Abstract
Within the frame of this article, briefly but comprehensively, we present the existing knowledge, perspectives, and challenges for the utilization of Layered Double Hydroxides (LDHs) as adsorbents against a plethora of pollutants in aquatic matrixes. The use of LDHs as adsorbents was established by considering their significant physicochemical features, including their textural, structural, morphological, and chemical composition, as well as their method of synthesis, followed by their advantages and disadvantages as remediation media. The utilization of LDHs towards the adsorptive removal of dyes, metals, oxyanions, and emerging pollutants is critically reviewed, while all the reported kinds of interactions that gather the removal are collectively presented. Finally, future perspectives on the topic are discussed. It is expected that this discussion will encourage researchers in the area to seek new ideas for the design, development, and applications of novel LDHs-based nanomaterials as selective adsorbents, and hence to further explore the potential of their utilization also for analytic approaches to detect and monitor various pollutants.
1. General Background
Freshwater quality has been threatened over the few last decades due to rapid urbanization. To meet the enhanced demand for products, the expansion of industrial and agricultural activities is taking place at an accelerated pace, which ends up releasing a large number of contaminants into water bodies [1,2]. In turn, these activities can lead to water contamination with various types of chemical substances, which are constantly and well reported in the specialized literature; namely dyes, metals, refining substances, pharmaceuticals, fertilizers, personal care products, pathogenic bacteria, and more. The most common between of them are:
Dyes: Various colored substances are widely utilized as dyes by the textile, food, paper, and pharmaceutical industries. Among these sectors, the textile industry releases 10 to 15% of the dyes produced in the environment. These elements, even in low concentrations, can pose a threat to the environment and living beings. The presence of dyes in water resources reduces the penetration of light through the water, affecting photosynthesis and dissolved oxygen levels, and harming the aquatic biota. In addition, some dyes can degrade to produce highly toxic and carcinogenic compounds [3,4,5].
Metals: Effluents from industries such as battery manufacturing, electroplating, mining, smelting, and many other industrial activities consist of heavy metals mixtures (such as Cd, Pb, Hg, Cu, Ni, Cr, As). The presence of these metals in water bodies is harmful to human health, as they are bioaccumulative and toxic. Among the negative effects of these elements in living beings, even at a low concentration are diarrhea, stomach pain, headaches, chronic bronchitis, and lung cancer [3,6,7].
Pharmaceuticals: Drugs are widely used in human medicines, livestock, aquaculture, beekeeping, and poultry to aid species growth. Currently, the release of these elements into the environment is not always absolutely regulated/controlled; hence, it is difficult to monitor their effects on living beings. The discharge of drugs into water resources occurs through the excretion of compounds not metabolized by living beings and industrial and hospital effluents. The continued presence of these pharmaceutical pollutants can cause oxidative stress and negative effects on reproduction (sperm motility and abnormal fetal development), osmoregulation, and altered immune functions in aquatic biotics [8,9].
Emerging pollutants: In general, emerging pollutants are chemical or microbiological products that are not commonly monitored or regulated and can cause problems to the environment as well as to the health of living beings. Except for the abovementioned, substances that can end up acting as pollutants are usually found in personal care products, pharmaceuticals, industrial additives, pesticides, plasticizers, and solvents. Among these substances, exposure to emerging organic compounds such as phenol, benzene, toluene, and xylenes can cause serious health problems such as gastrointestinal disorders, lung and kidney damage, heart attacks, and cancer. Yet, the increased use of pesticides and personal care products has increased dramatically in recent years. Although these products appear in low concentrations in water bodies, they can negatively influence the environment due to their resistance to biodegradation and accumulation in tissues [3,10].
Among the several technologies applied for water treatment, adsorption using layered double hydroxides (LDHs) will be highlighted in this article. Adsorption is a unitary operation in which the separation process occurs due to contact between a fluid phase—in this case, liquid—containing one or more contaminants (adsorbate) to be adsorbed/removed, and a solid (adsorbent). Due to the imbalance of forces (attraction or repulsion) for adsorption, the contaminant is attracted to the solid surface by physical or chemical interactions. This mass transfer process occurs until the balance between the adsorbed contaminant and what remains in the liquid phase, the residual, is reached. This is termed equilibrium. Adsorption is recognized as an effective process for water treatment due to certain characteristics, such as high efficiency, feasibility, low cost, flexibility, simplicity, wide processing range, cost-effective applications for water treatment, easy operation and implementation, great availability, and the possibility of adsorbents’ regeneration. A very important aspect is that the adsorption-based processes do not result in the formation of hazardous substances/by-products [1,3,11,12,13].
Around 70% of the adsorption operation cost is related to the adsorbents [14]. Therefore, scientists involved in this area play a fundamental role in the development, characterization, and optimization of novel adsorbents. Hence, we seek materials with low cost and ease of production, high efficiency and selectivity towards the removal of the targeted contaminant, large surface area and volume of accessible pores, and good mechanical resistance. It is also desirable for the adsorbents to be regenerated/reused for numerous sufficient cycles to justify the costs.
Currently, and within this context, layered double hydroxides (LDHs), also known as anionic clays, have drawn great attention in their application as adsorbents. Several researchers have proposed the use of LDHs as efficient adsorbents against a plethora of organic and inorganic contaminants [4,15,16,17,18]. Furthermore, recent research demonstrates that biochar/LDH co-blends are highly promising, sustainable, and eco-economic materials for water treatment. Conventional adsorbents such as activated carbon have specific limitations such as high cost, low reuse performance, and low selectivity for water treatment. On the other hand, biochar/LDH blends can have a low cost, high surface area, an elevated amount of active adsorption sites, inherent interchangeability, a considerable increase in anions, and less toxicity for the removal of organic substances [19,20,21,22,23]. A wide variety of works have focused on evaluating the adsorptive capacity of LDHs against various pollutants such as dyes, drugs, arsenic, rare earth, radioactive substances, phosphate, metals, among other pollutants. Table 1 collects the most important advantages and disadvantages of using LDHs for water treatment compared to other materials [19,20,21,22,23,24,25,26].

Table 1.
Advantages and disadvantages of using LDH in water treatment.
Considering the enhanced surface chemical heterogeneity and the nanostructured nature of LDHs, their elevated remediation efficiency against different substances is linked in the literature to plenty of physical and/or chemical interactions, with the most reported ones collected in Figure 1 [15,27]. Among all the interactions/mechanisms involved in the removal of various pollutants by Layered Double Hydroxides (LDHs), the most predominantly reported are:
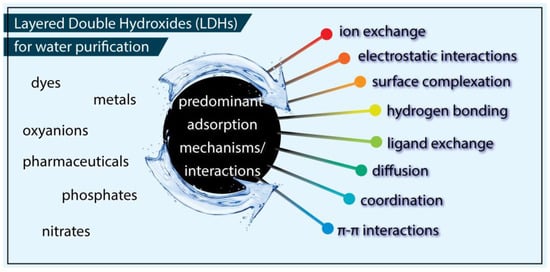
Figure 1.
All the reported interactions/mechanisms involved in the removal of various pollutants by Layered Double Hydroxides (LDHs).
- (1)
- Physical adsorption. LDHs can have a high specific surface area, and hence present high adsorption capacities due to the presence and availability of active adsorption sites. Furthermore, the specific surface area of LDHs can be increased through calcinating or modifying/depositing on supports with three-dimensional structures.
- (2)
- Ion exchange. Strongly negative molecules can be easily changed for the original anions in LDHs. In addition, positive ions can also be exchanged in the intermediate layer of LDHs, if pre-interleaved by some chelators.
- (3)
- Interleaving. This starts from a preparation process, such as co-precipitation. Furthermore, the capture of molecules via the intercalation process is faster and more complete than ion exchange.
2. LDHs Physicochemical Characteristics
For the design and utilization of a proper adsorbent, certain characteristics are essential, such as low production cost, thermal, mechanical, and chemical stabilities, desirable physicochemical characteristics (such as elevated textural properties and high surface functional groups availability), high efficiency and adsorption capacity, rapid kinetics, and regeneration/reuse potential [28]. Some of the mentioned properties can only be verified when applying the material in a specific process due to the dependence on operational conditions and adsorbate characteristics. Of course, it is very challenging to develop materials that possess all the abovementioned characteristics. Thus, scientists involved in the water purification area of research have developed and tested a broad range of materials. Among them, layered double hydroxides (LDHs) are prominent and meet many demands, considering the sustainability of the approach, high anion exchange capacity, high specific surface area, high ion exchange capacities, and regenerative adsorptivity [28]. In addition to their attractive properties as adsorbents, LDHs have applications in various fields such as drug carriers, catalysis, flame retardants, pharmaceutical transport systems, electrocatalytic water separation, additives for polymers, photocatalytic degradation, and medicine [6,18,29].
LDHs are two-dimensional (2D) nanostructures composed of stacked layers consisting of mixed hydroxides of di- and trivalent cations with hydrated anions in the spaces between the positively charged lamellae (Figure 2). LDHs are also called hydrotalcite compounds, due to the rigid layer structure derived from brucite with edge-sharing M(OH)6, similar to flexible graphene oxide nanosheets [30]. In general, such materials are represented by the formula: [M2+1−xM3+x(OH)2]x+. Am−x/m.nH2O [30], where M2+ represents a divalent metal cation; M3+, the trivalent cation; Am−, an anion intercalated with charge m; X, the ratio between divalent (M2+) and trivalent cations (M3+), for which the value of the molar ratio can be between 2.0 and 4.0; and n, the number of soft water molecules. Many divalent (such as Mg2+, Fe2+, Ca2+, Co2+, Cu2+, Ni2+, and Zn2+) and trivalent (such as Al3+, Cr3+, Ga3+, Mn3+, and Fe3+) metal cations can be used for the preparation of LDHs. Except for bivalent and trivalent metal ions, a wide range of monovalent and tetravalent metal ions (such as Li+, Ti4+, Sn4+, or Zr4+) may also be inserted in the octahedral sites [31]. In addition, the electroneutrality of LDHs can be due to the presence of hydrated organic or inorganic anions (, , , OH−, Cl−, Br−) in the interlamellar space [18]. Because of these and the different combinations between the cations that participate in the formation of the layers, LDHs can have numerous structural varieties. However, this combination must consider the octahedral coordination and the ionic radius (preferably between 0.50 and 0.74 Å), as distortions may occur with the use of cations. In addition, it is necessary to consider the relationship between the size and charge of the interlayer anion so that it is possible to balance the positive charges of the layer homogeneously [32].
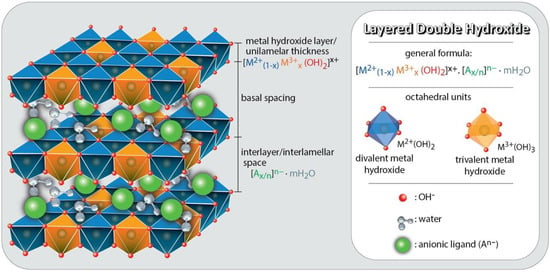
Figure 2.
A schematic presentation of the layered double hydroxides (LDHs) chemical composition and structure. Figure reproduced from reference [30]. Copyrights Elsevier, 2022.
For LDHs, the precisely controlled chemical composition of the lamellar layers and the interlayer composition provide a unique supramolecular nanometric structure with the ability to disperse active sites on an atomic scale, in addition to facilitating morphological manipulation [33,34]. Thus, the versatility of the chemical composition and nanostructure of LDHs establish them as promising adsorbents against a wide variety of pollutants. The most attractive properties of LDHs are the chemical compositions according to the metals used, the space between layers, and the high surface capable of adsorbing bioactive substances, which are dominated by the synthesis conditions [32]. All the above can be tuned on demand based on the synthetic approach/protocol. Another important feature of LDHs is the ease, low cost, and variety of available synthesis methods, which can be classified as direct and indirect methods:
- (i)
- Direct methods: The preparation of LDHs occurs via direct precipitation from the addition of tri- and divalent cations, in a solution in alkaline pH with the main methods of coprecipitation, salt–oxide, sol–gel, induced hydrolysis, and hydrothermal synthesis.
- (ii)
- Indirect methods: involve replacing an interlamellar anion from a previously produced precursor LDH. Examples of this substitution method are ion exchange in solution, ion exchange in acidic medium, double phase replacement, and regeneration through the delaminate precursor [35,36,37]. Therefore, the supramolecular structure, the facile manipulation of adsorption sites at the atomic scale, the versatility of compositions, in addition to the possibility of morphological manipulation create the possibility of tuning the amount and accessibility of the active adsorption sites, and hence the adsorption kinetics, as well as the efficiency for a specifically targeted pollutant [34]. As in any case, LDHs have some specific characteristics that can complicate their use as adsorbents. The low mechanical resistance is a problem for continuous water treatment units and in certain regeneration processes, as LDHs can be exfoliated. Therefore, there is a series of studies in the literature proposing to support LDHs in larger and recalcitrant particles [38,39,40]. In addition, in acidic media, the removal capacity of LDHs is compromised due to low structural stability at low pH [26]. In Table 2, we collected characteristics of methods of synthesis which can be followed for the preparation of pure LDHs, as well as their composites and hybrids [18,25,41,42].
 Table 2. Provides details of some of the methods used for the synthesis of LDH.
Table 2. Provides details of some of the methods used for the synthesis of LDH.
3. LDHs as Adsorbents
As already mentioned, LDHs are very promising materials for the removal of a wide variety of pollutants via adsorption. There are many reports in the literature; consequently, we will address some of the most important ones that gather the highest level of research attention for specific pollutants, with emphasis on the utilized LDHs.
Dyes: The presence of dyes in water and wastewater is highly undesirable, and LDHs have been reported as efficient remediation media of a large number of industrial dyes [4]. Dyes are mainly categorized into anionic and cationic dyes. LDHs can remove anionic dyes in the same order of magnitude as cationic dyes in activated carbon [43,44]. Examples of anionic dyes include methyl orange, amaranth, sunset yellow FCF, reactive blue 21, Eriochrome Black T, Congo red, and others. In the literature, it is common to find reports of high adsorption capacities regarding the removal of cationic dyes by LDHs [4,18,45].
El-Abboubi et al. [45] evaluated the double hydroxides of Mg-Al synthesized with dodecyl sulfate (Mg-Al-Ds) and carbonate (Mg-Al-CO3), through the coprecipitation method to remove methyl orange from aqueous solution. The prepared LDHs showed the intercalary-like structure with (00l) reflections and a nanoscale nature, as well as the presence of the desired anions in the interlayer space. The maximum capacities were 185.06 mg·g−1 for Mg-Al-Ds and 97.5 mg·g−1 for Mg-Al-CO3. They also observed the influence of the pH of the solution on the adsorption capacity of the dye in the case of Mg-Al-CO3. The adsorptive capacity of Mg-Al-Ds was not influenced by the pH of the solution, while Mg-Al-CO3 showed greater efficiency at pH in the range of 3–7. According to the authors, this behavior may be associated with two mechanisms: (1) anion exchange of carbonate anions for dye anions; (2) association of the positively charged surface groups of LDHs and dye anions.
Kostić et al. [18] synthesized MgCoAl-CO3-LDH double hydroxides using the coprecipitation method for the removal of dye RB19 from an aqueous solution. The material presented a surface area of around 48 m2·g−1, high crystallinity and the presence of carbonate, hydroxyl, and metal ions groups within its structure. The proposed adsorption mechanisms involved in the process were electrostatic attraction, physisorption, and chemical bonding. The maximum capacity was found to be equal to 367.93 mg·g−1.
Ahmed et al. [4] prepared Mg/Fe-LDHs nanoparticles through the precipitation method for adsorption of Congo red dye from effluents. The Mg/Fe-LDHs showed high crystallinity and the presence of hydroxyl functional groups. The adsorption process was governed both by physical and chemical interactions, with the maximum capacity reaching above 9000 mg per gram.
Metals: The discharge of heavy metals into water bodies results in harmful effects to the environment and human health due to their toxicity and persistence. The presence of these elements in water systems has caused concern in recent years; hence, the interest in novel and efficient remediation media is still elevated [9,16]. In the literature, it is quoted that the work of Satoshi Fujji et al. [46], from 1992, was the first in which LDHs were used for metal removal (Pb2+, Cu2+ and Zn2+) [47]. There is no established mechanism for sorption/adsorption of metals in each LDH; hence, the study of the involved mechanism of interaction is still a major challenge for researchers in the field. However, several possible mechanisms/kinds of interactions are proposed, such as surface complexation, isomorphic substitution, surface precipitation, and electrostatic interactions, and chelation with the binding anion has been reported [16,29,48].
Dinari and Neamati [48] synthesized Ca/Fe double hydroxides (LDHs) modified with 3-aminopropyl triethoxysilane as the silane coupling agent. Polyaniline nanocomposites with 5 and 10% by weight of modified silane Ca/Fe LDH-Cit were also studied. Pure polyaniline and that modified with NCs were used as adsorbent to remove Pb2+ ions from an aqueous solution. The results showed that NC10% showed higher adsorptive capacity (110 mg·g−1; 0.52 mmol·g−1) compared to NC5% (56 mg·g−1; 0.27 mmol·g−1) and NIBP (47 mg·g−1; 0.22 mmol·g−1), demonstrating the favoring of adsorption in the presence of Ca/Fe double hydroxides.
Guo et al. [29] synthesized ZnNiCr double-layer hydroxides (ZnNiCr-LDHs) using the microwave hydrothermal method to remove Cr6+ from an aqueous solution. The material presented a surface area of 354 m2·g−1, and the SEM analysis showed an irregular block structure. In this study, it was proposed that predominantly electrostatic interaction between the metal ion and the adsorbent took place with a maximum capacity of 28.2 mg·g−1 (0.54 mmol·g−1). The results demonstrate potential application prospects in the removal of Cr6+ in wastewater.
Zhang et al. [16] synthesized sodium alginate intercalated with MgAl-LDH (SA-LDH) for adsorption of Cd2+, Pb2+, and Cu2+ from an aqueous solution. The characterization results showed characteristic peaks at 2θ = 13.24°, 22.88°, 35.09°, 39.19°, 47.23°, and 60.97°, while CO, COO− and CH surface functional groups were also detected in its structure. The maximum adsorption capacities of SA-LDH were 60 mg·g−1 (0.945 mmol·g−1) for Cu2+, 243.66 mg·g−1 (1.176 mmol·g−1) for Pb2+, and 95.55 mg·g−1 (0.850 mmol·g−1) for Cd2+. The results showed that the possible mechanisms involved in the adsorption process are: (1) bonding or complexation with SurOH or Sur-O- of SA-LDH; (2) precipitation of metal hydroxides or carbonates; (3) isomorphic substitution, and (4) chelation with COO− in the interlayers.
Oxyanions: High levels of oxyanions found in the environment are reported all over the world. Oxyanions (e.g., arsenate, chromate, phosphate, selenite, selenate, borate, nitrate, etc.) are considered dangerous to humans and wildlife, even at very low concentrations [17,49]. In the case of oxyanions, the primarily reported mechanisms are ion exchange, electrostatic attraction, and coordination [17,50].
Motandi et al. [51] synthesized zirconium-modified Mg-Al-LDH layered double hydroxides (Zr-LDH) using the coprecipitation method, and then used calcination to obtain an oxide (Zr-LDO), and the materials were used as adsorbents for the removal of phosphate from aqueous solution. The materials showed a well-developed layered structure. In addition, Zr was homogeneously distributed in the adsorbent with a well-defined crystallinity and the presence of brucite, while hydroxyl groups and M–O elongation were detected. The results showed that Zr-LDH and Zr-LDO are excellent adsorbents for phosphate, obtaining maximum adsorption capacities of 99.35 mg/g for Zr-LDH and 80.33 mg·g−1 for Zr-LDO.
Jung et al. [17] synthesized Mg-Al double hydroxides (Mg-Al LDHs-FHC) via the one-pot in situ hydrothermal method for the monocomponent and multicomponent adsorption of arsenate and phosphate from aqueous solution. Initially, the influence of the Mg:Al ratio and temperature on the preparation of the adsorbent material was investigated. The results showed that Mg:Al molar ratios and temperature influenced the Mg-Al LDHs-FHC structural properties. The best adsorption efficiency was for Mg:Al molar ratio of 2:1 and temperature of 150 °C. In the one-component system, the maximum adsorption capacities were 56.30 mg·g−1 and 33.21 mg·g−1 for arsenate and phosphate, respectively. For the multicomponent system, the maximum adsorption capacities were 16.22 mg·g−1 for arsenate and 20.26 mg·g−1 for phosphate. In the single-component system, a possible adsorption mechanism is the ion exchange between the nitrate interlayer and the arsenate or phosphate groupings. In the multicomponent system, coordinated bonds are also possibly responsible for the competition of arsenate or phosphate in the adsorptive process.
Zhou et al. [50] prepared FeMgMn-LDH via the co-precipitation method for the removal of nitrate from an aqueous solution. The surface area of the material was 47 m2·g−1; the presence of –OH, N=O, M–OH and M–O–M was detected in its structure. The maximum amount of nitrate adsorption was 10.56 mg·g−1. The adsorptive process was spontaneous and exothermic. The possible proposed adsorption mechanisms of nitrate removal using FeMgMn-LDH were an electrostatic attraction and ion exchange.
Emerging pollutants: Plenty of synthetic substances that have recently been detected at low concentrations (ng·L−1 or µL−1) are assumed to be emerging pollutants nowadays. These components are used in different industrial processes for the production of pharmaceuticals, personal care products (PCPs), beverages, foods, and others [11,12,15,52], and as a result are discarded in aquatic environments. Although LDHs have been reported as efficient adsorbents for various emerging pollutants, such as sodium diclofenac, caffeine, inorganic endocrine disruptor, hormones, and bisphenol A, among others, there are still few studies in the literature.
Santamaría et al. [53] synthesized by the co-precipitation method Zinc–Titanium–Aluminum (ZnTiAl) double layer hydroxides (LDHs) with a Zn/(Al-Ti) molar ratio of 3:1 and studied them for the adsorption of diclofenac and salicylic acid. The study also evaluated the use of commercial aluminum (Al) and aluminum extracted from saline slag. It was shown that the increase in Ti content negatively affected the crystallinity of the material and that the increase in temperature decreased the surface area of the material due to the increase in amorphous mixed oxides. The results showed a great potential of the synthesized hydrotalcites for the adsorption of diclofenac (409 μmol/g) compared to salicylic acid (80 μmol·g−1)
Kumari et al. [11] used a double layer of Zn-Al hydroxide (LDH) loaded with Bi2O3 as an adsorbent for the removal of diclofenac from an aqueous solution. The material presented the main diffraction peaks 003, 006, 012, 041, 111, 241, and 110, and a surface area of 102 m2·g−1. The Zn-Al-LDH.xBi2O3 presented a capacity of 574.71 mg·g−1 for the removal of diclofenac. The results indicated that the adsorptive process occurs in a monolayer and that the diffusion of diclofenac occurs mainly on the external surface.
Other kinds of pollutants: There are few reports of the use of LDHs for the adsorptive removal of bacteria and viruses, per- and poly-fluoroalkyl substances, and rare earth and radioactive substances; such as Cs, Sr, and Th (excluding Uranium, which is well reported). Therefore, exploring the use of LDHs in the removal of this type of contaminant is very promising and important for environmental health protection.
Table 3 summarizes some cited articles in which LDH were studied as adsorbents against eclectic organic and inorganic substances from aqueous solutions.

Table 3.
Some characteristic works in which LDHs are used as adsorbents for organic and inorganic substances.
Utilization in real-life applications: Industrial effluents contain various pollutants simultaneously, such as dyes, metals, pesticides, and antibiotics, which impose a high physicochemical complexity on the real system. Therefore, it is necessary to understand the interaction between the adsorbates and the adsorbent in the water decontamination process [1,3]. Despite the importance of multicomponent adsorption, there are few references in the literature evaluating the use of LDHs to remove dyes, metals, and rare earth elements in multicomponent systems. Consequently, it is necessary to study the multicomponent adsorption, as well as the evaluation of the selectivity or affinity of a given LDH for each adsorbate and the competition between them. Another developing field is for LDH containing hybrids of two types—nanocomposites and organically modified LDH hybrids—which have been recently reported [54] for the removal of metal ions and dyes from wastewater, with particularly high capacities.
Considering the adsorption mode, batch experiments are well reported in the literature. On the contrary, although the design and efficiency are well approached in lab-scale batch studies, the fixed beds procedure has received little attention regarding exploration of the use of LDHs as adsorbents. The fixed bed columns are applied for continuous flow, and are effective for the treatment of large volumes of effluents, allowing application in industries, adsorption–desorption cycles, efficient fluid–particle contact and easy phase separation, which makes the treatment process cheaper and more sustainable [28,55,56]. In this context, the main challenge is to overcome the LDHs’ poor mechanical resistance for continuous use, because they can be sprayed or exfoliated [40]. In addition, leaching tests should be always considered when studying LDHs in aquatic matrixes.
Fixed beds are employed primarily for commercial water treatment scale-up. Discovering and proposing new operational modes to improve fluid–particle interaction and phase separation is a key issue. Therefore, a smaller treatment area, faster operation, larger water volumes treated, and cost reduction are important advantages. As a result, there may be benefits such as a smaller treatment area, faster operation, treatment of bigger quantities of water, and cheaper costs. Therein, the study of alternative contacting devices, such as fluidized bed, spouted bed, simulated moving bed, expanded bed, continuous stirred tanks, and multi-batch tanks, is much appreciated and warrants substantially further investigation [28].
4. Discussion
The studies presented and discussed above demonstrate the prosperity of utilizing LDHs as remediation media, and hence, the growing interest of researchers against various pollutants regarding interpretation and proposals for the involved mechanisms and interactions. The studies revealed elevated adsorptive capacities of organic and inorganic pollutants, even compared to benchmark materials such as porous carbons [4,16,45,48]. The high adsorptive capacity of LDHs is attributed to their high specific surface area, high thermal and chemical stability, but, more importantly, to their surface chemical composition. The characterization of LDHs via various analyses/techniques such as the pH of the point of zero charge (pHPZC), Fourier transform infrared spectroscopy (FT-IR), and X-ray diffraction (XRD) helps us to understand the adsorptive mechanism of the process. The most widely proposed interactions/mechanisms involved in the adsorption of organic compounds generally involve electrostatic interaction between the surface of the LDH (positively or negatively charged) with the cationic (positively charged) or anionic (negatively charged) compounds. The adsorptive mechanism associated with the adsorption of metal ions involves the ion exchange between the surface of the material and the metal ions. Other mechanisms are also involved in the adsorption of metal ions in LDHs, such as precipitation and complexation. Among the functional groups present in the structure of LDHs, the hydroxyl group favors the adsorption of both organic and inorganic compounds. Another important parameter in the adsorption process using LDHs is the pH of the solution. It is observed that, in general, the favorable pH for the adsorption of anionic compounds occurs at pH lower than 7 and for cationic compounds at pH above 7.
5. Future Prospects
Layered double hydroxides (LDHs) represent one category of materials with an increasing trend of interest toward the removal of water pollutants. To uplift the direction of LDHs-based adsorbent application on a commercial scale, novel approaches and considerations with emphasis on sustainability and low cost are presented. However, more adsorption investigations of LDHs need to be explored in multi-component systems rather than in the current trend, where most of the studies are limited to mono-component systems and lab-scale batch experiments. The parametric influence on the adsorption process requires an advanced optimization approach to attain maximum removal performance of water pollutants using LDHs-based adsorbents. The major setback of a “one time” use of the adsorbent must be overcome with the proper assessment of desorption strategies, followed by a good number of adsorbent recycle options, treated water re-use, and the adsorbate pollutant recovery and re-use. More mechanistic insights into water pollutant removal need to be understood in multicomponent simulated and real effluent systems to study the antagonistic effects targeting the development of adsorbents with high selectivity for the specific pollutant(s). To understand the complete application of layered double hydroxides in water pollutant elimination, the batch system followed by a continuous system needs to be performed at lab and pilot scale, with real effluents.
The simplicity and low cost of LDHs production (for instance, via straightforward coprecipitation of metal salts at mild basic conditions), in addition to the ability to tailor design their composition and structure, establish them as sustainable and attractive candidates for real-life applications. Moving a step forward regarding the advantages of nanomaterials, designing and synthesizing novel nanocomposites/hybrids should be assumed as a worthwhile effort to be further explored in the field of research. The development of synthesis methods capable of producing LDHs on a commercial scale is one of the current problems for the use of these materials on an industrial scale. In addition, LDHs syntheses are generally limited to the use of MgAl and MgFe, requiring the exploration of new compounds. Within the frame of circular (bio)economy and sustainability, the development of novel and advanced composited of LDHs with biomass-derived biochar/carbon and determining the optimum ratio between the counterparts is a prosperous topic of research, since still only a few studies exist.
Author Contributions
All authors contributed to the study conception and design. Conceptualization, B.M.V.d.G., D.A.G. and L.M.; writing—original draft preparation, B.M.V.d.G. and L.M.; writing—review and editing, B.M.V.d.G., R.S., D.A.G., K.S.T. and G.M. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank to National Council for Scientific and Technological Development (CNPq/Brazil), Coordination for the Improvement of Higher Education Personnel (CAPES/Brazil) and Foundation for Research Support of the State of Alagoas (FAPEAL/Brazil).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors have no relevant financial or non-financial interest to disclose.
References
- Nimbalkar, M.N.; Bhat, B.R. Simultaneous adsorption of methylene blue and heavy metals from water using Zr-MOF having free carboxylic group. J. Environ. Chem. Eng. 2021, 9, 106216. [Google Scholar] [CrossRef]
- Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Advances in decontamination of wastewater using biomass-basedcomposites: A critical review. Sci. Total Environ. 2021, 784, 147108. [Google Scholar] [CrossRef]
- Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Simultaneous adsorptive removal of conventional and emerging contaminants in multi-component systems for wastewater remediation: A critical review. Sci. Total Environ. 2021, 799, 149500. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.N.; Naji, L.A.; Faisal, A.; Al-Ansari, N.; Naushad, M. Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution. Sci. Rep. 2020, 10, 2042. [Google Scholar] [CrossRef]
- Solehudin, M.; Sirimahachai, U.; Ali, G.A.; Chong, K.F.; Wongnawa, S. One-pot synthesis of isotype heterojunction g-C3N4-MU photocatalyst for effective tetracycline hydrochloride antibiotic and reactive orange 16 dye removal. Adv. Powder Technol. 2020, 31, 1891–1902. [Google Scholar] [CrossRef]
- Mureseanu, M.; Eliescu, A.; Ignat, E.-C.; Carja, G.; Cioatera, N. Different routes of MgAl–LDH synthesis for tailoring the adsorption of Pb(II) pollutant from water. Comptes Rendus. Chim. 2022, 25, 1–12. [Google Scholar] [CrossRef]
- Gayathri, R.; Gopinath, K.; Kumar, P.S. Adsorptive separation of toxic metals from aquatic environment using agro waste biochar: Application in electroplating industrial wastewater. Chemosphere 2021, 262, 128031. [Google Scholar] [CrossRef] [PubMed]
- Nava-Andrade, K.; Carbajal-Arízaga, G.; Obregón, S.; Rodríguez-González, V. Layered double hydroxides and related hybrid materials for removal of pharmaceutical pollutants from water. J. Environ. Manag. 2021, 288, 112399. [Google Scholar] [CrossRef]
- Manjunath, S.; Baghel, R.S.; Kumar, M. Antagonistic and synergistic analysis of antibiotic adsorption on Prosopis juliflora activated carbon in multicomponent systems. Chem. Eng. J. 2020, 381, 122713. [Google Scholar] [CrossRef]
- Somma, S.; Reverchon, E.; Baldino, L. Water Purification of Classical and Emerging Organic Pollutants: An Extensive Review. ChemEngineering 2021, 5, 47. [Google Scholar] [CrossRef]
- Kumari, P.; Pal, B.; Das, R.K. Superior adsorptive removal of eco-toxic drug diclofenac sodium by Zn–Al LDH·xBi2O3 layer double hydroxide composites. Appl. Clay Sci. 2021, 208, 106119. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef]
- Quintela, D.U.; Henrique, D.C.; dos Santos Lins, P.V.; Ide, A.H.; Erto, A.; da Silva Duarte, J.L.; Meili, L. Waste of Mytella Falcata shells for removal of a triarylmethane biocide from water: Kinetic, equilibrium, regeneration and thermodynamic studies. Colloids Surf. B Biointerfaces 2020, 195, 111230. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Mu, B.; Yang, Y. Feasibility of industrial-scale treatment of dye wastewater via bio-adsorption technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.E.D.S.; dos Santos Lins, P.V.; de Magalhães Oliveira, L.M.T.; da Silva, E.O.; Anastopoulos, I.; Erto, A.; Giannakoudakis, D.A.; de Almeida, A.R.F.; da Silva Duarte, J.L.; Meili, L. Layered double hydroxides/biochar composites as adsorbents for water remediation applications: Recent trends and perspectives. J. Clean. Prod. 2021, 284, 124755. [Google Scholar] [CrossRef]
- Zhang, X.; Shan, R.; Li, X.; Yan, L.; Ma, Z.; Jia, R.; Sun, S. Effective removal of Cu(II), Pb(II) and Cd(II) by sodium alginate intercalated MgAl-layered double hydroxide: Adsorption properties and mechanistic studies. Water Sci. Technol. 2021, 83, 975–984. [Google Scholar] [CrossRef]
- Jung, K.-W.; Lee, S.Y.; Choi, J.-W.; Hwang, M.-J.; Shim, W.G. Synthesis of Mg–Al layered double hydroxides-functionalized hydrochar composite via an in situ one-pot hydrothermal method for arsenate and phosphate removal: Structural characterization and adsorption performance. Chem. Eng. J. 2021, 420, 129775. [Google Scholar] [CrossRef]
- Kostić, M.; Najdanović, S.; Velinov, N.; Vučić, M.R.; Petrović, M.; Mitrović, J.; Bojić, A. Ultrasound-assisted synthesis of a new material based on MgCoAl-LDH: Characterization and optimization of sorption for progressive treatment of water. Environ. Technol. Innov. 2022, 26, 102358. [Google Scholar] [CrossRef]
- Zubair, M.; Ihsanullah, I.; Aziz, H.A.; Ahmad, M.A.; Al-Harthi, M.A. Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook. Bioresour. Technol. 2021, 319, 124128. [Google Scholar] [CrossRef]
- Zubair, M.; Manzar, M.S.; Mu’Azu, N.D.; Anil, I.; Blaisi, N.I.; Al-Harthi, M.A. Functionalized MgAl-layered hydroxide intercalated date-palm biochar for Enhanced Uptake of Cationic dye: Kinetics, isotherm and thermodynamic studies. Appl. Clay Sci. 2020, 190, 105587. [Google Scholar] [CrossRef]
- Mu’Azu, N.D.; Zubair, M.; Jarrah, N.; Alagha, O.; Al-Harthi, M.A.; Essa, M.H. Sewage Sludge ZnCl2-Activated Carbon Intercalated MgFe–LDH Nanocomposites: Insight of the Sorption Mechanism of Improved Removal of Phenol from Water. Int. J. Mol. Sci. 2020, 21, 1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palapa, N.R.; Taher, T.; Rahayu, B.R.; Mohadi, R.; Rachmat, A.; Lesbani, A. CuAl LDH/Rice Husk Biochar Composite for Enhanced Adsorptive Removal of Cationic Dye from Aqueous Solution. Bull. Chem. React. Eng. Catal. 2020, 15, 525–537. [Google Scholar] [CrossRef]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’Azu, N.D.; Qureshi, A. Comparative Adsorptive Removal of Phosphate and Nitrate from Wastewater Using Biochar-MgAl LDH Nanocomposites: Coexisting Anions Effect and Mechanistic Studies. Nanomaterials 2020, 10, 336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Ehite, E.; Houston, R.; Li, Y.; Pope, C.; Labbé, N.; Abdoulmoumine, N. Synthesis and evaluation of layered double hydroxide based sorbent for hot gas cleanup of hydrogen chloride. Mater. Sci. Energy Technol. 2021, 4, 46–53. [Google Scholar] [CrossRef]
- Daniel, S.; Thomas, S. Layered Double Hydroxides: Fundamentals to Applications; Woodhead Publishing: Sawston, UK, 2020; pp. 1–76. [Google Scholar] [CrossRef]
- Santos, L.C.; da Silva, A.F.; dos Santos Lins, P.V.; da Silva Duarte, J.L.; Ide, A.H.; Meili, L. Mg-Fe layered double hydroxide with chloride intercalated: Synthesis, characterization and application for efficient nitrate removal. Environ. Sci. Pollut. Res. 2020, 27, 5890–5900. [Google Scholar] [CrossRef]
- Tang, S.; Yao, Y.; Chen, T.; Kong, D.; Shen, W.; Lee, H.K. Recent advances in the application of layered double hydroxides in analytical chemistry: A review. Anal. Chim. Acta 2020, 1103, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.L.; McKay, G. Current scenario and challenges in adsorption for water treatment. J. Environ. Chem. Eng. 2020, 8, 103988. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Y.; Zheng, J.; Shang, L.; Shi, Y.; Wu, Q.; Liu, X.; Wang, Y.; Shi, L.; Shao, Q. Synthesis and characterization of ZnNiCr-layered double hydroxides with high adsorption activities for Cr(VI). Adv. Compos. Hybrid Mater. 2021, 4, 819–829. [Google Scholar] [CrossRef]
- Gabriel, R.; de Carvalho, S.H.; da Silva Duarte, J.L.; Oliveira, L.M.; Giannakoudakis, D.A.; Triantafyllidis, K.S.; Soletti, J.I.; Meili, L. Mixed metal oxides derived from layered double hydroxide as catalysts for biodiesel production. Appl. Catal. A Gen. 2021, 630, 118470. [Google Scholar] [CrossRef]
- Shao, M.; Wei, M.; Evans, D.G.; Duan, X. Layered Double Hydroxide Materials in Photocatalysis. Photofunct. Layer. Mater. 2015, 166, 105–136. [Google Scholar] [CrossRef]
- de Sousa, A.L.M.D.; dos Santos, W.M.; de Souza, M.L.; Silva, L.C.P.B.B.; Yun, A.E.H.K.; Aguilera, C.S.B.; de França Chagas, B.; Rolim, L.A.; da Silva, R.M.F.; Neto, P.J.R. Layered Double Hydroxides as Promising Excipients for Drug Delivery Purposes. Eur. J. Pharm. Sci. 2021, 165, 105922. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, F.; Evans, D.G.; Duan, X. Layered double hydroxide films: Synthesis, properties and applications. Chem. Eng. J. 2014, 46, 5197–5210. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef] [PubMed]
- Rives, V.; Del Arco, M.; Martin, C. Intercalation of drugs in layered double hydroxides and their controlled release: A review. Appl. Clay Sci. 2014, 88–89, 239–269. [Google Scholar] [CrossRef]
- Conterosito, E.; Gianotti, V.; Palin, L.; Boccaleri, E.; Viterbo, D.; Milanesio, M. Facile preparation methods of hydrotalcite layered materials and their structural characterization by combined techniques. Inorganica Chim. Acta 2018, 470, 36–50. [Google Scholar] [CrossRef]
- Lins, P.V.S.; Henrique, D.C.; Ide, A.H.; da Silva Duarte, J.L.; Dotto, G.L.; Yazidi, A.; Sellaoui, L.; Erto, A.; e Silva Zanta, C.L.D.P.; Meili, L. Adsorption of a non-steroidal anti-inflammatory drug onto MgAl/LDH-activated carbon composite–Experimental investigation and statistical physics modeling. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124217. [Google Scholar] [CrossRef]
- Wan, S.; Wang, S.; Li, Y.; Gao, B. Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Ind. Eng. Chem. 2017, 47, 246–253. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.; Zanta, C.L.P.S.; Soletti, J.I.; Ribeiro, L.M.O.; Dornelas, C.B.; Silva, T.L.; Vieira, M.G.A. MgAl-LDH/Biochar composites for methylene blue removal by adsorption. Appl. Clay. Sci. 2019, 168, 11–20. [Google Scholar] [CrossRef]
- Harizi, I. Synthèse et Caractérisation des Matériaux à Base de Zéolithe et D’hydroxydes Doubles Lamellaires: Application à L’élimination des Colorants. Ph.D. Thesis, Université Ferhat Abbas, Sétif, El Bez, 2020. [Google Scholar]
- Jijoe, P.S.; Yashas, S.R.; Shivaraju, H.P. Fundamentals, synthesis, characterization and environmental applications of layered double hydroxides: A review. Environ. Chem. Lett. 2021, 19, 2643–2661. [Google Scholar] [CrossRef]
- Rojas, R. Applications of Layered Double Hydroxides on Environmental Remediation; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; pp. 39–71. [Google Scholar]
- Daud, M.; Hai, A.; Banat, F.; Wazir, M.; Habib, M.; Bharath, G.; Al-Harthi, M.A. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH)—Containing hybrids as promising adsorbents for dyes removal. J. Mol. Liq. 2019, 288, 110989. [Google Scholar] [CrossRef]
- El-Abboubi, M.; Taoufik, N.; Mahjoubi, F.; Oussama, A.; Kzaiber, F.; Barka, N. Sorption of methyl orange dye by dodecyl-sulfate intercalated Mg-Al layered double hydroxides. Mater. Today Proc. 2021, 37, 3894–3897. [Google Scholar] [CrossRef]
- Fujii, S.; Sugie, Y.; Kobune, M.; Touno, A.; Touji, J. Uptakes of Cu2+, Pb2+ and Zn2+ on Synthetic Hydrotalcite in Aqueous Solution. J. Jpn. Chem. Soc. 1992, 1992, 1504–1507. [Google Scholar] [CrossRef]
- Liang, X.; Zang, Y.; Xu, Y.; Tan, X.; Hou, W.; Wang, L.; Sun, Y. Sorption of metal cations on layered double hydroxides. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 122–131. [Google Scholar] [CrossRef]
- Dinari, M.; Neamati, S. Surface modified layered double hydroxide/polyaniline nanocomposites: Synthesis, characterization and Pb2+ removal. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124438. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Ma, Z.; Dong, X.; Wei, Z.; Liu, X.; Zhu, L. Mg/Al-layered double hydroxide modified biochar for simultaneous removal phosphate and nitrate from aqueous solution. Environ. Technol. Innov. 2021, 23, 101771. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; Gao, W.; Zhang, Y.; Yang, Y. Selective nitrate removal from aqueous solutions by a hydrotalcite-like absorbent FeMgMn-LDH. Sci. Rep. 2020, 10, 16126. [Google Scholar] [CrossRef]
- Motandi, M.K.; Zhang, Z.; Inkoua, S.; Yan, L. Application of zirconium modified layered double hydroxide and calcination product for adsorptive removal of phosphate from aqueous solution. Environ. Prog. Sustain. Energy 2022, 41, e13744. [Google Scholar] [CrossRef]
- dos Santos, G.E.D.S.; Ide, A.H.; Duarte, J.L.S.; McKay, G.; Silva, A.O.S.; Meili, L. Adsorption of anti-inflammatory drug diclofenac by MgAl/layered double hydroxide supported on Syagrus coronata biochar. Powder Technol. 2020, 364, 229–240. [Google Scholar] [CrossRef]
- Santamaría, L.; López-Aizpún, M.; García-Padial, M.; Vicente, M.; Korili, S.; Gil, A. Zn-Ti-Al layered double hydroxides synthesized from aluminum saline slag wastes as efficient drug adsorbents. Appl. Clay Sci. 2020, 187, 105486. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- de Sousa, F.W.; Moreira, S.A.; Oliveira, A.G.; Cavalcante, R.M.; Nascimento, R.F.; Rosa, M.F. Uso da casca de coco verde como adsorbente na remoção de metais tóxicos. Química Nova 2007, 30, 1153–1157. [Google Scholar] [CrossRef] [Green Version]
- Aksu, Z.; Gönen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).