Coffee Beverage: A New Strategy for the Synthesis of Polymethacrylates via ATRP
Abstract
:1. Introduction
2. Results
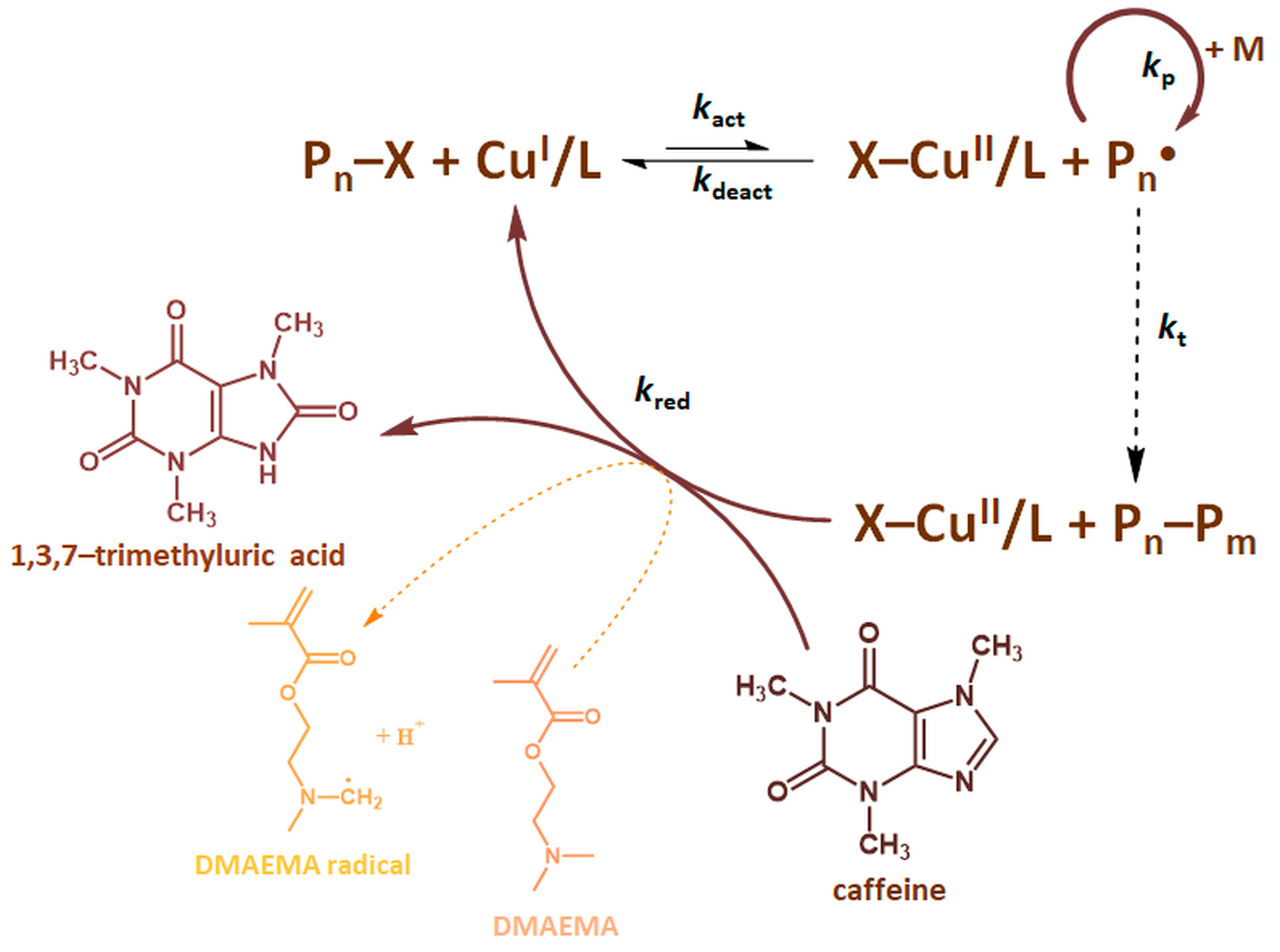
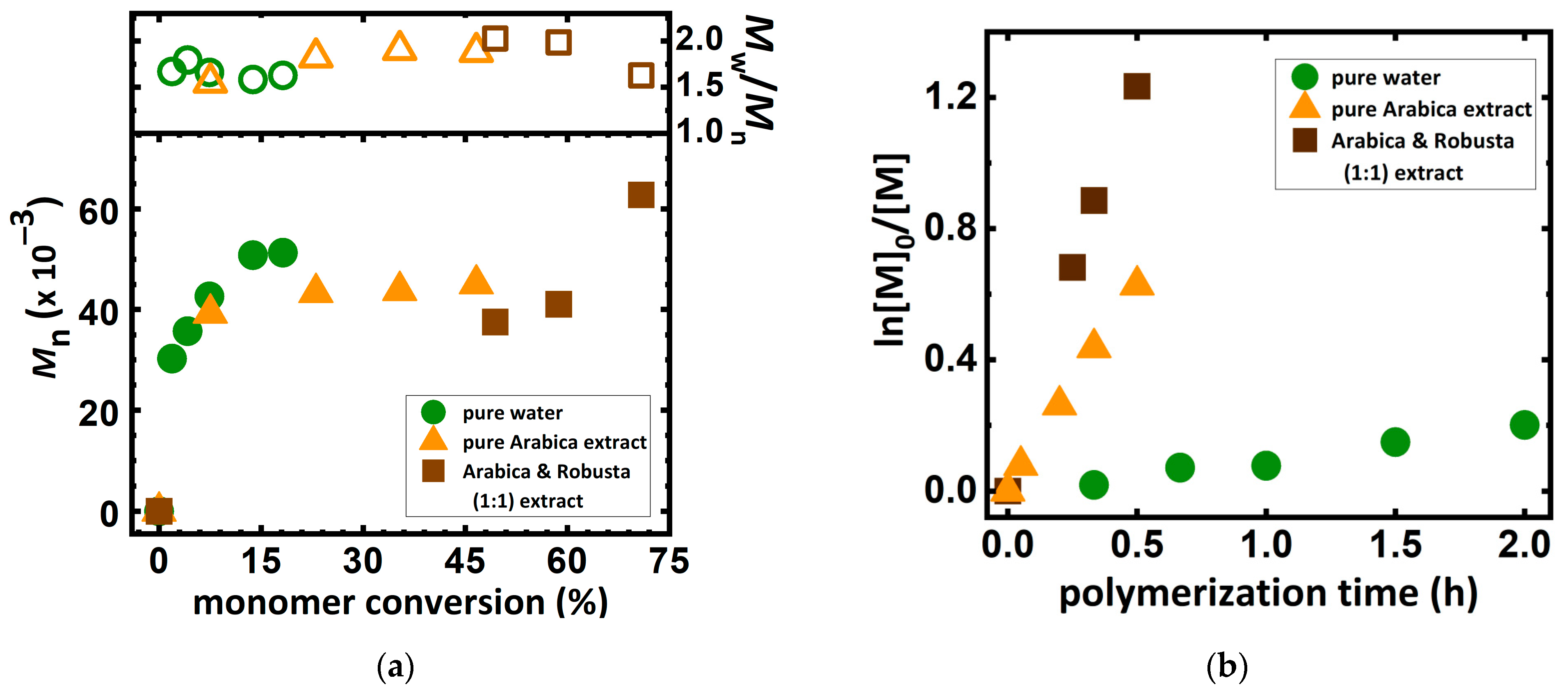
2.1. ARGET ATRP of DMAEMA in Various Coffee Bean Blend Solutions
2.2. ARGET ATRP of DMAEMA in Various Coffee Extracts Concentration Solutions
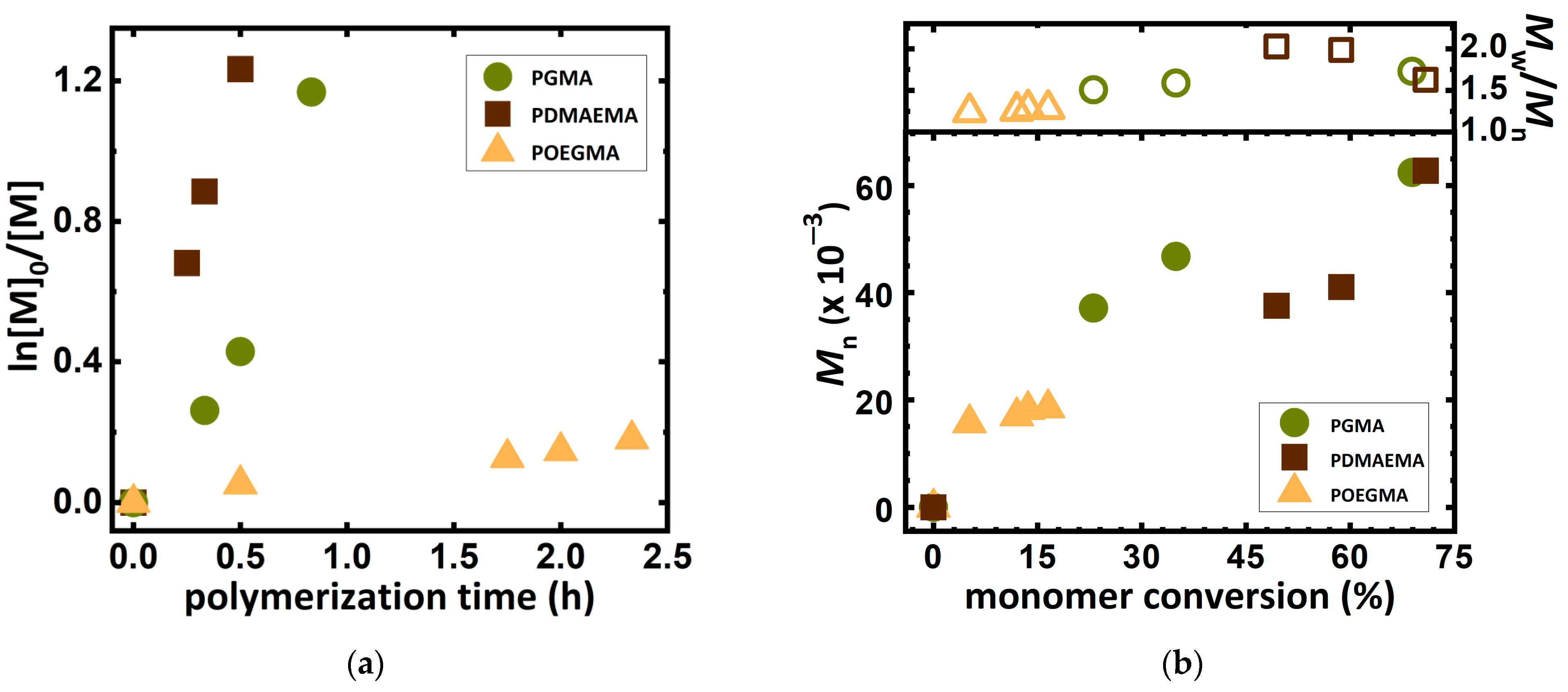
2.3. ARGET ATRP of Various Monomers
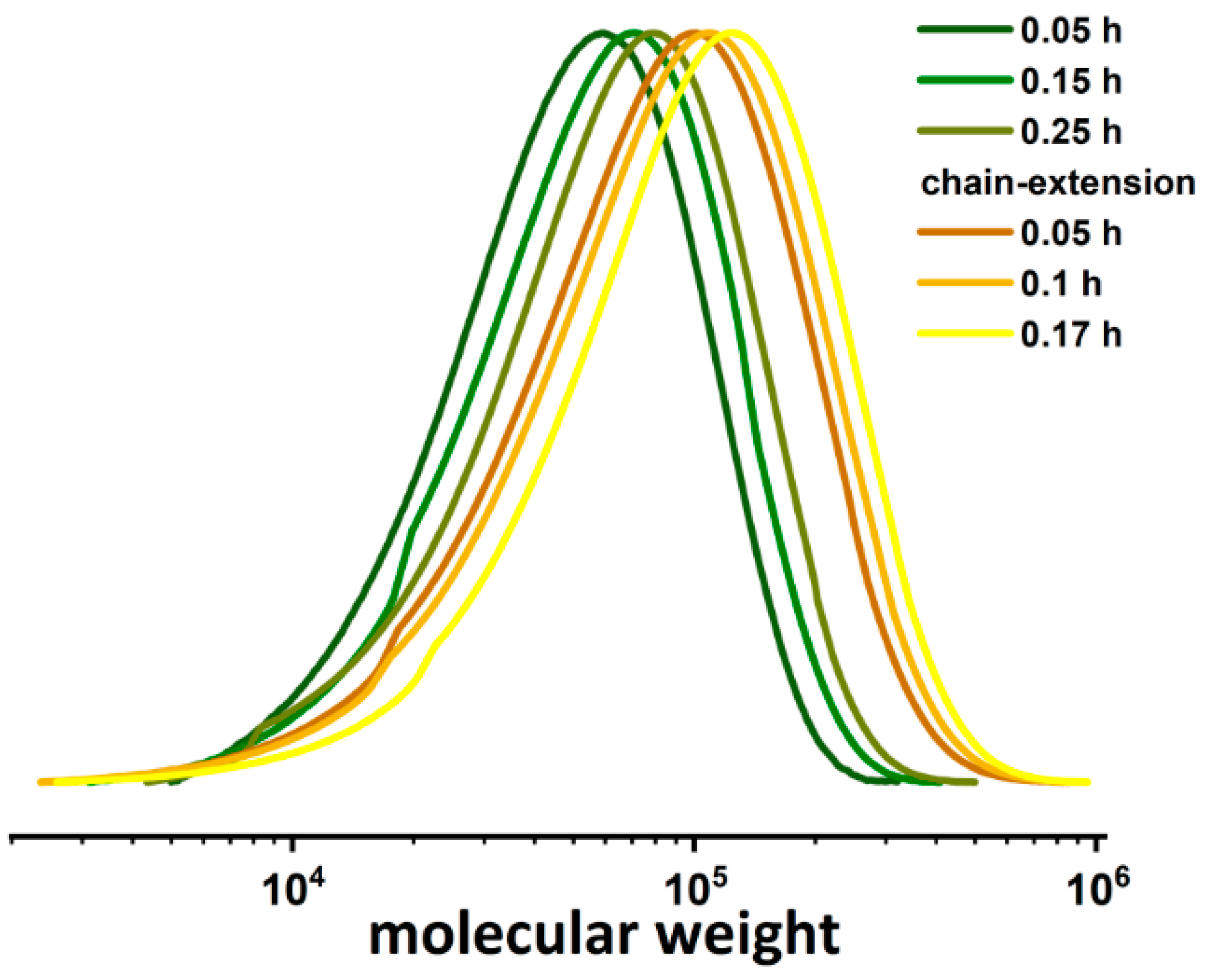
2.4. Chain-Extension Reaction of PDMAEMA-Br
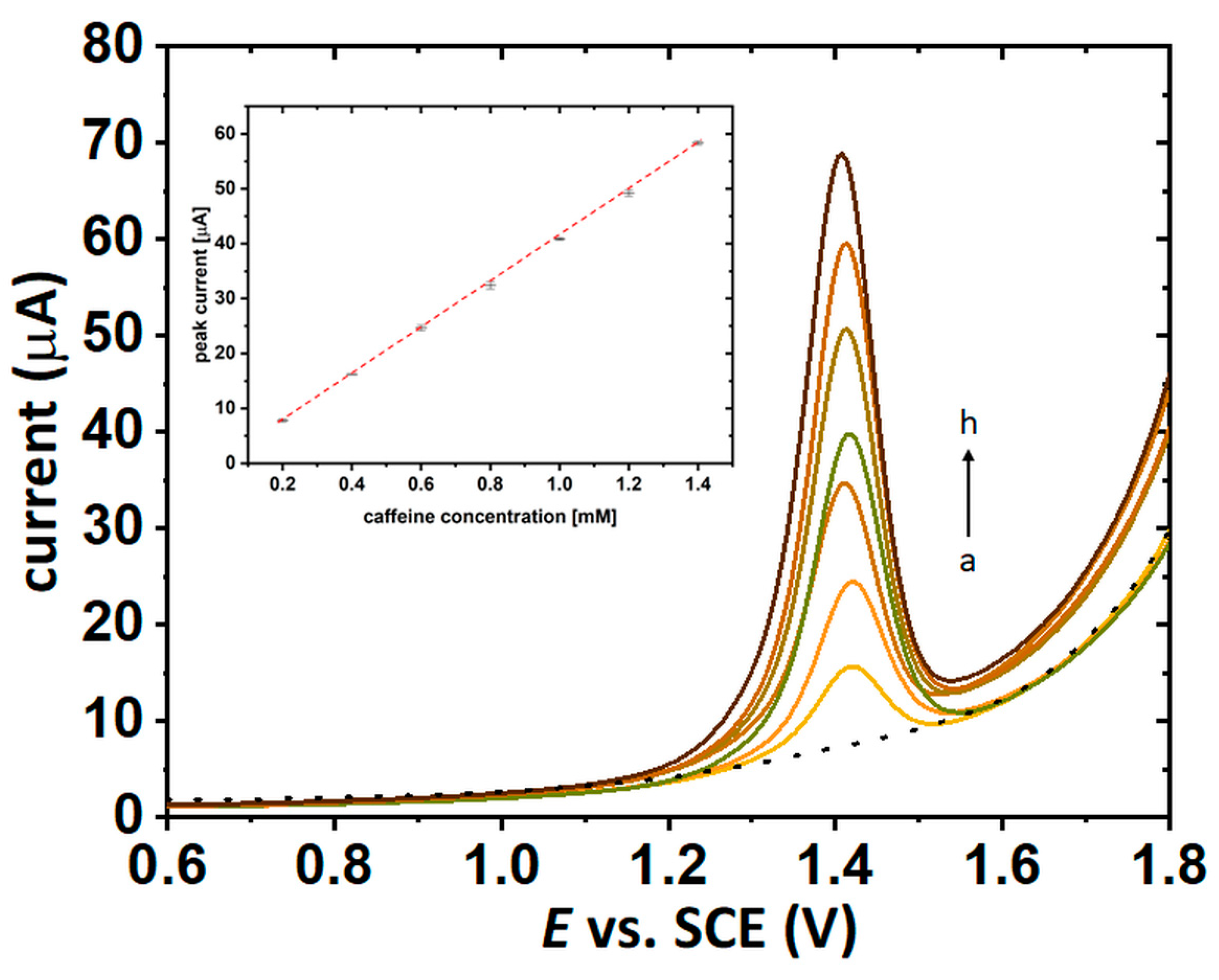
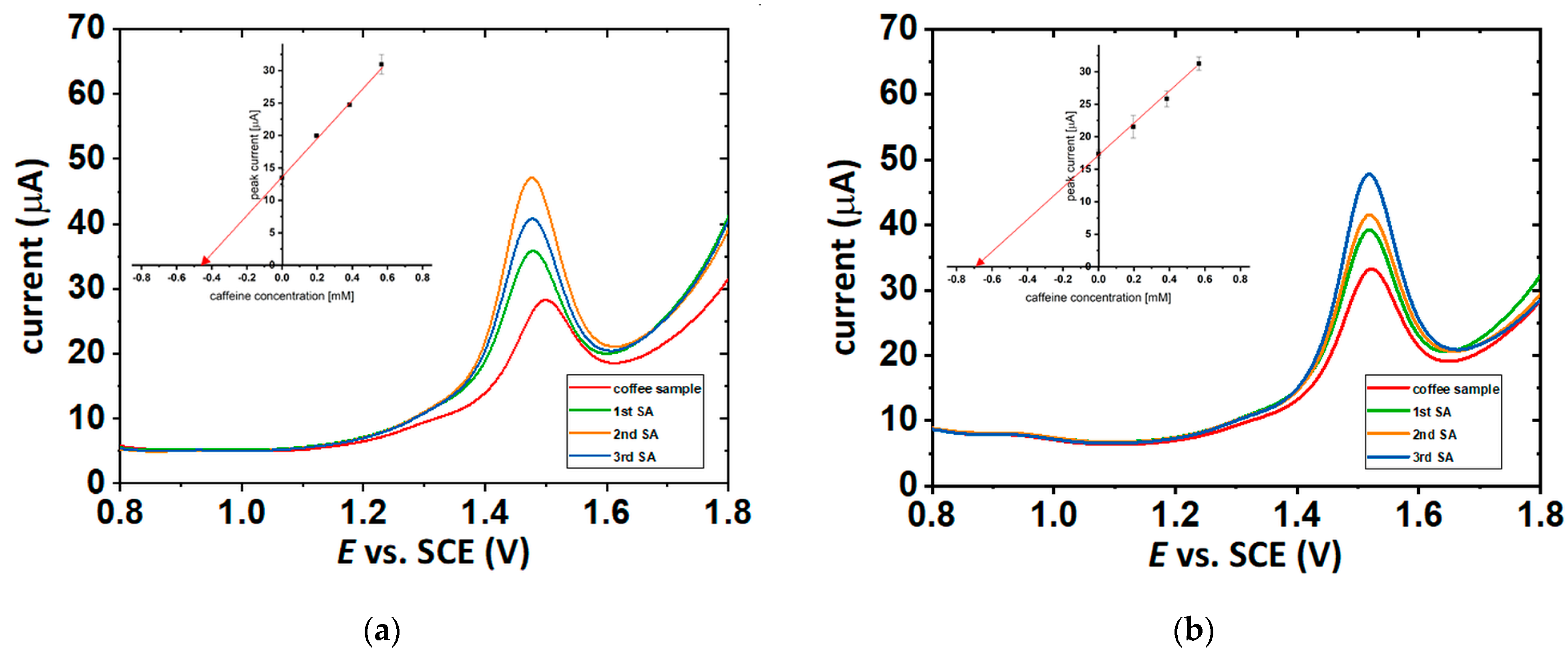
2.5. Electrochemical Measurements of Caffeine Concentration in the Coffee Solutions
3. Materials and Methods
3.1. Materials
3.2. Analysis
3.2.1. 1H-NMR
3.2.2. GPC
3.2.3. DPV
3.2.4. HPLC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Komes, D.; Busic, A. Antioxidants in coffee. In Processing and Impact on Antioxidants in Beverages; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 25–32. [Google Scholar]
- Iriondo-DeHond, A.; Elizondo, A.S.; Iriondo-DeHond, M.; Rios, M.B.; Mufari, R.; Mendiola, J.A.; Ibanez, E.; del Castillo, M.D. Assessment of healthy and harmful maillard reaction products in a novel coffee cascara beverage: Melanoidins and acrylamide. Foods 2020, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Aristizábal-Marulanda, V.; Chacón-Perez, Y.; Cardona Alzate, C.A. Chapter 3—The biorefinery concept for the industrial valorization of coffee processing by-products. In Handbook of Coffee Processing by-Products; Galanakis, C.M., Ed.; Academic Press: Chania, Greece, 2017; pp. 63–92. [Google Scholar]
- Ky, C.L.; Louarn, J.; Dussert, S.; Guyot, B.; Hamon, S.; Noirot, M. Caffeine, trigonelline, chlorogenic acids and sucrose diversity in wild Coffea arabica L. and C-canephora P. accessions. Food Chem. 2001, 75, 223–230. [Google Scholar] [CrossRef]
- Yen, W.J.; Wang, B.S.; Chang, L.W.; Duh, P.D. Antioxidant properties of roasted coffee residues. J. Agric. Food Chem. 2005, 53, 2658–2663. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, W.; Min, K.; Matyjaszewski, K. Activators regenerated by electron transfer for atom transfer radical polymerization of styrene. Macromolecules 2006, 39, 39–45. [Google Scholar] [CrossRef]
- Min, K.; Gao, H.F.; Matyjaszewski, K. Use of ascorbic acid as reducing agent for synthesis of well-defined polymers by ARGET ATRP. Macromolecules 2007, 40, 1789–1791. [Google Scholar] [CrossRef]
- Kwak, Y.; Matyjaszewski, K. ARGET ATRP of methyl methacrylate in the presence of nitrogen-based ligands as reducing agents. Polym. Int. 2009, 58, 242–247. [Google Scholar] [CrossRef]
- Li, X.H.; Wang, W.J.; Li, B.G.; Zhu, S.P. Kinetics and modeling of solution ARGET ATRP of styrene, butyl acrylate, and methyl methacrylate. Macromol. React. Eng. 2011, 5, 467–478. [Google Scholar] [CrossRef]
- Guazzelli, E.; Masotti, E.; Calosi, M.; Kriechbaum, M.; Uhlig, F.; Galli, G.; Martinelli, E. Single-chain folding and self-assembling of amphiphilic polyethyleneglycol-modified fluorinated styrene homopolymers in water solution. Polymer 2021, 231, 124107. [Google Scholar] [CrossRef]
- Chmielarz, P. Synthesis of inositol-based star polymers through low ppm ATRP methods. Polym. Adv. Technol. 2017, 28, 1804–1812. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P. Dually-functional riboflavin macromolecule as a supramolecular initiator and reducing agent in temporally-controlled low ppm ATRP. Express Polym. Lett. 2020, 14, 235–247. [Google Scholar] [CrossRef]
- Tanaka, K.; Matyjaszewski, K. Copolymerization of (meth)acrylates with olefins using activators regenerated by electron transfer for atom transfer radical polymerization (ARGET ATRP). Macromol. Symp. 2008, 261, 1–9. [Google Scholar] [CrossRef]
- Chan, N.; Cunningham, M.F.; Hutchinson, R.A. ARGET ATRP of methacrylates and acrylates with stoichiometric ratios of ligand to copper. Macromol. Chem. Phys. 2008, 209, 1797–1805. [Google Scholar] [CrossRef]
- Simakova, A.; Averick, S.E.; Konkolewicz, D.; Matyjaszewski, K. Aqueous ARGET ATRP. Macromolecules 2012, 45, 6371–6379. [Google Scholar] [CrossRef]
- He, H.K.; Luebke, D.; Nuwala, H.; Matyjaszewski, K. Synthesis of poly(ionic liquid)s by atom transfer radical polymerization with ppm of Cu catalyst. Macromolecules 2014, 47, 6601–6609. [Google Scholar] [CrossRef]
- Wang, Y.; Lorandi, F.; Fantin, M.; Chmielarz, P.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Miniemulsion ARGET ATRP via interfacial and ion-pair catalysis: From ppm to ppb of residual copper. Macromolecules 2017, 50, 8417–8425. [Google Scholar] [CrossRef]
- Borsari, M.; Braidi, N.; Buffagni, M.; Ghelfi, F.; Parenti, F.; Porcelli, N.; Serafini, G.; Isse, A.A.; Bonifaci, L.; Cavalca, G.; et al. Copper-catalyzed ARGET ATRP of styrene from ethyl alpha-haloisobutyrate in EtOAc/EtOH, using ascorbic acid/Na2CO3 as reducing system. Eur. Polym. J. 2021, 157, 110675. [Google Scholar] [CrossRef]
- Chen, H.; Chen, L.F.; Wang, C.H.; Qu, R.J. Atom transfer radical polymerization using activators regenerated by electron transfer of acrylonitrile in 1-(1-ethoxycarbonylethyl)-3-methylimidazolium hexafluorophosphate. J. Polym. Sci. A Polym. Chem. 2011, 49, 1046–1049. [Google Scholar] [CrossRef]
- Chen, H.; Yang, L.X.; Liang, Y.; Hao, Z.H.; Lu, Z.X. ARGET ATRP of acrylonitrile catalyzed by FeCl3/isophthalic acid in the presence of air. J. Polym. Sci. A Polym. Chem. 2009, 47, 3202–3207. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.W.; Xu, X.; Xing, T.L.; Chen, G.Q. Structure and properties of silk grafted with 2-hydroxyethyl methacrylate by ARGET ATRP. AMR 2012, 441, 332–336. [Google Scholar]
- Forbes, D.C.; Creixell, M.; Frizzell, H.; Peppas, N.A. Polycationic nanoparticles synthesized using ARGET ATRP for drug delivery. Eur. J. Pharm. Biopharm. 2013, 84, 472–478. [Google Scholar] [CrossRef]
- Shao, M.L.; Yue, X.A.; Yue, T.Q.; He, J. Regulating gelation time and kinetics analysis based on the ARGET ATRP mechanism. J. Polym. Sci. 2020, 58, 519–527. [Google Scholar] [CrossRef]
- Zaborniak, I.; Surmacz, K.; Flejszar, M.; Chmielarz, P. Triple-functional riboflavin-based molecule for efficient atom transfer radical polymerization in miniemulsion media. J. Appl. Polym. Sci. 2020, 137, e49275. [Google Scholar] [CrossRef]
- Jakubowski, W.; Matyjaszewski, K. Activators regenerated by electron transfer for atom transfer radical polymerization of (meth)acrylates and related block copolymers. Angew. Chem. Int. Ed. 2006, 45, 4482–4486. [Google Scholar] [CrossRef] [PubMed]
- Karkare, P.; Kumar, S.; Murthy, C.N. ARGET-ATRP using beta-CD as reducing agent for the synthesis of PMMA-b-PS-b-PMMA triblock copolymers. J. Appl. Polym. Sci. 2019, 136, 47117. [Google Scholar] [CrossRef]
- Wei, Q.; Sun, M.; Lorandi, F.; Yin, R.; Yan, J.; Liu, T.; Kowalewski, T.; Matyjaszewski, K. Cu-catalyzed atom transfer radical polymerization in the presence of liquid metal micro/nanodroplets. Macromolecules 2021, 54, 1631–1638. [Google Scholar] [CrossRef]
- Chmielarz, P.; Krys, P.; Park, S.; Matyjaszewski, K. PEO-b-PNIPAM copolymers via SARA ATRP and eATRP in aqueous media. Polymer 2015, 71, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Fantin, M.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Understanding the fundamentals of aqueous ATRP and defining conditions for better control. Macromolecules 2015, 48, 6862–6875. [Google Scholar] [CrossRef]
- Flejszar, M.; Chmielarz, P.; Smenda, J.; Wolski, K. Following principles of green chemistry: Low ppm photo-ATRP of DMAEMA in water/ethanol mixture. Polymer 2021, 228, 123905. [Google Scholar] [CrossRef]
- Sun, Y.; Zhai, G.Q. CuSO4-catalyzed self-initiated radical polymerization of 2-(N,N-dimethylamino) ethyl methacrylate as an intrinsically reducing inimer. Chin. J. Polym. Sci. 2013, 31, 1161–1172. [Google Scholar] [CrossRef]
- Chmielarz, P.; Krys, P.; Wang, Z.; Wang, Y.; Matyjaszewski, K. Synthesis of well-defined polymer brushes from silicon wafers via surface-initiated seATRP. Macromol. Chem. Phys. 2017, 218, 1700106. [Google Scholar] [CrossRef]
- Morits, M.; Hynninen, V.; Nonappa; Niederberger, A.; Ikkala, O.; Gröschel, A.H.; Müllner, M. Polymer brush guided templating on well-defined rod-like cellulose nanocrystals. Polym. Chem. 2018, 9, 1650–1657. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, S.Q.; Qian, Y.H.; Zhao, W.F.; Zhao, C.S. Photo-responsive membrane surface: Switching from bactericidal to bacteria-resistant property. Mater. Sci. Eng. C 2018, 84, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Zhang, X.F.; Peng, S.Y.; Gu, H.W.; Zhang, L.J. Smart pH-sensitive micelles based on redox degradable polymers as DOX/GNPs carriers for controlled drug release and CT imaging. Colloids Surf. B Biointerfaces 2018, 163, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, X.; Zhang, K.; Wu, M.; Wu, Q.; Liu, J.; Yang, J.; Zhang, J. Nano-hydroxyapatite particle brushes via direct initiator tethering and surface-initiated atom transfer radical polymerization for dual responsive pickering emulsion. Langmuir 2020, 36, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Yan, Q.; Yuan, S.J.; Zhuang, X.D.; Zhang, F. Enhanced antifouling and anticorrosion properties of stainless steel by biomimetic anchoring PEGDMA-cross-linking polycationic brushes. Ind. Eng. Chem. Res. 2019, 58, 7107–7119. [Google Scholar] [CrossRef]
- Chmielarz, P.; Park, S.; Simakova, A.; Matyjaszewski, K. Electrochemically mediated ATRP of acrylamides in water. Polymer 2015, 60, 302–307. [Google Scholar] [CrossRef]
- Michieletto, A.; Lorandi, F.; De Bon, F.; Isse, A.A.; Gennaro, A. Biocompatible polymers via aqueous electrochemically mediated atom transfer radical polymerization. J. Polym. Sci. 2020, 58, 114–123. [Google Scholar] [CrossRef]
- Fawzy, A.; Zaafarany, I.A.; Khairou, K.S.; Almazroai, L.S.; Bawazeer, T.M.; Al-Jahdali, B.A. Oxidation of caffeine by permanganate Ion in perchloric and sulfuric acids solutions: A comparative kinetic study. Sci. J. Chem. 2016, 4, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Krys, P.; Matyjaszewski, K. Kinetics of atom transfer radical polymerization. Eur. Polym. J. 2017, 89, 482–523. [Google Scholar] [CrossRef] [Green Version]
- Fantin, M.; Chmielarz, P.; Wang, Y.; Lorandi, F.; Isse, A.A.; Gennaro, A.; Matyjaszewski, K. Harnessing the interaction between surfactant and hydrophilic catalyst to control eATRP in miniemulsion. Macromolecules 2017, 50, 3726–3732. [Google Scholar] [CrossRef]
- Ribelli, T.G.; Lorandi, F.; Fantin, M.; Matyjaszewski, K. Atom transfer radical polymerization: Billion times more active catalysts and new initiation systems. Macromol. Rapid Commun. 2019, 40, 1800616. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P.; Sobkowiak, A.; Matyjaszewski, K. A simplified electrochemically mediated ATRP synthesis of PEO-b-PMMA copolymers. Polymer 2015, 77, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Burton, I.W.; Farina, C.F.M.; Ragupathy, S.; Arunachalam, T.; Newmaster, S.; Berrue, F. Quantitative NMR methodology for the authentication of roasted coffee and prediction of blends. J. Agric. Food Chem. 2020, 68, 14643–14651. [Google Scholar] [CrossRef] [PubMed]
- Švorc, L.U.; Tomčík, P.; Svítková, J.; Rievaj, M.; Bustin, D. Voltammetric determination of caffeine in beverage samples on bare boron-doped diamond electrode. Food Chem. 2012, 135, 1198–1204. [Google Scholar] [CrossRef]
- Redivo, L.; Stredansky, M.; De Angelis, E.; Navarini, L.; Resmini, M.; Svorc, L. Bare carbon electrodes as simple and efficient sensors for the quantification of caffeine in commercial beverages. R. Soc. Open Sci. 2018, 5, 172146. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.H.; Matyjaszewski, K. Controlled/”living” radical polymerization. Atom transfer radical polymerization using multidentate amine ligands. Macromolecules 1997, 30, 7697–7700. [Google Scholar] [CrossRef]
- Chmielarz, P.; Sobkowiak, A. Synthesis of poly(butyl acrylate) using an electrochemically mediated atom transfer radical polymerization. Polimery 2016, 61, 585–590. [Google Scholar] [CrossRef]
- Noein, L.; Haddadi-Asl, V.; Salami-Kalajahi, M. Grafting of pH-sensitive poly (N,N-dimethylaminoethyl methacrylate-co-2-hydroxyethyl methacrylate) onto HNTS via surface-initiated atom transfer radical polymerization for controllable drug release. Int. J. Polym. Mater. Polym. Biomat. 2017, 66, 123–131. [Google Scholar] [CrossRef]
- Zhang, M.M.; Xiong, Q.Q.; Chen, J.Q.; Wang, Y.S.; Zhang, Q.Q. A novel cyclodextrin-containing pH-responsive star polymer for nanostructure fabrication and drug delivery. Polym. Chem. 2013, 4, 5086–5095. [Google Scholar] [CrossRef]
- Ding, A.S.; Xu, J.; Gu, G.X.; Lu, G.L.; Huang, X.Y. PHEA-g-PMMA well-defined graft copolymer: ATRP synthesis, self-assembly, and synchronous encapsulation of both hydrophobic and hydrophilic guest molecules. Sci. Rep. 2017, 7, 12601. [Google Scholar] [CrossRef] [Green Version]
- Monakhova, Y.B.; Ruge, W.; Kuballa, T.; Ilse, M.; Winkelmann, O.; Diehl, B.; Thomas, F.; Lachenmeier, D.W. Rapid approach to identify the presence of Arabica and Robusta species in coffee using H-1 NMR spectroscopy. Food Chem. 2015, 182, 178–184. [Google Scholar] [CrossRef] [PubMed]

| Entry 1 | Type of Coffee 2 | Conv 3 (%) | DPapp 3 | kpapp 3 (h−1) | Mn,th4 (×10−3) | Mn,app5 (×10−3) | Ð5 | f6 (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | pure water | 18.2 | 36 | 0.094 | 5.9 | 51.3 | 1.63 | 863 |
| 2 | Arabica & Robusta (50/50%) | 70.9 | 142 | 2.55 | 22.6 | 62.8 | 1.63 | 278 |
| 3 | Arabica (100%) | 46.6 | 93 | 1.28 | 14.9 | 45.1 | 1.88 | 302 |
| Entry 1 | Concentration of Coffee Extract 2 (%) | Conv 3 (%) | DPapp 3 | kpapp 3 (h−1) | Mn,th4 (×10−3) | Mn,app5 (×10−3) | Ð5 |
|---|---|---|---|---|---|---|---|
| 1 | 10 | 57.5 | 115 | 1.73 | 18.4 | 65.0 | 1.58 |
| 2 | 7.5 | 70.9 | 142 | 2.55 | 22.6 | 62.8 | 1.63 |
| 3 | 5 | 48.2 | 96 | 1.34 | 15.4 | 49.3 | 1.85 |
| Entry 1 | Monomer | t (h) | Conv 2 (%) | DPapp 3 | kpapp 2 (h−1) | Mn,th3 (×10−3) | Mn,app4 (×10−3) | Ð4 |
|---|---|---|---|---|---|---|---|---|
| 1 | DMAEMA | 0.5 | 70.9 | 142 | 2.55 | 22.6 | 62.8 | 1.63 |
| 2 | GMA | 1 | 79.1 | 158 | 1.38 | 22.7 | 69.5 | 1.78 |
| 3 | OEGMA | 2.33 | 16.5 | 33 | 0.08 | 16.7 | 18.5 | 1.28 |
| Coffee Solution | Caffeine Concentration (mM) | |
|---|---|---|
| DPV Analysis | Reference HPLC | |
| 7.5% Arabica | 4.47 (s *: 7.64 × 10−7) | 4.31 (s: 7.28 × 10−2) |
| 5% Arabica and Robusta (50/50%) | 4.65 (s: 1.20 × 10−6) | - |
| 7.5% Arabica and Robusta (50/50%) | 7.00 (s: 1.14 × 10−6) | 7.08 (s: 1.56 × 10−2) |
| 10% Arabica and Robusta (50/50%) | 7.23 (s: 1.35 × 10−6) | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surmacz, K.; Błoniarz, P.; Chmielarz, P. Coffee Beverage: A New Strategy for the Synthesis of Polymethacrylates via ATRP. Molecules 2022, 27, 840. https://doi.org/10.3390/molecules27030840
Surmacz K, Błoniarz P, Chmielarz P. Coffee Beverage: A New Strategy for the Synthesis of Polymethacrylates via ATRP. Molecules. 2022; 27(3):840. https://doi.org/10.3390/molecules27030840
Chicago/Turabian StyleSurmacz, Karolina, Paweł Błoniarz, and Paweł Chmielarz. 2022. "Coffee Beverage: A New Strategy for the Synthesis of Polymethacrylates via ATRP" Molecules 27, no. 3: 840. https://doi.org/10.3390/molecules27030840








