Rapid and Sensitive Detection of Rutin in Food Based on Nitrogen-Doped Carbon Quantum Dots as Fluorescent Probe
Abstract
:1. Introduction
2. Results and Discussion
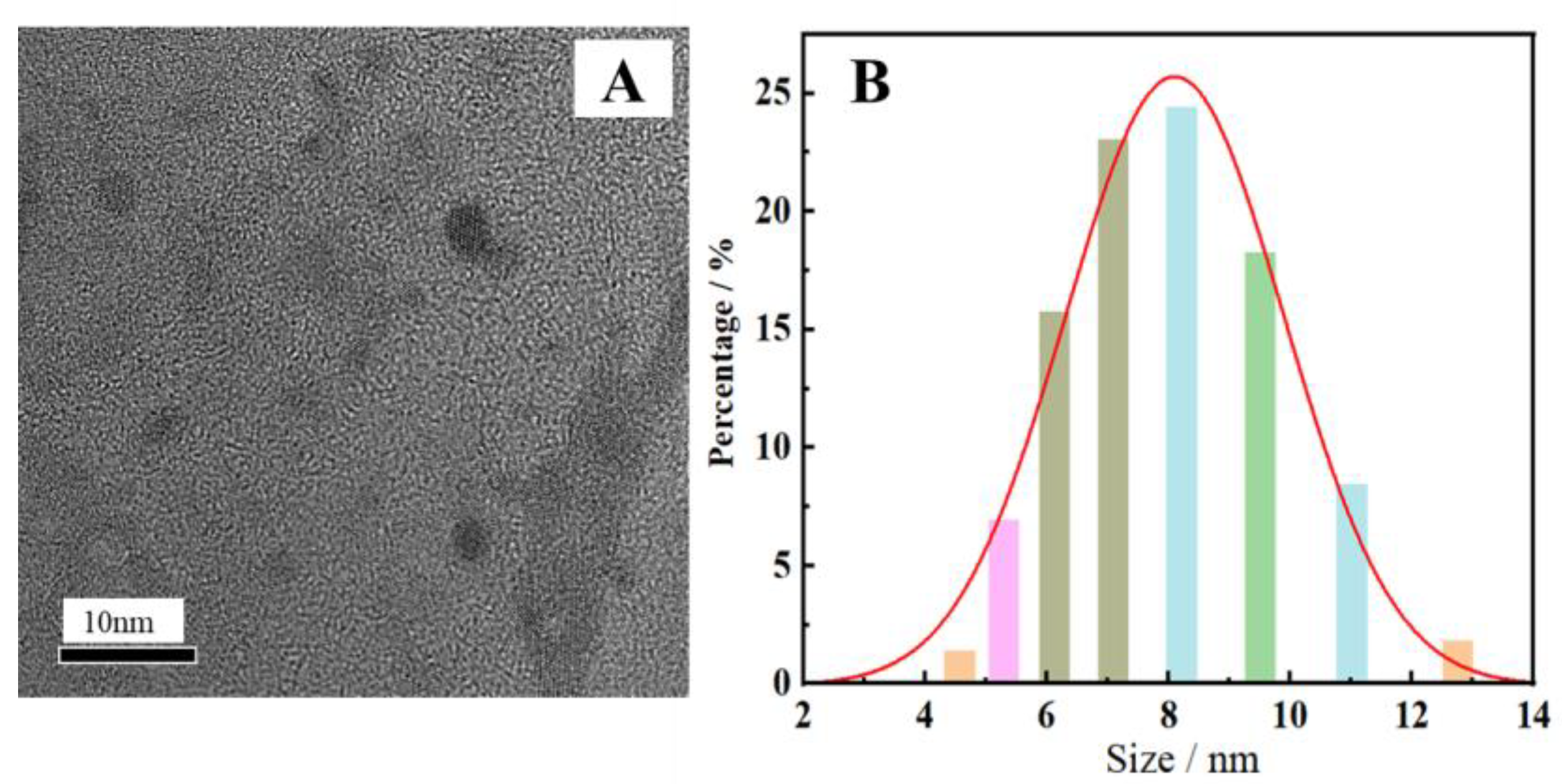
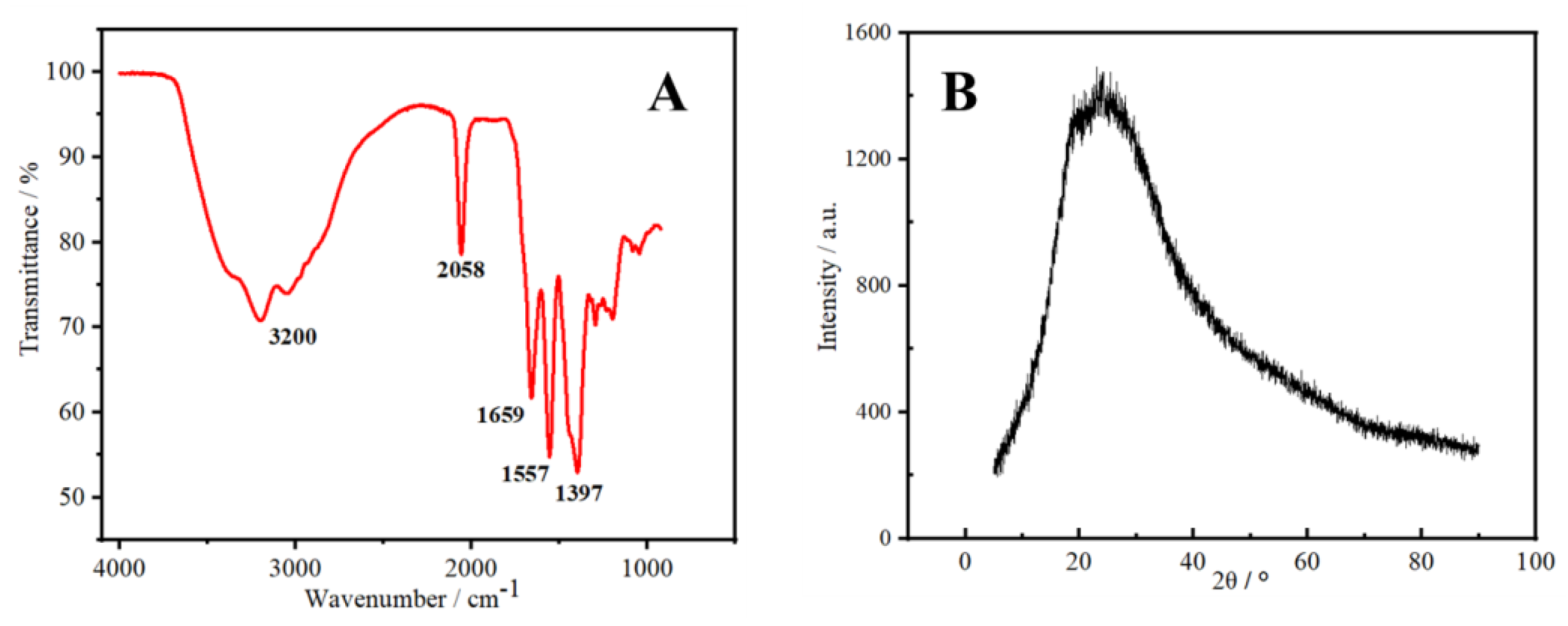
2.1. Characterization of N-CDs
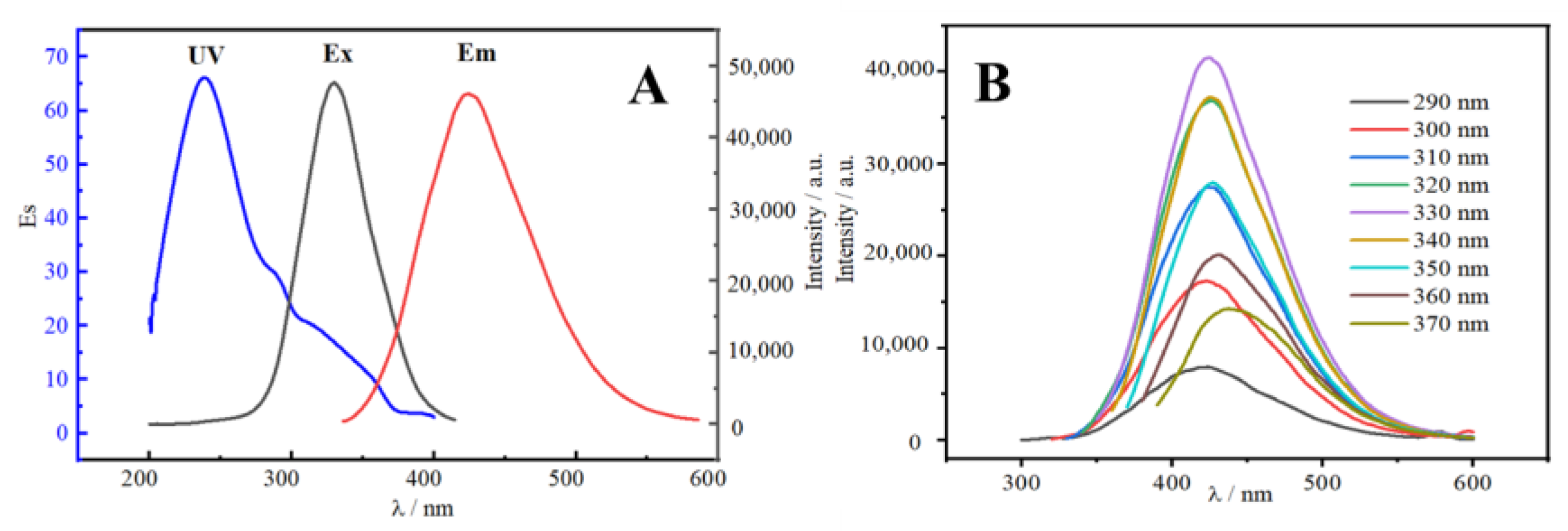
2.2. Optical Properties of N-CDs
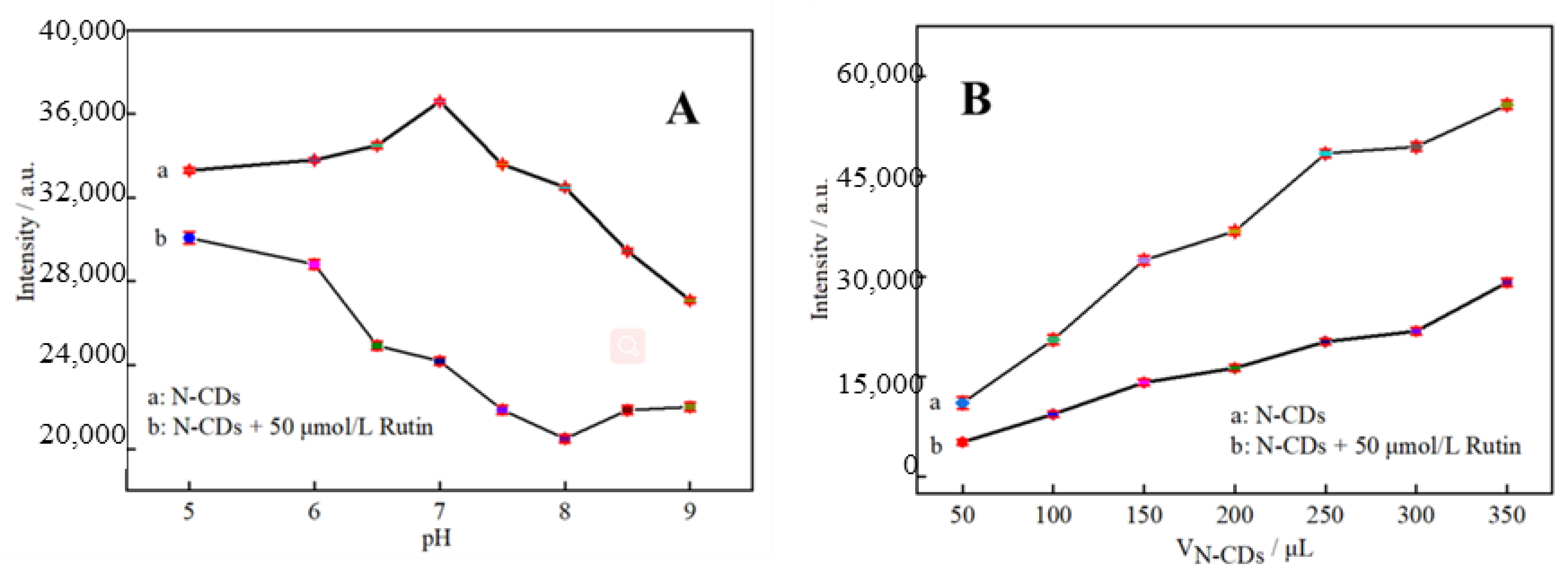
2.3. Condition Optimization for Rutin Determination by Fluorescent Probe
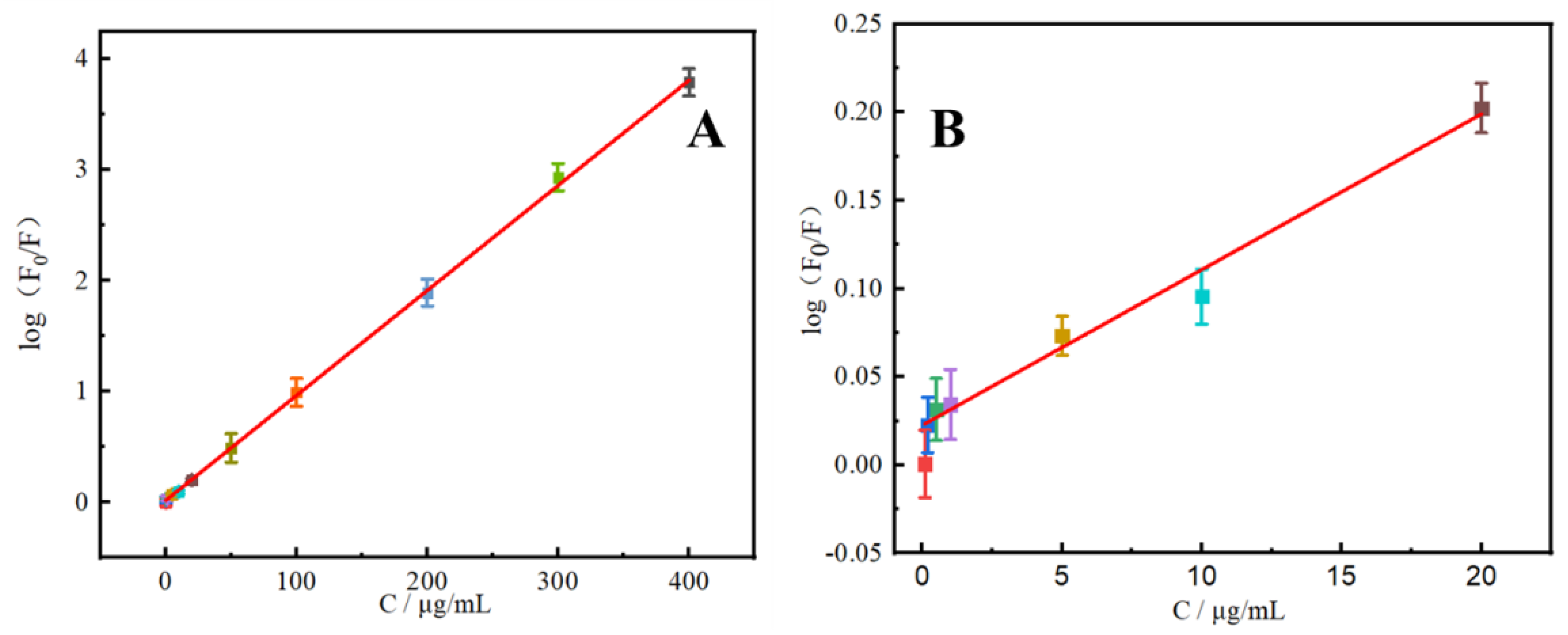
2.4. Performance Analysis
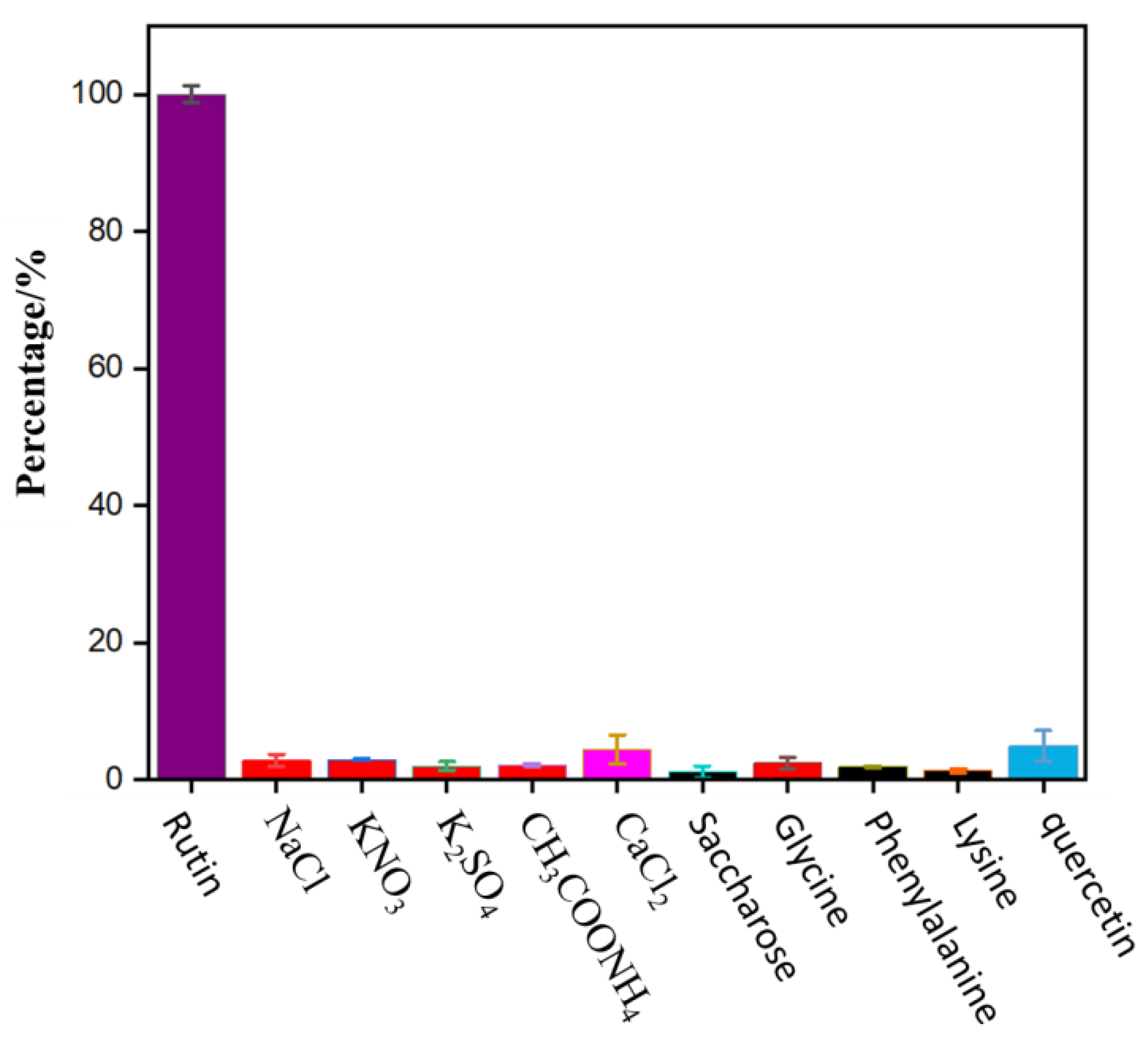
2.5. Selectivity, Reproducibility, and Stability
2.6. Sample Analysis
3. Materials and Methods
3.1. Instruments
3.2. Materials
3.3. Preparation of N-CDs
3.4. Characterization of N-CDs
3.5. Optical Performance Test of N-CDs
3.6. Sample Pretreatment and Determination of Rutin Content
3.7. Data Analysis and Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosseinzadeh, H.; Nassiri-Asl, M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J. Endocrinol. Investig. 2014, 37, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Imani, A.; Maleki, N.; Bohlouli, S.; Kouhsoltani, M.; Sharifi, S.; Maleki, D.S. Molecular mechanisms of anticancer effect of rutin. Phytother. Res. 2021, 35, 2500–2513. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxidative Med. Cell Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budzynska, B.; Faggio, C.; Kruk-Slomka, M.; Samec, D.; Nabavi, S.F.; Sureda, A.; Devi, K.P.; Nabavi, S.M. Rutin as neuroprotective agent: From bench to bedside. Curr. Med. Chem. 2019, 26, 5152–5164. [Google Scholar] [CrossRef] [PubMed]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki, D.S.; Rahbar, S.Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani, J.N.; Raeesi, S. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as cell signaling pathway modulator: Prospects in treatment and chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef]
- He, X.R.; Bai, Y.J.; Zhao, Z.F.; Wang, X.X.; Fang, J.C.; Huang, L.H.; Zeng, M.; Zhang, Q.; Zhang, J.Y.; Zheng, X.H. Local and traditional uses, phytochemistry, and pharmacology of Sophora japonica L.: A review. J. Ethnopharmacol. 2016, 187, 160–182. [Google Scholar] [CrossRef]
- Zou, L.; Wu, D.T.; Ren, G.X.; Hu, Y.C.; Peng, L.X.; Zhao, J.L.; Garcia-Perez, P.; Carpena, M.; Prieto, M.A.; Cao, H.; et al. Bioactive compounds, health benefits, and industrial applications of Tartary buckwheat (Fagopyrum tataricum). Crit. Rev. Food Sci. Nutr. 2021, 62, 1–17. [Google Scholar] [CrossRef]
- Zhu, F. Chemical composition and health effects of Tartary buckwheat. Food Chem. 2016, 203, 231–245. [Google Scholar] [CrossRef]
- Liu, D.G.; Mei, Q.; Wan, X.L.; Que, H.L.; Li, L.Y.; Wan, D.R. Determination of rutin and isoquercetin contents in Hibisci mutabilis Folium in different collection periods by HPLC. J. Chromatogr. Sci. 2015, 53, 1680–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Lin, P.; Ma, L.H.; Xu, K.X.; Lin, X.L. Separation and determination of flavonoids in three traditional chinese medicines by capillary electrophoresis with amperometric detection. J. Sep. Sci. 2016, 39, 1357–1362. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.F.; Li, T.H.; Li, X.N.; Feng, J.; Tang, T.F.; Cheng, H. A facile fabrication of a hierarchical ZIF-8/MWCNT nanocomposite for the sensitive determination of rutin. Anal. Methods 2021, 13, 5450–5457. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.N.; Zhao, Y.X.; Xu, X.T.; Sun, Y.; Li, Y.H.; Du, J.X. A covalent triazine framework as an oxidase mimetic in the luminol chemiluminescence system: Application to the determination of the antioxidant rutin. Mikrochim. Acta 2019, 187, 42. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.J.; Qin, X.Z.; Lu, M.C.; Ma, Q.J. Water soluble silicon nanoparticles as a fluorescent probe for highly sensitive detection of rutin. ACS Omega 2022, 7, 28588–28596. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Lee, S.T. Carbon dots: Advances in nanocarbon applications. Nanoscale 2019, 11, 19214–19224. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.H.; Wei, Q.; Sun, D.W. Carbon dots: Principles and their applications in food quality and safety detection. Crit. Rev. Food Sci. Nutr. 2018, 58, 2466–2475. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Saravanan, A.; Das, P.; Luong, J.; Gedanken, A. Microbial inhibition and biosensing with multifunctional carbon dots: Progress and perspectives. Biotechnol. Adv. 2021, 53, 107843. [Google Scholar] [CrossRef]
- Park, Y.; Yoo, J.; Lim, B.; Kwon, W.; Rhee, S.W. Improving the functionality of carbon nanodots: Doping and surface functionalization. J. Mater. Chem. A 2016, 30, 11582–11603. [Google Scholar] [CrossRef]
- Qi, H.J.; Teng, M.; Liu, M.; Liu, S.X.; Li, J.; Yu, H.P.; Teng, C.B.; Huang, Z.H.; Liu, H.; Shao, Q.; et al. Biomass-derived nitrogen-doped carbon quantum dots: Highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Sahu, S.; Sinha, N.; Bhutia, S.K. Synthesis of a carbon-dot-based photoluminescent probe for selective and ultrasensitive detection of Hg2+ in water and living cells. Analyst 2015, 140, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Shelar, A.; Sangshetti, J.; Chakraborti, S.; Singh, A.V.; Patil, R.; Gosavi, S. Helminthicidal and larvicidal potentials of biogenic silver nanoparticles synthesized from medicinal plant Momordica charantia. Med. Chem. 2019, 15, 781–789. [Google Scholar] [CrossRef]
- Aslam, M.; Fozia, F.; Gul, A.; Ahmad, I.; Ullah, R.; Bari, A.; Mothana, R.A.; Hussain, H. Phyto-extract-mediated synthesis of silver nanoparticles using aqueous extract of sanvitalia procumbens, and characterization, optimization and photocatalytic degradation of azo dyes orange g and direct blue-15. Molecules 2021, 26, 6144. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Zhang, P.; Zhai, X.Y.; Tian, F.; Li, W.C.; Yang, J.H.; Liu, Y.; Wang, H.B.; Wang, W.; Liu, W.G. Nano-carrier for gene delivery and bioimaging based on carbon dots with PEI-passivation enhanced fluorescence. Biomaterials 2012, 33, 3604–3613. [Google Scholar] [CrossRef]
- Ansari, F.; Kahrizi, D. Hydrothermal synthesis of highly fluorescent and non-toxic carbon dots using Stevia rebaudiana Bertoni. Cell Mol. Biol. 2018, 64, 32–36. [Google Scholar] [CrossRef]
- Gao, X.H.; Du, C.; Zhuang, Z.H.; Chen, W. Carbon quantum dot-based nanoprobes for metal ion detection. J. Mater. Chem. C 2016, 4, 6927–6945. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Guo, Z.; Liu, W.; Ma, Q.; Feng, F.; Dong, C. A novel fluorescent sensor based on sulfur and nitrogen co-doped carbon dots with excellent stability for selective detection of doxycycline in raw milk. RSC Adv. 2017, 7, 12827–12834. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.T.; Yang, C.L.; Chen, Y.X.; Zhu, Z.B.; Zhou, L.H. Cuttlefish ink-based N and S co-doped carbon quantum dots as a fluorescent sensor for highly sensitive and selective para-nitrophenol detection. Anal. Methods 2021, 13, 5351–5359. [Google Scholar] [CrossRef]
- Feng, Z.B.; Li, Z.L.; Zhang, X.W.; Shi, Y.P.; Zhou, N. Nitrogen-doped carbon quantum dots as fluorescent probes for sensitive and selective detection of nitrite. Molecules 2017, 22, 2061. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, Y.C.; Huang, H.; Wang, A.J.; Chen, J.R.; Feng, J.J. Facile synthesis of N, S-codoped fluorescent carbon nanodots for fluorescent resonance energy transfer recognition of methotrexate with high sensitivity and selectivity. Biosens. Bioelectron. 2015, 64, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Sinduja, B.; Abraham, J.S. Sensitive determination of rutin by spectrofluorimetry using carbon dots synthesized from a non-essential amino acid. Spectrochim. Acta Part A 2018, 193, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Shang, S.Z.; Zheng, X.D.; Lei, P.; Han, J.M.; Yuan, L.D.; Li, Z.; Wang, R.; Gong, W.M.; Tang, J.G.; et al. Fluorescent sensors based on Cu-doped carbon quantum dots for the detection of rutin. J. Brazil. Chem. Soc. 2019, 30, 988–996. [Google Scholar] [CrossRef]
- Li, H.Y.; Xu, Y.; Zhao, L.; Ding, J.; Chen, M.Y.; Chen, G.R.; Li, Y.; Ding, L. Synthesis of tiny carbon dots with high quantum yield using multi-1 walled carbon nanotubes as support for selective “turn-off-on” detection of rutin and Al3+. Carbon 2019, 143, 391–401. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhang, Y.C.; Ma, H.Y.; Liu, X.L.; Wang, Q. Synthesized of Carbon Quantum Dots from Red Dates and as A “Off-On ” Fluorescence Probe for High Sensitive Detection of Rutin. Chin. J. Lumin 2018, 39, 600–608. [Google Scholar] [CrossRef]
- Zhang, T.; Ji, Q.X.; Song, J.Y.; Li, H.M.; Wang, X.; Shi, H.Q.; Niu, M.H.; Chu, T.T.; Zhang, F.S.; Guo, Y.Z. Preparation of Nitrogen and Sulfur Co-Doped Fluorescent Carbon Dots from Cellulose Nanocrystals as a Sensor for the Detection of Rutin. Molecules 2022, 27, 8021. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shao, F.Q.; Huang, H.; Feng, J.L.; Wang, A.J. Eco-friendly and rapid microwave synthesis of green fluorescent graphitic carbon nitride quantum dots for vitro bioimaging. Sens. Actuators B 2016, 226, 506–511. [Google Scholar] [CrossRef]

| Elements | Mass Percentage (%) | Atom Percentage (%) |
|---|---|---|
| C | 88.46 | 86.95 |
| N | 5.04 | 4.25 |
| O | 5.93 | 4.37 |
| S | 0.57 | 0.21 |
| CDs | Linear Range | Detection Limit | Sample | Reference |
|---|---|---|---|---|
| CDs | 0.5–15 (μmol/L) | 61 (μg/L) | Drugs | [32] |
| Cu-CDs | 0.1–15 (μg/mL) | 0.05 (μg/mL) | Drugs | [33] |
| T-CDs | 0.06–130 (mg/L) | 0.02 (mg/L) | Tablets, human serum, human urine | [34] |
| N-CDs | 0.25–10 (μmol/L) | 0.15 (μmol/L) | Tablets | [35] |
| N, S-CDs | 0–145 (mg/L) | 0.02 (μmol/L) | Lake water Human urine | [37] |
| N-CDs | 0.1–400 (μg/mL) | 0.03 (μg/mL) | Black buckwheat tea Wolfberry | This work |
| Sample | Added (μg/mL) | Detected (μg/mL) ( ± S) | Recovery (%) | RSD (%) | Detected by T/QAS 013-2020 (μg/mL) ( ± S) |
|---|---|---|---|---|---|
| Black buckwheat tea | 0 | 106.73 ± 0.38 | - | 0.35 | 106.84 ± 0.24 |
| 1 | 107.73 ± 0.38 | 93.98–103.88 | 4.11 | 107.66 ± 0.27 | |
| 10 | 116.71 ± 0.22 | 96.78–102.51 | 2.24 | 116.72 ± 0.21 | |
| 100 | 206.81 ± 2.35 | 96.22–103.10 | 2.35 | 207.11 ± 2.36 | |
| Wolfberry | 0 | 43.89 ± 0.67 | - | 1.53 | 44.01 ± 0.75 |
| 1 | 44.89 ± 0.03 | 97.00–103.83 | 3.04 | 44.91 ± 0.03 | |
| 10 | 53.94 ± 0.34 | 96.71–104.92 | 3.43 | 53.99 ± 0.21 | |
| 100 | 144.23 ± 1.95 | 97.06–102.38 | 1.95 | 144.06 ± 1.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Si, X.; Han, M.; Bai, C. Rapid and Sensitive Detection of Rutin in Food Based on Nitrogen-Doped Carbon Quantum Dots as Fluorescent Probe. Molecules 2022, 27, 8834. https://doi.org/10.3390/molecules27248834
Huang Y, Si X, Han M, Bai C. Rapid and Sensitive Detection of Rutin in Food Based on Nitrogen-Doped Carbon Quantum Dots as Fluorescent Probe. Molecules. 2022; 27(24):8834. https://doi.org/10.3390/molecules27248834
Chicago/Turabian StyleHuang, Yue, Xiaojing Si, Mei Han, and Chen Bai. 2022. "Rapid and Sensitive Detection of Rutin in Food Based on Nitrogen-Doped Carbon Quantum Dots as Fluorescent Probe" Molecules 27, no. 24: 8834. https://doi.org/10.3390/molecules27248834




