17N4OS (265.1123)2 {144, 122} Kaempferol-di-glucoside: C27H30O16 (611.1611) {287} |
| IND_AFG | GR_MOR | IR | IT | KSH | |
|---|---|---|---|---|---|
| IND_AFG | 307.1003_4.95 | 151.1105_3.67 | 151.1105_3.67 | 151.1105_3.67 | |
| 365.1196_3.36 | 169.1211_3.66 | 169.1211_3.66 | 151.1105_4.86 | ||
| 369.15_2.95 | 169.1211_4.86 | 353.1548_3.66 | 169.1211_3.66 | ||
| 383.1298_2.85 | 369.15_2.95 | 365.1196_3.36 | 169.1211_4.86 | ||
| 472.1734_3.24 | 472.1734_3.24 | 369.15_2.95 | 611.157_4.14 | ||
| GR_MOR | 252.1061_0.59 | 123.1156_3.66 | 169.1211_3.66 | 151.1105_3.67 | |
| 367.135_3.42 | 151.1105_3.67 | 177.1629_9.84 | 151.1105_4.86 | ||
| 369.1508_1.49 | 169.1211_3.66 | 353.1548_3.66 | 169.1211_3.66 | ||
| 305.0824_0.71 | 169.1211_4.86 | 167.0115_24.08 | 169.1211_4.86 | ||
| 167.1057_1.49 | 369.15_2.95 | 265.1216_0.58 | 611.157_4.14 | ||
| IR | 252.1061_0.59 | 155.1057_2.77 | 169.1211_3.66 | 252.1061_0.59 | |
| 294.1526_0.79 | 169.1212_4.39 | 177.1629_9.84 | 294.1526_0.79 | ||
| 280.1373_0.73 | 294.1526_0.79 | 353.1548_3.66 | 611.157_4.14 | ||
| 265.1216_0.58 | 317.1579_2.81 | 167.0115_24.08 | 280.1373_0.73 | ||
| 606.229_4.15 | 265.1216_0.58 | 265.1216_0.58 | 265.1216_0.58 | ||
| IT | 139.1108_7.13 | 123.1156_3.66 | 139.1108_7.13 | 169.1212_4.39 | |
| 169.1212_4.39 | 151.1105_3.67 | 169.1212_4.39 | 252.1061_0.59 | ||
| 252.1061_0.59 | 169.1211_4.86 | 369.1508_1.49 | 369.1508_1.49 | ||
| 369.1508_1.49 | 348.1994_3.66 | 203.082_4.75 | 611.157_4.14 | ||
| 203.082_4.75 | 369.15_2.95 | 171.1005_3.58 | 203.082_4.75 | ||
| KSH | 155.1057_2.77 | 155.1057_2.77 | 169.1211_3.66 | 169.1211_3.66 | |
| 307.1003_4.95 | 183.1005_3.33 | 307.1003_4.95 | 307.1003_4.95 | ||
| 317.1579_2.81 | 307.1003_4.95 | 331.173_3.66 | 331.173_3.66 | ||
| 331.173_3.66 | 317.1579_2.81 | 369.15_2.95 | 353.1548_3.66 | ||
| 364.1944_2.96 | 331.173_3.66 | 511.1749_4.94 | 369.15_2.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gikas, E.; Koulakiotis, N.S.; Tsarbopoulos, A. Phytochemical Differentiation of Saffron (Crocus sativus L.) by High Resolution Mass Spectrometry Metabolomic Studies. Molecules 2021, 26, 2180. https://doi.org/10.3390/molecules26082180
Gikas E, Koulakiotis NS, Tsarbopoulos A. Phytochemical Differentiation of Saffron (Crocus sativus L.) by High Resolution Mass Spectrometry Metabolomic Studies. Molecules. 2021; 26(8):2180. https://doi.org/10.3390/molecules26082180
Chicago/Turabian StyleGikas, Evangelos, Nikolaos Stavros Koulakiotis, and Anthony Tsarbopoulos. 2021. "Phytochemical Differentiation of Saffron (Crocus sativus L.) by High Resolution Mass Spectrometry Metabolomic Studies" Molecules 26, no. 8: 2180. https://doi.org/10.3390/molecules26082180






