Porphyrin Functionalized Carbon Quantum Dots for Enhanced Electrochemiluminescence and Sensitive Detection of Cu2+
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Nanomaterials
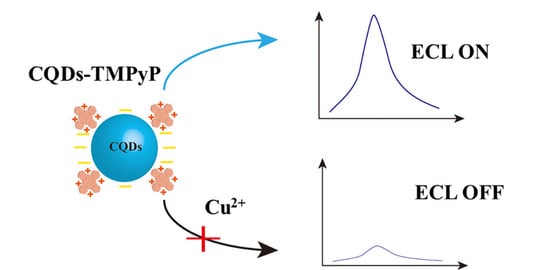
2.2. ECL Mechanism
2.3. Optimization of Experimental Conditions
2.4. Performance of the ECL Biosensor
2.5. Stability and Selectivity
2.6. Real Sample Analysis
3. Materials and Methods
3.1. Reagents and Materials
3.2. Apparatus
3.3. Synthesis of the CQD-TMPyP Nanocomposite
3.4. Electrode Cleaning and Preparation
3.5. ECL Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Li, Q.; Ohulchanskyy, T.Y.; Liu, R.; Koynov, K.; Wu, D.; Best, A.; Kumar, R.; Bonoiu, A.; Prasad, P.N. Photoluminescent Carbon Dots as Biocompatible Nanoprobes for Targeting Cancer Cells in Vitro. J. Phys. Chem. C 2010, 114, 12062–12068. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Cui, L.; Wang, K.; Zhan, H. Development of a highly sensitive electrochemiluminescence sophoridine sensor using Ru(bpy)(3)(2+) integrated carbon quantum dots—Polyvinyl alcohol composite film. Sens. Actuators B-Chem. 2017, 248, 402–410. [Google Scholar] [CrossRef]
- Lei, J.P.; Ju, H.X. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012, 41, 2122–2134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Chi, Y.; Dong, Y.; Lin, J.; Wang, B. Electrochemiluminescence of Water-Soluble Carbon Nanocrystals Released Electrochemically from Graphite. J. Am. Chem. Soc. 2009, 131, 4564–4565. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sun, H.; Liang, Z. Nanomaterials in Electrochemiluminescence Sensors. Chemelectrochem 2017, 4, 1651–1662. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, R.; Li, G.; Chen, C.; Chi, Y.; Chen, G. Polyamine-Functionalized Carbon Quantum Dots as Fluorescent Probes for Selective and Sensitive Detection of Copper Ions. Anal. Chem. 2012, 84, 6220–6224. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Lin, J.; Chen, Y.; Fu, F.; Chi, Y.; Chen, G. Graphene quantum dots, graphene oxide, carbon quantum dots and graphite nanocrystals in coals. Nanoscale 2014, 6, 7410–7415. [Google Scholar] [CrossRef] [PubMed]
- Milosavljevic, V.; Nguyen, H.V.; Michalek, P.; Moulick, A.; Kopel, P.; Kizek, R.; Adam, V. Synthesis of carbon quantum dots for DNA labeling and its electrochemical, fluorescent and electrophoretic characterization. Chem. Pap. 2015, 69, 192–201. [Google Scholar] [CrossRef]
- Zhu, K.; Hu, X.; Ge, Q.; Sun, Q. Fluorescent recognition of deoxyribonucleic acids by a quantum dotimeso-tetrakis(N-methylpyridinium-4-yl)porphyrin complex based on a photo induced electron-transfer mechanism. Anal. Chim. Acta 2014, 812, 199–205. [Google Scholar] [CrossRef]
- Cai, W.-R.; Zhang, G.-Y.; Lu, K.-K.; Zeng, H.-B.; Cosnier, S.; Zhang, X.-J.; Shan, D. Enhanced Electrochemiluminescence of One-Dimensional Self-Assembled Porphyrin Hexagonal Nanoprisms. ACS Appl. Mater. Interfaces 2017, 9, 20904–20912. [Google Scholar] [CrossRef]
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A Water-Stable Porphyrin-Based Metal-Organic Framework Active for Visible-Light Photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 7440–7444. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, C.; Li, Z.Z.; Wu, J.; Liu, P.K.; Mo, F.; Fu, Y. Multifunctional Zinc Oxide Promotes Electrochemiluminescence of Porphyrin Aggregates for Ultrasensitive Detection of Copper Ion. Anal. Chem. 2020, 92, 3324–3331. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.-K.; Cai, W.; Yin, Z.; Cheng, M.; Kong, Y. Self-assembly of covalent porphyrin compound and its enhanced electrochemiluminescence performance. Bull. Korean Chem. Soc. 2022, 43, 1373–1382. [Google Scholar] [CrossRef]
- Li, Y.-X.; Li, J.; Zhu, D.; Wang, J.Z.; Shu, G.F.; Li, J.; Zhang, S.L.; Zhang, X.J.; Cosnier, S.; Zeng, H.B.; et al. 2D Zn-Porphyrin-Based Co(II)-MOF with 2-Methylimidazole Sitting Axially on the Paddle–Wheel Units: An Efficient Electrochemiluminescence Bioassay for SARS-CoV-2. Adv. Funct. Mater. 2022, 32, 2209743. [Google Scholar] [CrossRef]
- Li, L.; Ning, X.; Qian, Y.; Pu, G.; Wang, Y.; Zhang, X.; Wang, H.; Chen, J.; Shan, D.; Lu, X. Porphyrin nanosphere–graphene oxide composite for ehanced electrochemiluminescence and sensitive detection of Fe3+ in human serum. Sens. Actuators B Chem. 2018, 257, 331–339. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, C.; Bai, Y.; Wang, Q.; Ma, P.; Ma, X.; Zhu, P. Electrochemiluminescence Enhanced by the Synergetic Effect of Porphyrin and Multi-walled Carbon Nanotubes for Uric Acid Detection. Electroanalysis 2022, 34, 302–309. [Google Scholar] [CrossRef]
- Liu, X.; Lu, J.; Chen, J.; Zhang, M.; Chen, Y.; Xing, F.; Feng, L. Chiral Self-Assembly of Porphyrins Induced by Chiral Carbon Dots. Front. Chem. 2020, 8, 670. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z.; Yan, X.; Zhang, X.; Amblard, F.; Chen, Y.; Feng, L. Artificial tongue based on carbon dots and a porphyrin derivative for pattern recognition of metal ions. Sens. Diagn. 2023, 2, 188–193. [Google Scholar] [CrossRef]
- Parsaee, Z.; Haratipour, P.; Lariche, M.J.; Vojood, A. A novel high performance nano chemosensor for copper (II) ion based on an ultrasound-assisted synthesized diphenylamine-based Schiff base: Design, fabrication and density functional theory calculations. Ultrason. Sonochem. 2018, 41, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Raju, C.V.; Kalaiyarasan, G.; Paramasivam, S.; Joseph, J.; Kumar, S.S. Phosphorous doped carbon quantum dots as an efficient solid state electrochemiluminescence platform for highly sensitive turn-on detection of Cu2+ ions. Electrochim. Acta 2020, 331, 135391. [Google Scholar]
- Wang, J.; Zhang, Z.; Zha, S.; Zhu, Y.; Wu, P.; Ehrenberg, B.; Chen, J.-Y. Carbon nanodots featuring efficient FRET for two-photon photodynamic cancer therapy with a low fs laser power density. Biomaterials 2014, 35, 9372–9381. [Google Scholar] [CrossRef]
- Qin, D.; Jiang, X.; Mo, G.; Feng, J.; Yu, C.; Deng, B. A Novel Carbon Quantum Dots Signal Amplification Strategy Coupled with Sandwich Electrochemiluminescence Immunosensor for the Detection of CA15-3 in Human Serum. ACS Sens. 2019, 4, 504–512. [Google Scholar] [CrossRef]
- Jiao, M.; Li, Y.; Jia, Y.; Yang, Z.; Luo, X. Aqueously synthesized color-tunable quaternary Cu-In-Zn-S quantum dots for Cu(II) detection via mild and rapid cation exchange. Sens. Actuators B-Chem. 2019, 294, 32–39. [Google Scholar] [CrossRef]
- Cho, S.W.; Rao, A.S.; Bhunia, S.; Reo, Y.J.; Singha, S.; Ahn, K.H. Ratiometric fluorescence detection of Cu(II) with a keto-dipicolylamine ligand: A mechanistic implication. Sens. Actuators B-Chem. 2019, 279, 204–212. [Google Scholar] [CrossRef]
- Shen, Q.; Tang, S.; Li, W.; Nie, Z.; Liu, Z.; Huang, Y.; Yao, S. A novel DNA-templated click chemistry strategy for fluorescent detection of copper(II) ions. Chem. Commun. 2012, 48, 281–283. [Google Scholar] [CrossRef]
- Zhou, M.; Han, L.; Deng, D.; Zhang, Z.; He, H.; Zhang, L.; Luo, L. 4-mercaptobenzoic acid modified silver nanoparticles-enhanced electrochemical sensor for highly sensitive detection of Cu2+. Sens. Actuators B-Chem. 2019, 291, 164–169. [Google Scholar] [CrossRef]
- Cheng, B.; Zhou, L.; Lu, L.; Liu, J.; Dong, X.; Xi, F.; Chen, P. Simultaneous label-free and pretreatment-free detection of heavy metal ions in complex samples using electrodes decorated with vertically-ordered silica nanochannels. Sens. Actuators B-Chem. 2018, 259, 364–371. [Google Scholar] [CrossRef]
- Amjadi, M.; Manzoori, J.L.; Hallaj, T.; Azizi, N. Sulfur and nitrogen co-doped carbon quantum dots as the chemiluminescence probe for detection of Cu2+ ions. J. Lumin. 2017, 182, 246–251. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Li, B. Chemiluminescence of CdTe quantum dots using K3Fe(CN)(6) as oxidant and its capping ligand-dependent effect. Microchem. J. 2010, 95, 186–191. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, F.; Yang, X. Label-free colorimetric biosensing of copper(II) ions with unimolecular self-cleaving deoxyribozymes and unmodified gold nanoparticle probes. Nanotechnology 2010, 21, 205502. [Google Scholar] [CrossRef]
- Dordevic, L.; Arcudi, F.; D’Urso, A.; Cacioppo, M.; Micali, N.; Bürgi, T.; Purrello, R.; Prato, M. Design principles of chiral carbon nanodots help convey chirality from molecular to nanoscale level. Nat. Commun. 2018, 9, 3442. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, X.; Zhang, X.; Liu, S.; Pei, Q.; Zheng, M.; Xie, Z. Porphyrin-Based Carbon Dots for Photodynamic Therapy of Hepatoma. Adv. Healthc. Mater. 2017, 6, 1600924. [Google Scholar] [CrossRef] [PubMed]






| Analytical Method | Linear Range/mM | Detection Limit/nM | Linear Range/mM | Ref. |
|---|---|---|---|---|
| Fluorescence | 0.020–20 | 6.7 | 0.020–20 | [23] |
| Fluorescence | 0–10 | 89 | 0–10 | [24] |
| Fluorescence | 0.5–10 | 290 | 0.5–10 | [25] |
| Electrochemical | 0.1–100 | 0.08 | 0.1–100 | [26] |
| Electrochemical | 0.1–30 | 20 | 0.1–30 | [27] |
| Chemiluminescence | 0.15–7.8 | 31.5 | 0.15–7.8 | [28] |
| Chemiluminescence | 0.12–900 | 100 | 0.12–900 | [29] |
| Chemiluminescence | 0.625–15 | 290 | 0.625–15 | [30] |
| Electrochemiluminescence | 0.01–10 | 2.78 | 0.01–10 | Our work |
| Sample | Added Cu2+/nM | Detected Cu2+/nM | Recovery/% | RSD/% (n = 3) |
|---|---|---|---|---|
| 1 | 10 | 12.27 | 122.7 | 1.06 |
| 2 | 100 | 98.22 | 98.22 | 8.20 |
| 3 | 500 | 496.85 | 99.37 | 6.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hou, X.; Lu, D.; Chen, Y.; Feng, L. Porphyrin Functionalized Carbon Quantum Dots for Enhanced Electrochemiluminescence and Sensitive Detection of Cu2+. Molecules 2023, 28, 1459. https://doi.org/10.3390/molecules28031459
Zhang X, Hou X, Lu D, Chen Y, Feng L. Porphyrin Functionalized Carbon Quantum Dots for Enhanced Electrochemiluminescence and Sensitive Detection of Cu2+. Molecules. 2023; 28(3):1459. https://doi.org/10.3390/molecules28031459
Chicago/Turabian StyleZhang, Xinying, Xialing Hou, Decheng Lu, Yingying Chen, and Lingyan Feng. 2023. "Porphyrin Functionalized Carbon Quantum Dots for Enhanced Electrochemiluminescence and Sensitive Detection of Cu2+" Molecules 28, no. 3: 1459. https://doi.org/10.3390/molecules28031459