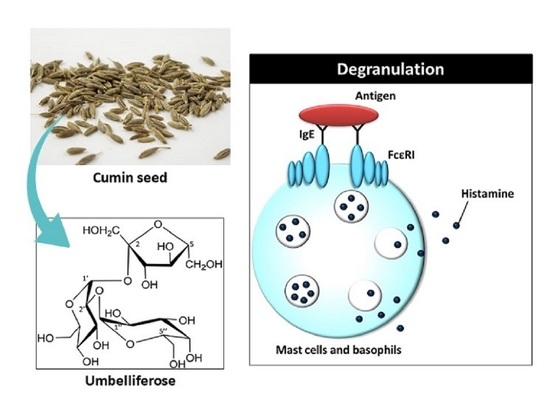
Umbelliferose Isolated from Cuminum cyminum L. Seeds Inhibits Antigen-Induced Degranulation in Rat Basophilic Leukemia RBL-2H3 Cells
Abstract
:1. Introduction
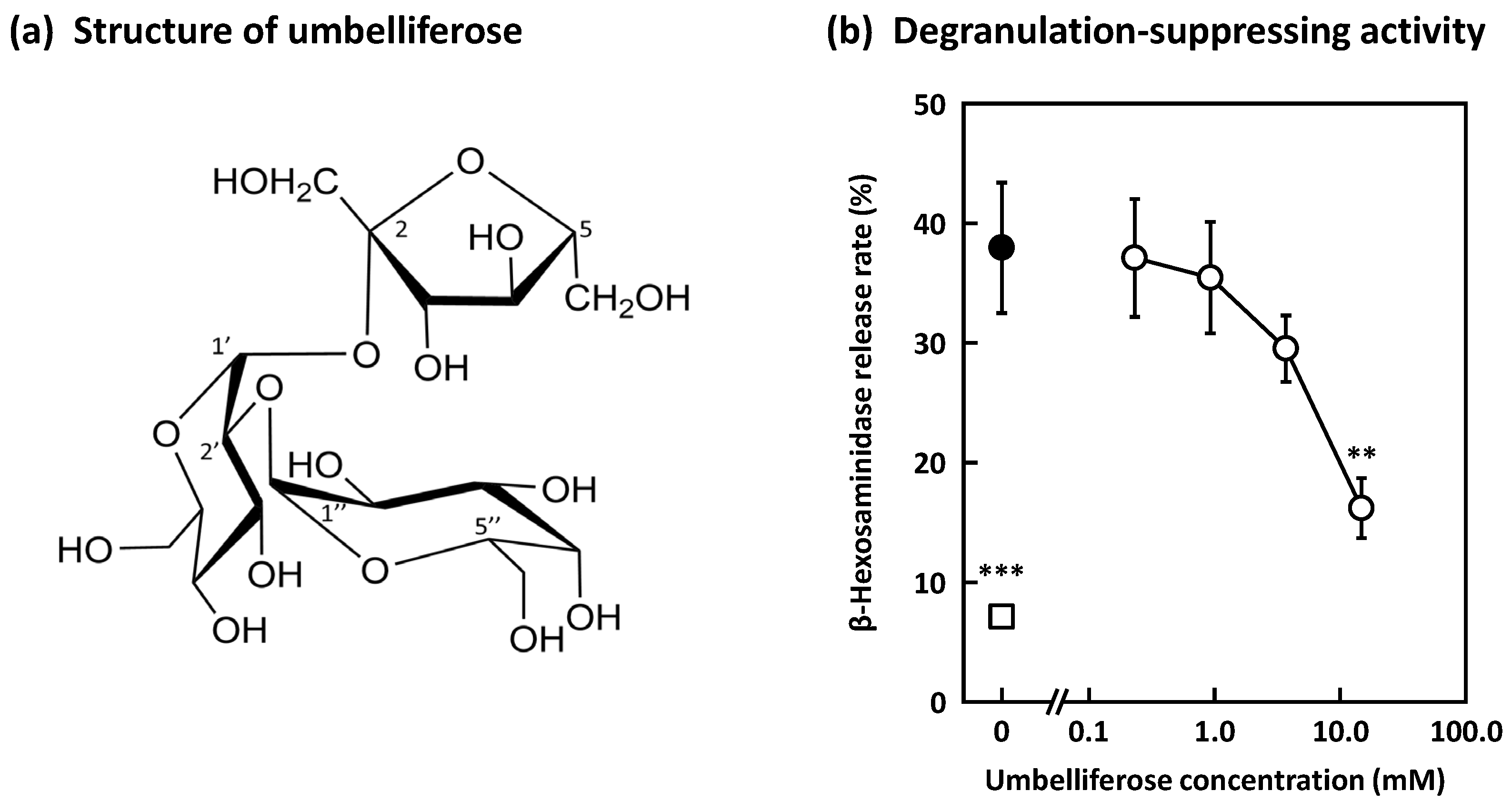
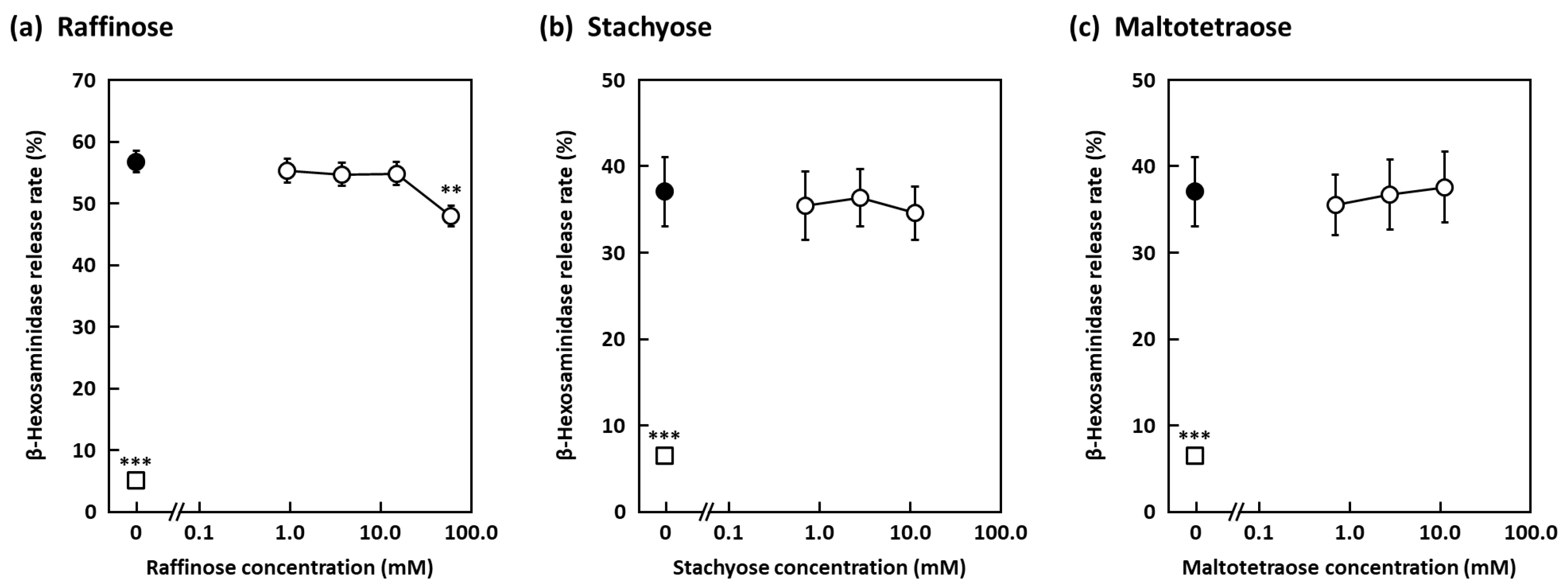
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
3.2. General Experimental Procedures
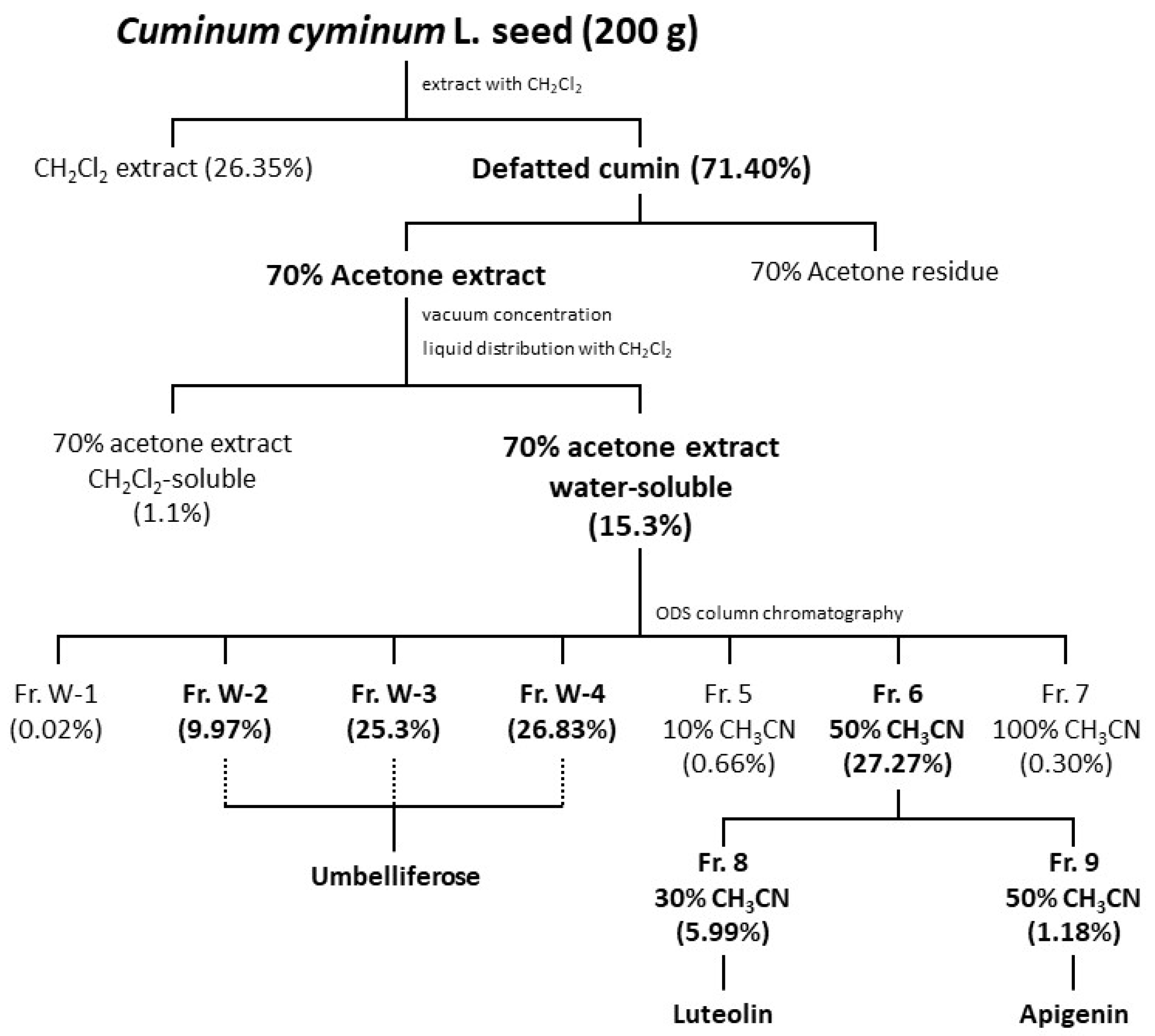
3.3. Extraction
3.4. Fractionating the Water-Soluble Fraction
3.5. Isolating and Identifying the Components in the Water-Soluble Fraction
3.6. Quantifying Umbelliferose
3.7. Preparation of Sugars
3.8. Cells and Cell Cultures
3.9. β-Hexosaminidase Release Assay
3.10. Cell Viability
3.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Turner, H.; Kinet, J.P. Signalling through the high-affinity IgE receptor FcεRI. Nature 1999, 402, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Peavy, R.D.; Gilfillan, A.M. Mechanisms of mast cell signaling in anaphylaxis. J. Allergy Clin. Immunol. 2009, 124, 639–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, M. Mediators of inflammation and the inflammatory process. J. Allergy Clin. Immunol. 1999, 103, 378–381. [Google Scholar] [CrossRef]
- Sano, M.; Suzuki, M.; Miyase, T.; Yoshino, K.; Maeda-Yamamoto, M. Novel antiallergic catechin derivatives isolated from oolong tea. J. Agric. Food Chem. 1999, 47, 1906–1910. [Google Scholar] [CrossRef]
- Fujimura, Y.; Umeda, D.; Kiyohara, Y.; Sunada, Y.; Yamada, K.; Tachibana, H. The involvement of the 67 kDa laminin receptor-mediated modulation of cytoskeleton in the degranulation inhibition induced by epigallocatechin-3-O-gallate. Biochem. Biophys. Res. Commun. 2006, 348, 524–531. [Google Scholar] [CrossRef]
- Onishi, S.; Nishi, K.; Yasunaga, S.; Muranaka, A.; Maeyama, K.; Kabota, A.; Sugahara, T. Nobiletin, a polymethoxy flavonoid, exerts anti-allergic effect by suppressing activation of phosphoinositide 3-kinase. J. Funct. Foods 2014, 6, 606–614. [Google Scholar] [CrossRef]
- Morishita, Y.; Saito, M.; Takemura, E.; Fujikawa, R.; Yamamoto, R.; Kuroyanagi, M.; Shirota, O.; Muto, N. Flavonoid glucuronides isolated from spinach inhibit IgE-mediated degranulation in basophilic leukemia RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. Integr. Mol. Med. 2015, 2, 99–105. [Google Scholar]
- Yasunaga, S.; Kadota, A.; Kikuchi, T.; Kubo, C.; Nishi, K.; Sugahara, T. Effect of concurrent administration of nobiletin and β-lactoglobulin on the symptoms of Japanese cedar pollinosis models in mice. J. Funct. Foods 2016, 22, 389–397. [Google Scholar] [CrossRef]
- Korinek, M.; Handoussa, H.; Tsai, Y.H.; Chen, Y.Y.; Chen, M.H.; Chiou, Z.W.; Fang, Y.; Chang, F.R.; Yen, C.H.; Hsieh, C.F.; et al. Anti-inflammatory and antimicrobial volatile oils: Fennel and cumin inhibit neutrophilic inflammation via regulating calcium and MAPKs. Front. Pharmacol. 2021, 12, 674095. [Google Scholar] [CrossRef]
- Patil, S.B.; Takalikar, S.S.; Joglekar, M.M.; Haldavnekar, V.S.; Arvindekar, A.Y. Insulinotropic and β-cell protective action of cuminaldehyde, cuminol and an inhibitor isolated from Cuminum cyminum in streptozotocin-induced diabetic rats. Br. J. Nutr. 2013, 110, 1434–1443. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.R.; Ansari, S.H. Aromatic aldehyde compound cuminaldehyde protects nonalcoholic fatty liver disease in rats feeding high fat diet. Hum. Exp. Toxicol. 2019, 38, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Neto, V.; Souza, C.D.; Gonzaga, L.F.; Silveira, B.C.; Sousa, N.C.F.; Pontes, J.P.; Santos, D.M.; Martins, W.C.; Pessoa, J.F.V.; Carvalho Júnior, A.R.; et al. Cuminaldehyde potentiates the antimicrobial actions of ciprofloxacin against Staphylococcus aureus and Escherichia coli. PLoS ONE 2020, 15, e0232987. [Google Scholar] [CrossRef]
- Hada, M.; Nishi, K.; Ishida, M.; Onda, H.; Nishimoto, S.; Sugahara, T. Inhibitory effect of aqueous extract of Cuminum cyminum L., seed on degranulation of RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. Cytotechnology 2019, 71, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.H.; Kim, H.S.; Kang, H.J.; Lee, H.S.; Jeong, S.I.; Kim, S.J.; Jang, S.I. Anti-inflammatory and antipruritic effects of luteolin from Perilla (P. frutescens L.) leaves. Molecules 2014, 19, 6941–6951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, Y. Luteolin as a potential preventive and therapeutic candidate for Alzheimer’s disease. Exp. Gerontol. 2017, 95, 39–43. [Google Scholar] [CrossRef]
- Cicek, M.; Unsal, V.; Doganer, A.; Demir, M. Investigation of oxidant/antioxidant and anti-inflammatory effects of apigenin on apoptosis in sepsis-induced rat lung. J. Biochem. Mol. Toxicol. 2021, 35, e22743. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Imran, M.; Gondal, T.A.; Atif, A.; Shahbaz, M.; Qaisarani, T.B.; Mughal, M.H.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phytother. Res. 2020, 34, 1812–1828. [Google Scholar] [CrossRef]
- Goodarzi, S.; Tabatabaei, M.J.; Jafari, M.H.; Shemirani, F.; Tavakoli, S.; Mofasseri, M.; Tofighi, Z. Cuminum cyminum fruits as source of luteolin-7-O-glucoside, potent cytotoxic flavonoid against breast cancer cell lines. Nat. Prod. Res. 2020, 34, 1602–1606. [Google Scholar] [CrossRef]
- Mastuda, H.; Morikawa, T.; Ueda, K.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of antigen-Induced degranulation, TNF-alpha and IL-4 production from RBL-2H3 cells. Bioorg. Med. Chem. 2002, 10, 3123–3128. [Google Scholar] [CrossRef]
- Park, C.H.; Min, S.Y.; Yu, H.W.; Kim, K.; Kim, S.; Lee, H.J.; Kim, J.H.; Park, Y.J. Effects of apigenin on RBL-2H3, RAW264.7, and HaCaT cells: Anti-allergic, anti-inflammatory, and skin-protective activities. Int. J. Mol. Sci. 2020, 21, 4620. [Google Scholar] [CrossRef]
- Liang, K.L.; Yu, S.J.; Huang, W.C.; Yen, H.R. Luteolin attenuates allergic nasal inflammation via inhibition of interleukin-4 in an allergic rhinitis mouse model and peripheral blood from human subjects with allergic rhinitis. Front. Pharmacol. 2020, 11, 291. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.; Che, D.; Yu, Y.; Liu, L.; Mi, S.; Zhang, Y.; Hao, J.; Li, W.; Ji, M.; Geng, S.; et al. Luteolin inhibits FcεRI- and MRGPRX2-mediated mast cell activation by regulating calcium signaling pathways. Phytother. Res. 2022, 36, 2197–2206. [Google Scholar] [CrossRef]
- Hopf, H.; Kandler, O. Physiology of umbelliferose. Biochem. Physiol. Pflanz. 1976, 169, 5–36. [Google Scholar] [CrossRef]
- Hopf, H.; Kandler, O. Biosynthesis of umbelliferose in Aegopodium podagraria. Plant Physiol. 1974, 54, 13–14. [Google Scholar] [CrossRef] [Green Version]
- Pistelli, L.; Marsili, A.; Morelli, I.; Barili, P.L.; Pizza, C. Umbelliferose from Cachrys ferulacea seeds: Determination of the sugar sequence by NMR 2D-COLOC technique. Planta Med. 1990, 56, 230–231. [Google Scholar] [CrossRef]
- Oliveira, C.R.; Spindola, D.G.; Garcia, D.; Erustes, A.; Bechara, A.; Palmeira-Dos-Santos, C.; Smaili, S.S.; Pereira, G.J.S.; Hinsberger, A.; Viriato, E.P.; et al. Medicinal properties of Angelica archangelica root extract: Cytotoxicity in breast cancer cells and its protective effects against in vivo tumor development. J. Integr. Med. 2019, 17, 132–140. [Google Scholar] [CrossRef]
- Watanabe, H.; Sonoyama, K.; Watanabe, J.; Yamaguchi, N.; Kikuchi, H.; Nagura, T.; Aritsuka, T.; Fukumoto, K.; Kasai, T. Reduction of allergic airway eosinophilia by dietary raffinose in Brown Norway rats. Br. J. Nutr. 2004, 92, 247–255. [Google Scholar] [CrossRef]
- Sonoyama, K.; Watanabe, H.; Watanabe, J.; Yamaguchi, N.; Yamashita, A.; Hashimoto, H.; Kishino, E.; Fujita, K.; Okada, M.; Mori, S.; et al. Allergic airway eosinophilia is suppressed in ovalbumin-sensitized Brown Norway rats fed raffinose and alpha-linked galactooligosaccharide. J. Nutr. 2005, 135, 538–548. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Tanabe, S. Evaluation of the anti-allergic activity of Citrus unshiu using rat basophilic leukemia RBL-2H3 cells as well as basophils of patients with seasonal allergic rhinitis to pollen. Int. J. Mol. Med. 2006, 17, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Abe, T.; Kikuchi, H.; Aritsuka, T.; Takata, Y.; Fukushi, E.; Fukushi, Y.; Kawabata, J.; Ueno, K.; Onodera, S.; Shiomi, N. Structural confirmation of novel oligosaccharides isolated from sugar beet molasses. Food Chem. 2016, 202, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Cuong, D.T.D.; Dat, H.T.; Duan, N.T.; Thuong, P.D.; Phat, N.T.; Tri, M.D.; Son, D.V.; Hoa, N.T.; Tuyen, P.N.K.; Phung, N.K.P. Isolation and characterization of six flavonoids from the leaves of Sterculia foetida Linn. Vietnam J. Chem. 2019, 57, 438–442. [Google Scholar] [CrossRef]
- Ersöz, T.; Harput, S.; Saracoğlu, İ.; Çaliş, İ. Phenolic compounds from Scutellaria pontica. Turk. J. Chem. 2002, 26, 581–588. [Google Scholar]


| Sample | Solvent | Activity | Identified Compound |
|---|---|---|---|
| 70% Acetone extract water-soluble fraction | D.W. | + | |
| Fr. W-1 | D.W. | Unrated | |
| Fr. W-2 | D.W. | + | Umbelliferose |
| Fr. W-3 | D.W. | + | |
| Fr. W-4 | D.W. | + | |
| Fr. 5 10% CH3CN eluate | DMSO | − | |
| Fr. 6 50% CH3CN | DMSO | + | |
| Fr. 7 100% CH3CN | DMSO | Unrated | |
| Fr. 8 30% CH3CN eluate | DMSO | + | Luteolin |
| Fr. 9 50% CN3CN eluate | DMSO | + | Apigenin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishida, M.; Ohara, R.; Miyagawa, F.; Kikuzaki, H.; Nishi, K.; Onda, H.; Yoshino, N.; Sugahara, T. Umbelliferose Isolated from Cuminum cyminum L. Seeds Inhibits Antigen-Induced Degranulation in Rat Basophilic Leukemia RBL-2H3 Cells. Molecules 2022, 27, 4101. https://doi.org/10.3390/molecules27134101
Ishida M, Ohara R, Miyagawa F, Kikuzaki H, Nishi K, Onda H, Yoshino N, Sugahara T. Umbelliferose Isolated from Cuminum cyminum L. Seeds Inhibits Antigen-Induced Degranulation in Rat Basophilic Leukemia RBL-2H3 Cells. Molecules. 2022; 27(13):4101. https://doi.org/10.3390/molecules27134101
Chicago/Turabian StyleIshida, Momoko, Rika Ohara, Fuka Miyagawa, Hiroe Kikuzaki, Kosuke Nishi, Hiroyuki Onda, Nanami Yoshino, and Takuya Sugahara. 2022. "Umbelliferose Isolated from Cuminum cyminum L. Seeds Inhibits Antigen-Induced Degranulation in Rat Basophilic Leukemia RBL-2H3 Cells" Molecules 27, no. 13: 4101. https://doi.org/10.3390/molecules27134101