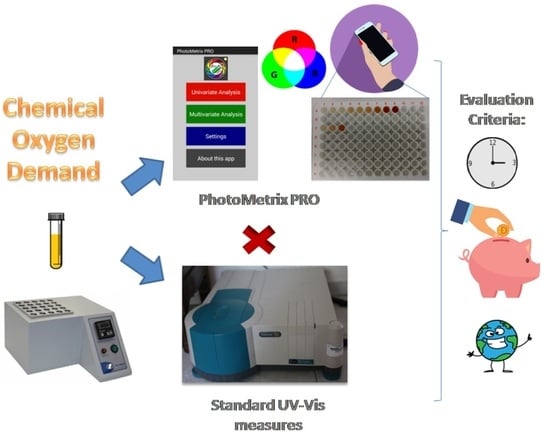
Miniaturized Method for Chemical Oxygen Demand Determination Using the PhotoMetrix PRO Application
Abstract
:1. Introduction
2. Results
2.1. Standard Colorimetric Method versus PhotoMetrix PRO Analysis
2.2. Cost and Time Reductions and Greenness Improvement Due to Analysis Miniaturization
3. Discussion
4. Materials and Methods
4.1. Chemicals and Water Matrices
4.2. Sample Digestion and Standard Colorimetric Method
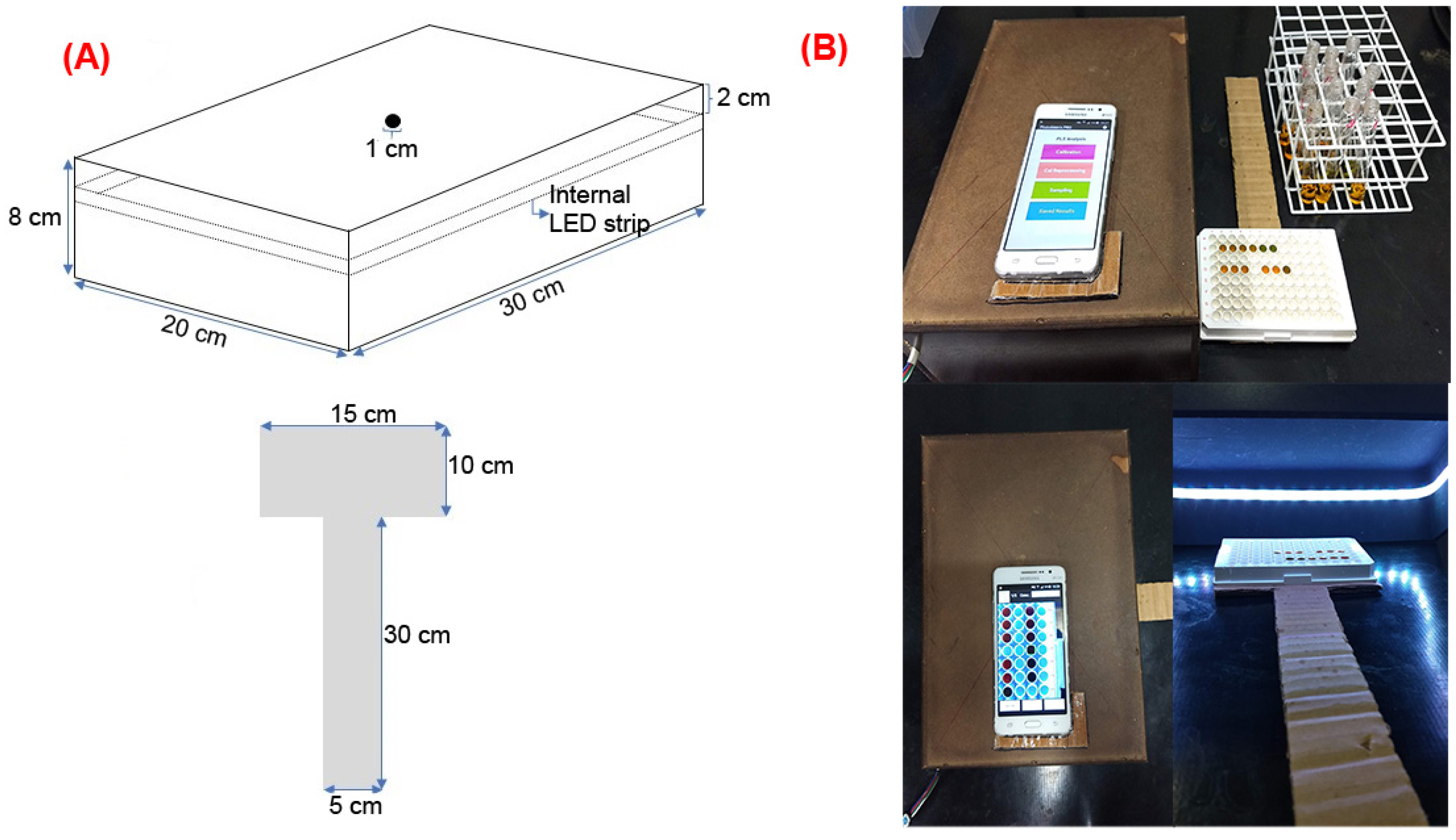
4.3. PhotoMetrix PRO Analysis
4.4. COD Method Miniaturization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- United Nations. World Population Prospects 2019; United Nations: New York, NY, USA, 2019; ISBN 9789211483161. [Google Scholar]
- Hoornweg, D.; Sugar, L.; Gómez, C.L.T. Cities and Greenhouse Gas Emissions: Moving Forward. Environ. Urban. 2011, 23, 207–227. [Google Scholar] [CrossRef]
- Tun, M.M.; Juchelková, D. Estimation of Greenhouse Gas Emissions: An Alternative Approach to Waste Management for Reducing the Environmental Impacts in Myanmar. Environ. Eng. Res. 2019, 24, 618–629. [Google Scholar] [CrossRef]
- Sharp, J.H. Gross Analyses of Organic Matter in Seawater: Why, How, and from Where. In Marine Chemistry in the Coastal Environment; Church, T.M., Ed.; American Chemical Society: Washington, DC, USA, 1975; pp. 682–696. ISBN 9780841202375. [Google Scholar]
- Chen, S.C.; Tzeng, J.H.; Tien, Y.; Wu, L.F. Rapid Determination of Chemical Oxygen Demand (COD) Using Focused Microwave Digestion Followed by a Titrimetric Method. Anal. Sci. 2001, 17, 551–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- APHA; AWWA; WEF 5220 D. Closed Reflux, Colorimetric Method. In Standard Methods for the Examination of Water and Wastewater, American Public Health Association; Baird, R.B., Eaton, A.D., Rice, E.W., Eds.; APHA: Washington, DC, USA, 2017; pp. 21–23. [Google Scholar]
- Geerdink, R.B.; Sebastiaan van den Hurk, R.; Epema, O.J. Chemical Oxygen Demand: Historical Perspectives and Future Challenges. Anal. Chim. Acta 2017, 961, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cuervo Lumbaque, E.; Cardoso, R.M.; de Araújo Gomes, A.; Malato, S.; Sánchez Pérez, J.A.; Sirtori, C. Removal of Pharmaceuticals in Hospital Wastewater by Solar Photo-Fenton with Fe3+-EDDS Using a Pilot Raceway Pond Reactor: Transformation Products and in Silico Toxicity Assessment. Microchem. J. 2021, 164, 106014. [Google Scholar] [CrossRef]
- Prado, T.; Silva, D.M.; Guilayn, W.C.; Rose, T.L.; Gaspar, A.M.C.; Miagostovich, M.P. Quantification and Molecular Characterization of Enteric Viruses Detected in Effluents from Two Hospital Wastewater Treatment Plants. Water Res. 2011, 45, 1287–1297. [Google Scholar] [CrossRef]
- Gönder, Z.B.; Kara, E.M.; Celik, B.O.; Vergili, I.; Kaya, Y.; Altinkum, S.M.; Bagdatli, Y.; Yilmaz, G. Detailed Characterization, Antibiotic Resistance and Seasonal Variation of Hospital Wastewater. Environ. Sci. Pollut. Res. 2021, 28, 16380–16393. [Google Scholar] [CrossRef]
- Meo, M.I.; Haydar, S.; Nadeem, O.; Hussain, G.; Rashid, H. Characterization of Hospital Wastewater, Risk Waste Generation and Management Practices in Lahore. Proc. Pakistan Acad. Sci. 2014, 51, 317. [Google Scholar]
- Okpala, C.O.R.; Sardo, G.; Vitale, S.; Bono, G.; Arukwe, A. Hazardous Properties and Toxicological Update of Mercury: From Fish Food to Human Health Safety Perspective. Crit. Rev. Food Sci. Nutr. 2018, 58, 1986–2001. [Google Scholar] [CrossRef]
- Labra, M.; Bernasconi, M.; Grassi, F.; De Mattia, F.; Sgorbati, S.; Airoldi, R.; Citterio, S. Toxic and Genotoxic Effects of Potassium Dichromate in Pseudokirchneriella Subcapitata Detected by Microscopy and AFLP Marker Analysis. Aquat. Bot. 2007, 86, 229–235. [Google Scholar] [CrossRef]
- 5220 Chemical Oxygen Demand (COD). In Standard Methods For the Examination of Water and Wastewater; Baird, R.B.; Eaton, A.D.; Rice, E.W. (Eds.) American Public Health Association: Washington, DC, USA, 2017; p. 1. [Google Scholar]
- Anastas, P.T. Green Chemistry and the Role of Analytical Methodology Development. Crit. Rev. Anal. Chem. 1999, 29, 167–175. [Google Scholar] [CrossRef]
- Kolb, M.; Bahadir, M.; Teichgräber, B. Determination of Chemical Oxygen Demand (COD) Using an Alternative Wet Chemical Method Free of Mercury and Dichromate. Water Res. 2017, 122, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-Palacios, P.; Balderas-Hernández, P.; Ibanez, J.G.; Roa-Morales, G. Replacing Dichromate with Hydrogen Peroxide in the Chemical Oxygen Demand (COD) Test. Water Sci. Technol. 2012, 66, 1069–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, I.H.A.; Hassan, H.H.; Hamed, E.; Abdel-Aziz, A.M. Sensitive and Green Method for Determination of Chemical Oxygen Demand Using a Nano-Copper Based Electrochemical Sensor. Electroanalysis 2017, 29, 2401–2409. [Google Scholar] [CrossRef]
- Kim, H.; Lim, H.; Colosimo, M.F. Determination of Chemical Oxygen Demand (COD) Using Ultrasound Digestion and Oxidation-Reduction Potential-Based Titration. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2007, 42, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kim, J.; Awofeso, O.; Kim, H.; Regnier, F.; Bae, E. Smartphone-Based Colorimetric Analysis for Detection of Saliva Alcohol Concentration. Appl. Opt. 2015, 54, 9183. [Google Scholar] [CrossRef]
- Shen, L.; Hagen, J.A.; Papautsky, I. Point-of-Care Colorimetric Detection with a Smartphone. Lab Chip 2012, 12, 4240–4243. [Google Scholar] [CrossRef]
- Oncescu, V.; O’Dell, D.; Erickson, D. Smartphone Based Health Accessory for Colorimetric Detection of Biomarkers in Sweat and Saliva. Lab Chip 2013, 13, 3232–3238. [Google Scholar] [CrossRef]
- Helfer, G.A.; Magnus, V.S.; Böck, F.C.; Teichmann, A.; Ferrão, M.F.; Da Costa, A.B. PhotoMetrix: An Application for Univariate Calibration and Principal Components Analysis Using Colorimetry on Mobile Devices. J. Braz. Chem. Soc. 2017, 28, 328–335. [Google Scholar] [CrossRef]
- Gorziza, R.P.; Carvalho, C.M.B.; González, M.; Ortiz, R.S.; Helfer, G.A.; Ferrão, M.F.; Limberger, R.P. Blue Ballpoint Pen Inks Differentiation Using Multivariate Image Analysis of Digital Images Captured with PhotoMetrix PRO®. Braz. J. Forensic Sci. Med. Law Bioeth. 2020, 9, 331–355. [Google Scholar] [CrossRef]
- Pappis, C.; Librelotto, M.; Baumann, L.; Parckert, A.B.; Santos, R.O.; Teixeira, I.D.; Helfer, G.A.; Lobo, E.A.; da Costa, A.B. Point-of-Use Determination of Fluoride and Phosphorus in Water through a Smartphone Using the PhotoMetrix® App. Braz. J. Anal. Chem. 2019, 6, 58–66. [Google Scholar] [CrossRef]
- Lumbaque, E.C.; Becker, R.W.; Araújo, D.S.; Dallegrave, A.; Fracari, T.O.; Lavayen, V.; Sirtori, C. Degradation of Pharmaceuticals in Different Water Matrices by a Solar Homo/Heterogeneous Photo-Fenton Process over Modified Alginate Spheres. Environ. Sci. Pollut. Res. 2019, 26, 6532–6544. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.A.; Tischer, B.; Filoda, P.F.; Parckert, A.B.; dos Santos, R.B.; Vinciguerra, L.L.; Ferrão, M.F.; Barin, J.S.; Ben da Costa, A. A New Tool for Interpretation of Thermal Stability of Raw Milk by Means of the Alizarol Test Using a PLS Model on a Mobile Device. Food Anal. Methods 2018, 11, 2022–2028. [Google Scholar] [CrossRef]
- de Jesus, J.R.; Guimarães, I.C.; Arruda, M.A.Z. Quantifying Proteins at Microgram Levels Integrating Gel Electrophoresis and Smartphone Technology. J. Proteom. 2019, 198, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Radtke, J.F.; Martins, J.S.; Machado, E.L. Determinação da Demanda Química de Oxigênio (DQO) em Efluentes a Partir da Aquisição de Imagens Digitais Utilizando Smartphone (Determination of Chemical Oxygen Demand (COD) in Wastewater by the Acquisition of Digital Images Using Smartphone). Rev. Jovens Pesqui. 2019, 9, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Comissão Técnica de Química (CT-05). Orientação Sobre Validação de Métodos Analíticos, Inmetro. (2011) 20. Available online: http://www.inmetro.gov.br/Sidoq/Arquivos/Cgcre/DOQ/DOQ-Cgcre-8_04.pdf (accessed on 19 February 2021).
- Kim, B.S.; Vogelpohl, A. Degradation of Organic Pollutants by the Photo-Fenton-Process. Chem. Eng. Technol. 1998, 21, 187–191. [Google Scholar] [CrossRef]
- Hermosilla, D.; Cortijo, M.; Pao, C. Optimizing the Treatment of Landfill Leachate by Conventional Fenton and Photo-Fenton Processes. Sci. Total Environ. 2009, 407, 3473–3481. [Google Scholar] [CrossRef]
- Sirtori, C.; Zapata, A.; Oller, I.; Gernjak, W.; Agüera, A.; Malato, S. Decontamination industrial pharmaceutical wastewater by combining solar photo-Fenton and biological treatment. Water Res. 2009, 43, 661–668. [Google Scholar] [CrossRef]
- Sarria, V.; Parra, S.; Adler, N.; Peringer, P.; Benitez, N.; Pulgarin, C. Recent developments in the coupling of photoassisted and aerobic biological processes for the treatment of biorecalcitrant compounds. Catal. Today 2002, 76, 301–315. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green Analytical Chemistry. TrAC Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Kumar, V.; Sikarwar, D.; Majumder, A.; Kumar, A. An Assessment of Hospital Wastewater and Biomedical Waste Generation, Existing Legislations, Risk Assessment, Treatment Processes, and Scenario during COVID-19. J. Environ. Manag. 2022, 308, 114609. [Google Scholar] [CrossRef]
- OECD. Test No. 303: Simulation Test—Aerobic Sewage Treatment—A: Activated Sludge Units; B: Biofilms; OECD Guidelines for the Testing of Chemicals, Section 3; OECD Publishing: Paris, France, 2001. [Google Scholar] [CrossRef] [Green Version]
- Lumbaque, E.C.; da Silva, B.A.; Böck, F.C.; Helfer, G.A.; Ferrão, M.F.; Sirtori, C. Total Dissolved Iron and Hydrogen Peroxide Determination Using the PhotoMetrixPRO Application: A Portable Colorimetric Analysis Tool for Controlling Important Conditions in the Solar Photo-Fenton Process. J. Hazard. Mater. 2019, 378, 120740. [Google Scholar] [CrossRef] [PubMed]
- Särkkä, H.; Vepsäläinen, M.; Sillanpää, M. Natural Organic Matter (NOM) Removal by Electrochemical Methods—A Review. J. Electroanal. Chem. 2015, 755, 100–108. [Google Scholar] [CrossRef]
- Taebi, A.; Droste, R.L. Pollution Loads in Urban Runoff and Sanitary Wastewater. Sci. Total Environ. 2004, 327, 175–184. [Google Scholar] [CrossRef]
- Zaporozec, A. Ground-Water Pollution and Its Sources. GeoJournal 1981, 5, 457–471. [Google Scholar] [CrossRef]

| Parameter | Standard Colorimetric Method | Miniaturized Method (PhotoMetrix PRO) |
|---|---|---|
| Range | 60–450 mg L−1 | |
| Slope | 0.0002 | 1.4035 |
| Intercept | 0.052 | 0.001 |
| Regression coefficient (r²) | 0.9932 | 0.9999 |
| LOD (mg L−1) | 9.42 | 2.28 |
| *LOQ (mg L−1) | 60 | |
| Sample | COD Results (mg L−1 O2) |
|---|---|
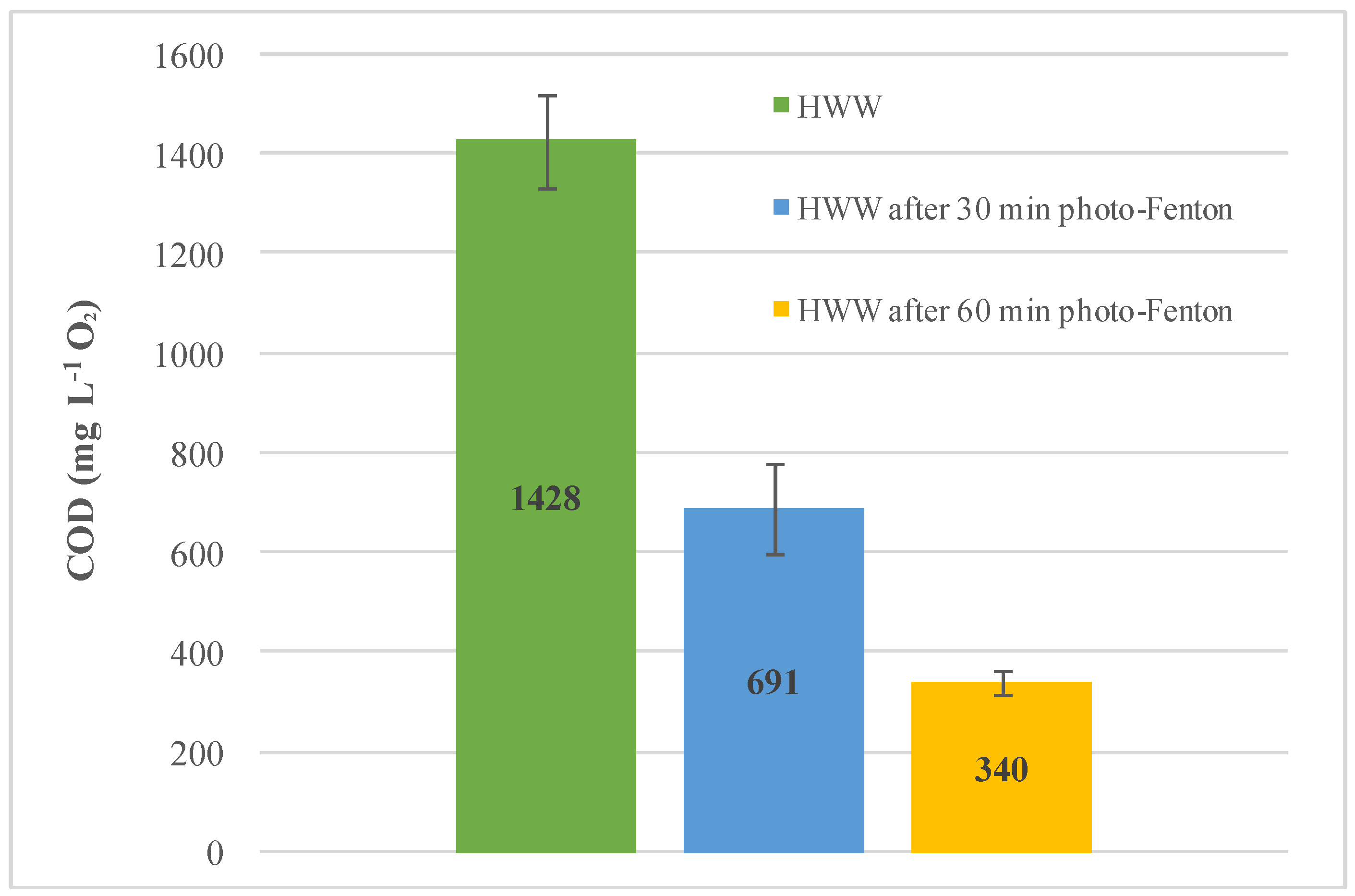
| HWW | 442 ± 11 |
| SWW | 399 ± 60 |
| GW | 243 ± 6 |
| SW | 616 ± 28 |
| Spiked TW | 166 ± 9 |
| Spiked UPW | 150 ± 8 |
| Cost per Sample Analysis ($US) | |||
|---|---|---|---|
| Item | Value (a) | Standard Colorimetric Method | Miniaturized Method |
| K2Cr2O7 | 982.00/kg | 0.0150 | 0.0050 |
| Ag2SO4 | 7360.00/kg | 0.2570 | 0.0859 |
| H2SO4 | 493.42/L | 1.85 | 0.6168 |
| HgSO4 | 694.98/kg | 0.0347 | 0.0116 |
| KHP | 330.69/kg | 0.0008 (b) | 0.0003 (b) |
| Total cost for 1 sample ($US) (c) | 15.11 | 5.05 | |
| Total cost for 10 samples ($US) (c) | 34.53 | 11.51 | |
| Criteria | Standard Colorimetric Method | Miniaturized Method |
|---|---|---|
| 1. Direct analytical techniques should be applied to avoid sample treatment. | Off-line analysis | |
| 2. Minimal sample size and minimal number of samples are goals. | 2.500 mL sample size | 0.833 mL sample size |
| 3. In situ measurements should be performed. | Analytical device is positioned off-line | |
| 4. Integration of analytical processes and operations saves energy and reduces the use of reagents. | 3 or fewer sample preparation steps | |
| 5. Automated and miniaturized methods should be selected. | Manual method—not miniaturized | Manual method—none or miniaturized |
| 6. Derivatization should be avoided. | Derivatization not needed | |
| 7. Generation of a large volume of analytical waste should be avoided and proper management of analytical waste should be provided. | 7.5 mL of waste per sample | 2.5 mL of wasteper sample |
| 8. Multianalyte or multiparameter methods are preferred to methods using one analyte at a time. | 1 parameter 12 samples per hour | 1 parameter 60 samples per hour |
| 9. The use of energy should be minimized (most energy-intensive technique). | UV–Vis spectrometry | Non-instrumental detection |
| 10. Reagents obtained from renewable sources should be preferred. | No reagents from bio-based sources | |
| 11. Toxic reagents should be eliminated or replaced. | 5.000 mL of toxic reagents used | 1.666 mL of toxic reagents used |
| 12. The safety of the operator should be increased. | Threats not avoided: toxic to aquatic life; bioacumulative; persistent; corrosive | |
| Method Description | Advantages | Disadvantages |
|---|---|---|
| Miniaturized PhotoMetrix PRO method | Reduced volumes of reagents, sample, and waste generated; more time- and cost-effective. | Use of dichromate and mercury. |
| Use of KMnO4 as an oxidizer [16] | Free of mercury and dichromate. | 55 mL of waste generated. |
| Use of H2O2 as an oxidizer [17] | Free of dichromate. | 75 mL of waste generated. Use of mercury. |
| Use of nano-Cu/GCE sensors [18] | Direct analysis. | Sensors not commercially available. |
| Ultrasound-assisted digestion [19] | Reductions of temperature and digestion time. | Sonication not sufficient to digest all of the organic matter. Use of dichromate and mercury. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
von Mühlen, L.; Prestes, O.D.; Ferrão, M.F.; Sirtori, C. Miniaturized Method for Chemical Oxygen Demand Determination Using the PhotoMetrix PRO Application. Molecules 2022, 27, 4721. https://doi.org/10.3390/molecules27154721
von Mühlen L, Prestes OD, Ferrão MF, Sirtori C. Miniaturized Method for Chemical Oxygen Demand Determination Using the PhotoMetrix PRO Application. Molecules. 2022; 27(15):4721. https://doi.org/10.3390/molecules27154721
Chicago/Turabian Stylevon Mühlen, Lisandro, Osmar D. Prestes, Marco F. Ferrão, and Carla Sirtori. 2022. "Miniaturized Method for Chemical Oxygen Demand Determination Using the PhotoMetrix PRO Application" Molecules 27, no. 15: 4721. https://doi.org/10.3390/molecules27154721