Synthesis, Characterization, Thermal Analysis, DFT, and Cytotoxicity of Palladium Complexes with Nitrogen-Donor Ligands
Abstract
:1. Introduction
2. Results
2.1. Characterization of Palladium Complexes
2.1.1. Spectral Data
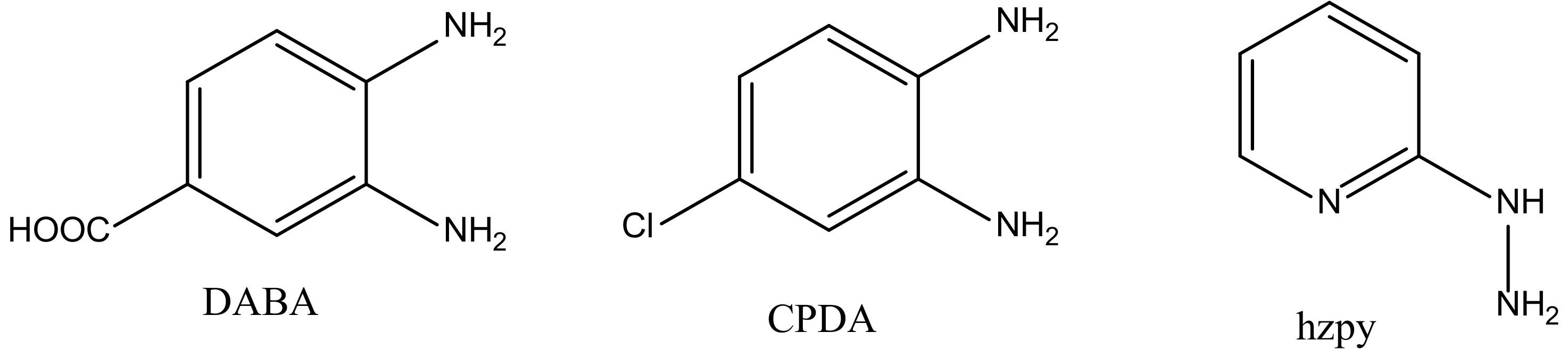
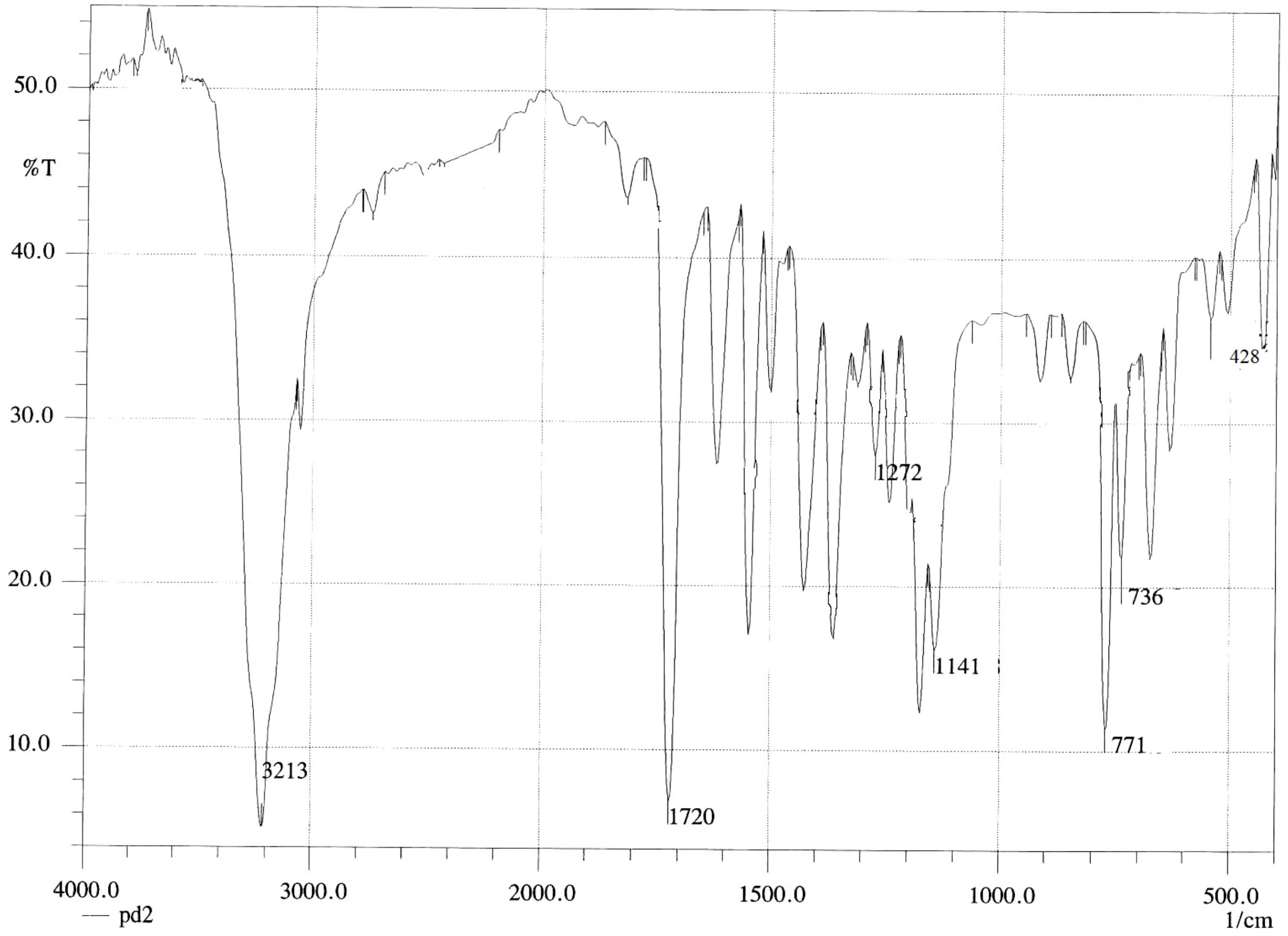
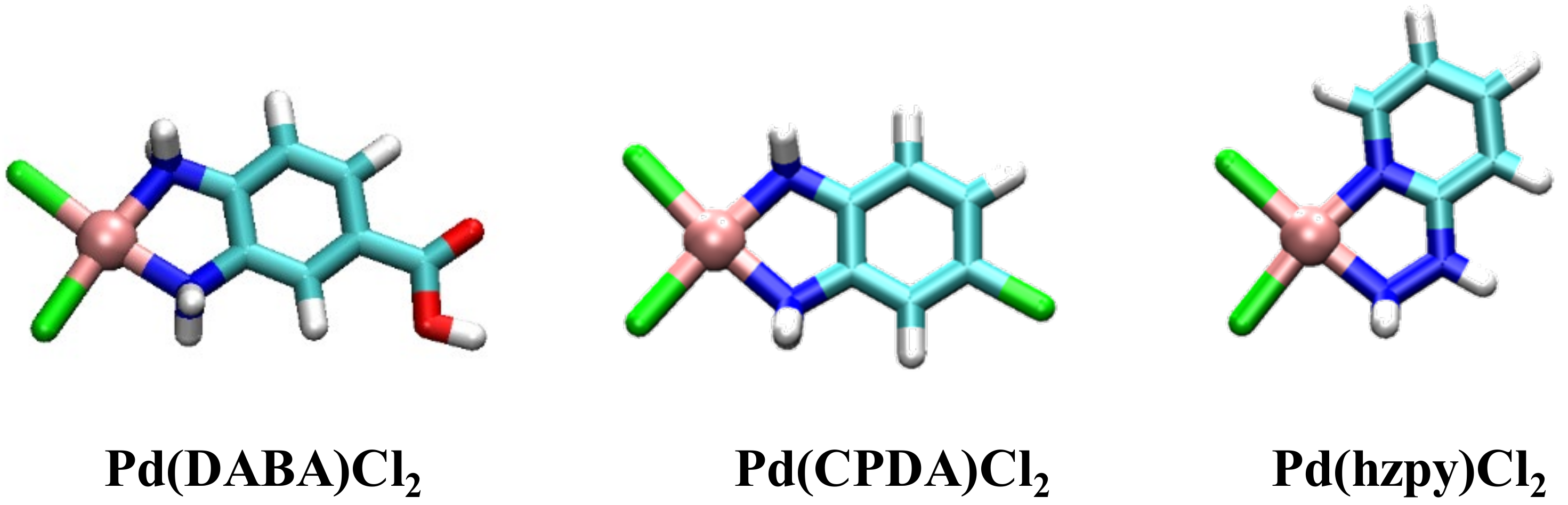
[Pd(DABA)Cl2] Complex
[Pd(CPDA)Cl2] Complex
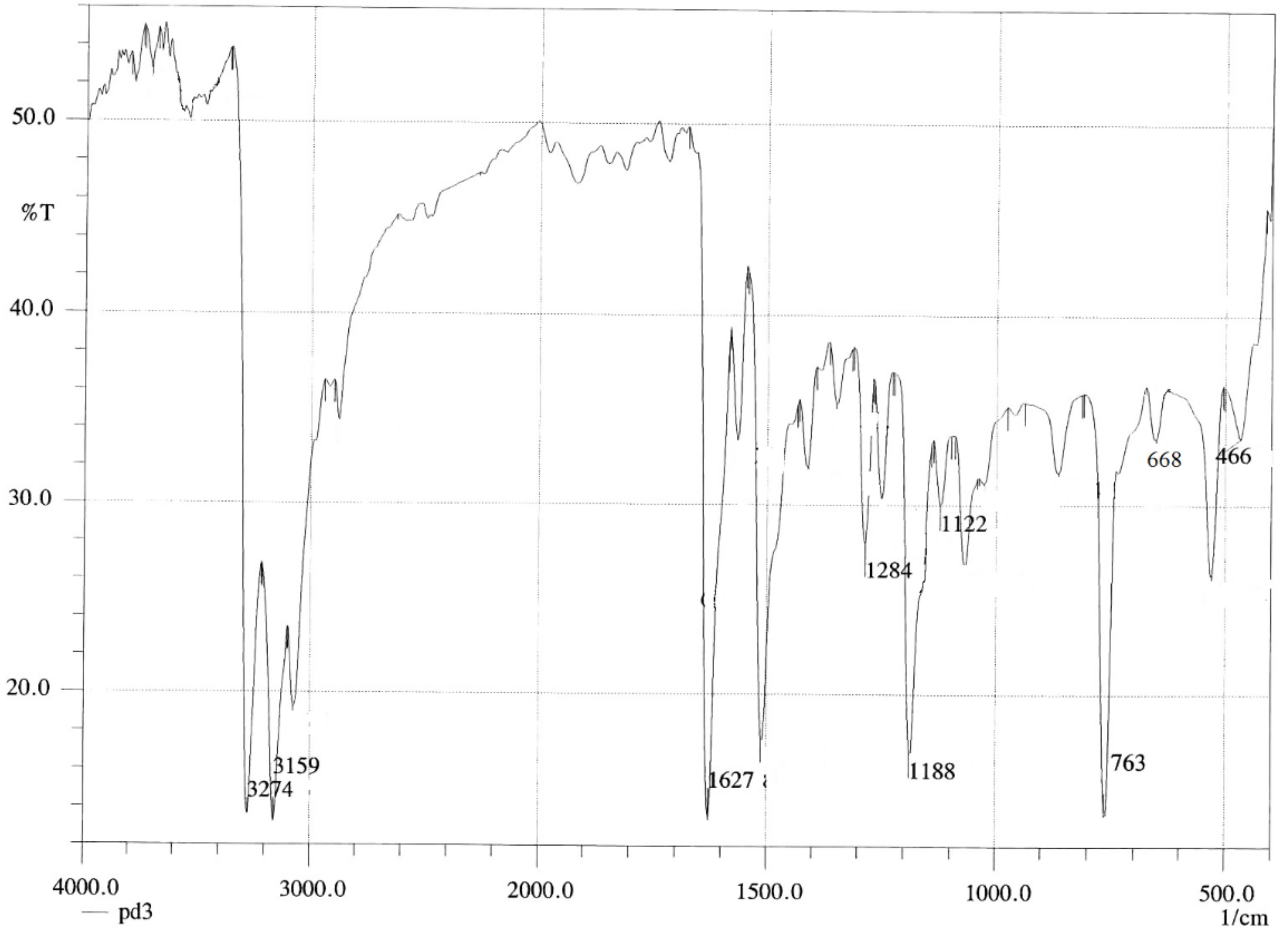
[Pd(hzpy)Cl2] Complex
2.1.2. 1HNMR Spectra
2.2. Thermal Studies
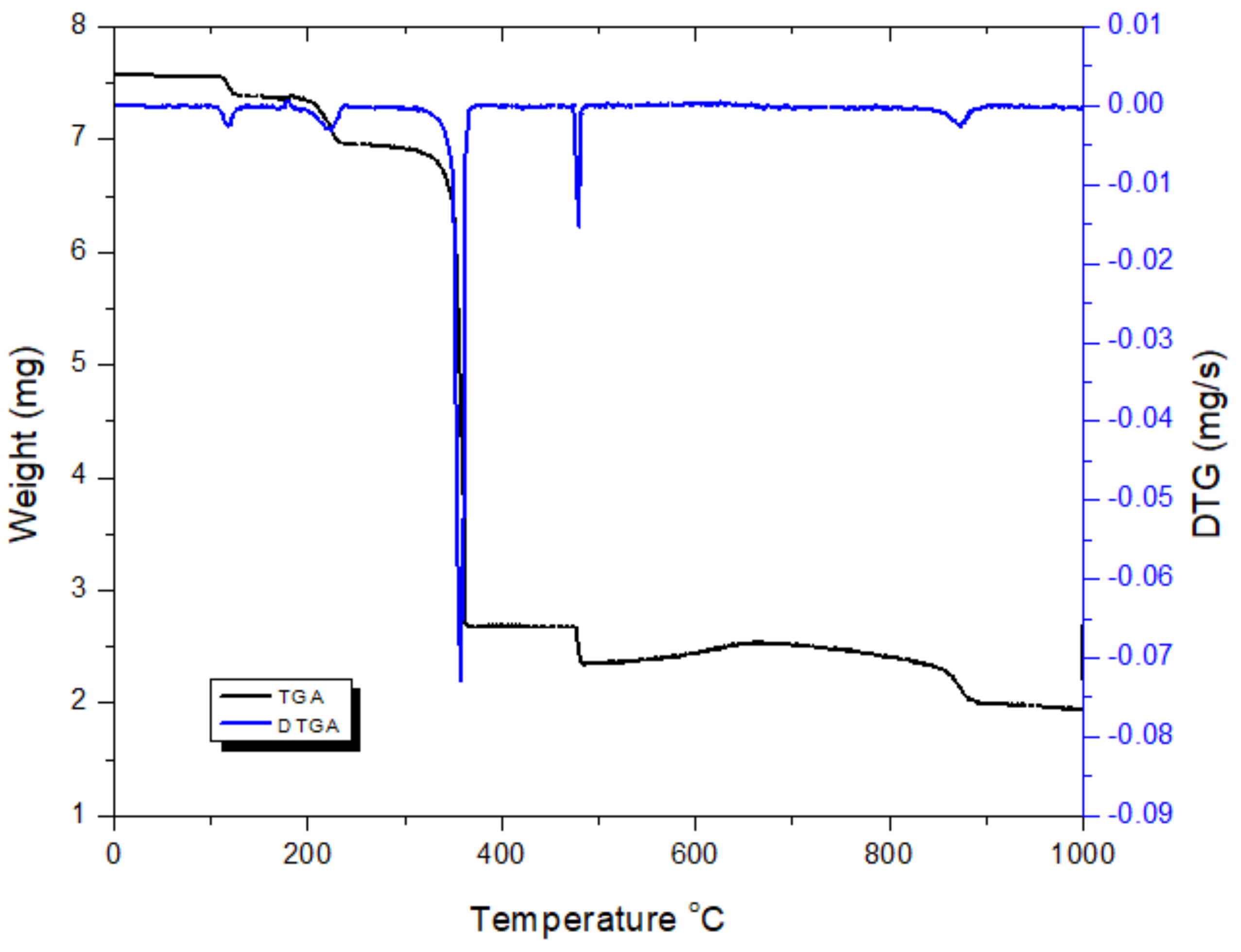
2.2.1. Thermal Analysis of the [Pd(DABA)Cl2] Complex
2.2.2. Thermal analysis of the [Pd(CPDA)Cl2] Complex
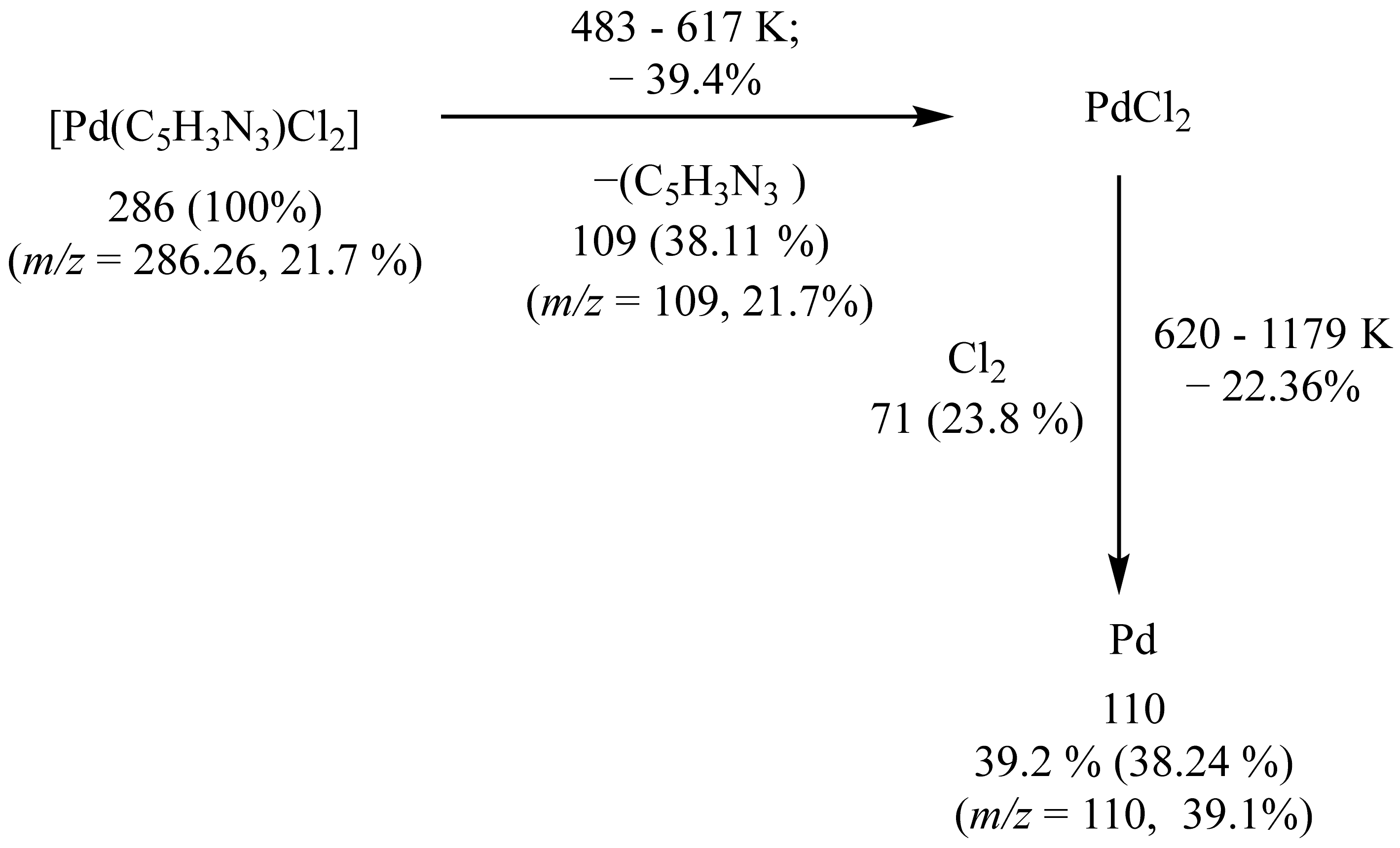
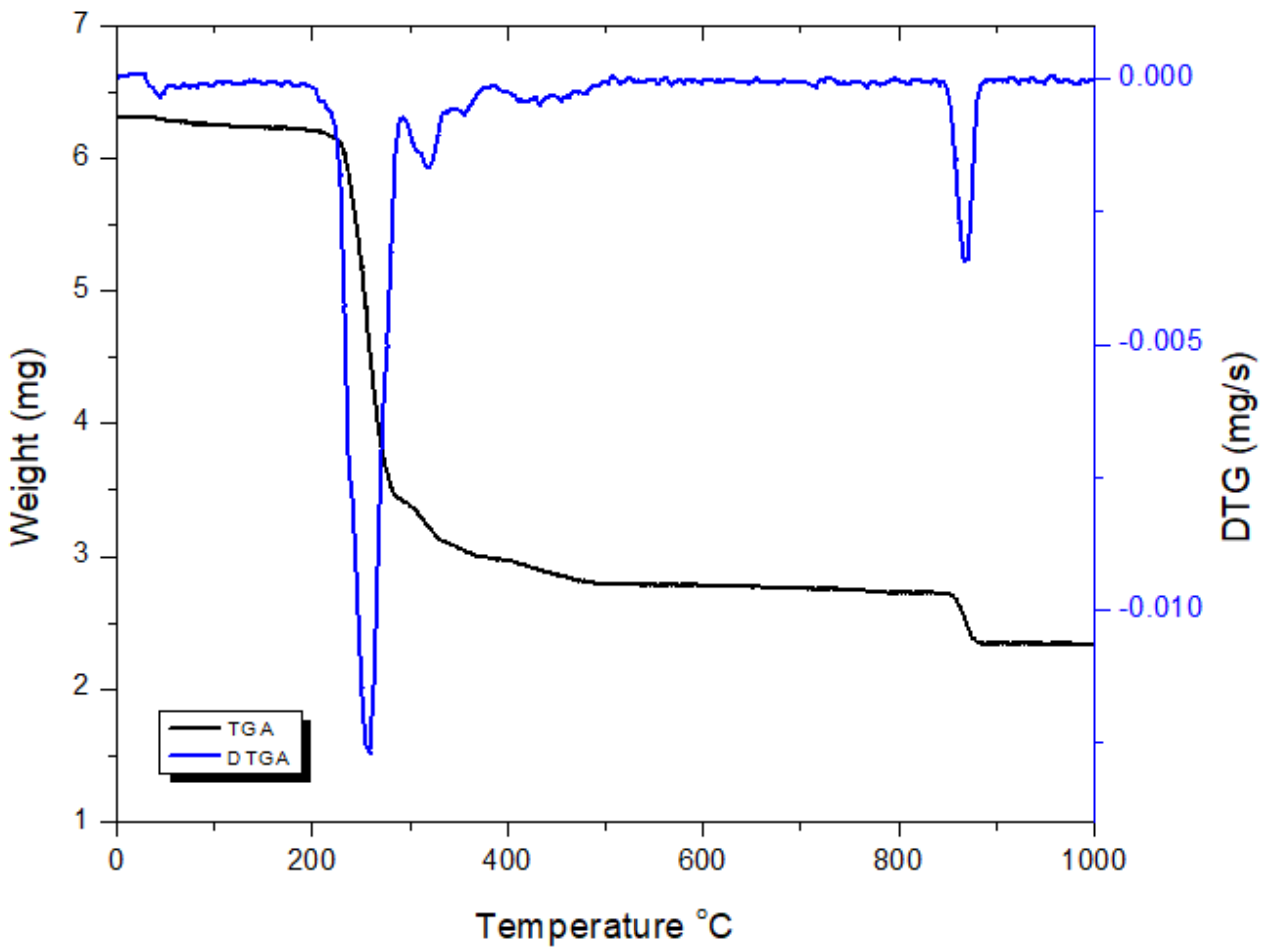
2.2.3. Thermal analysis of the [Pd(hzpy)Cl2] Complex
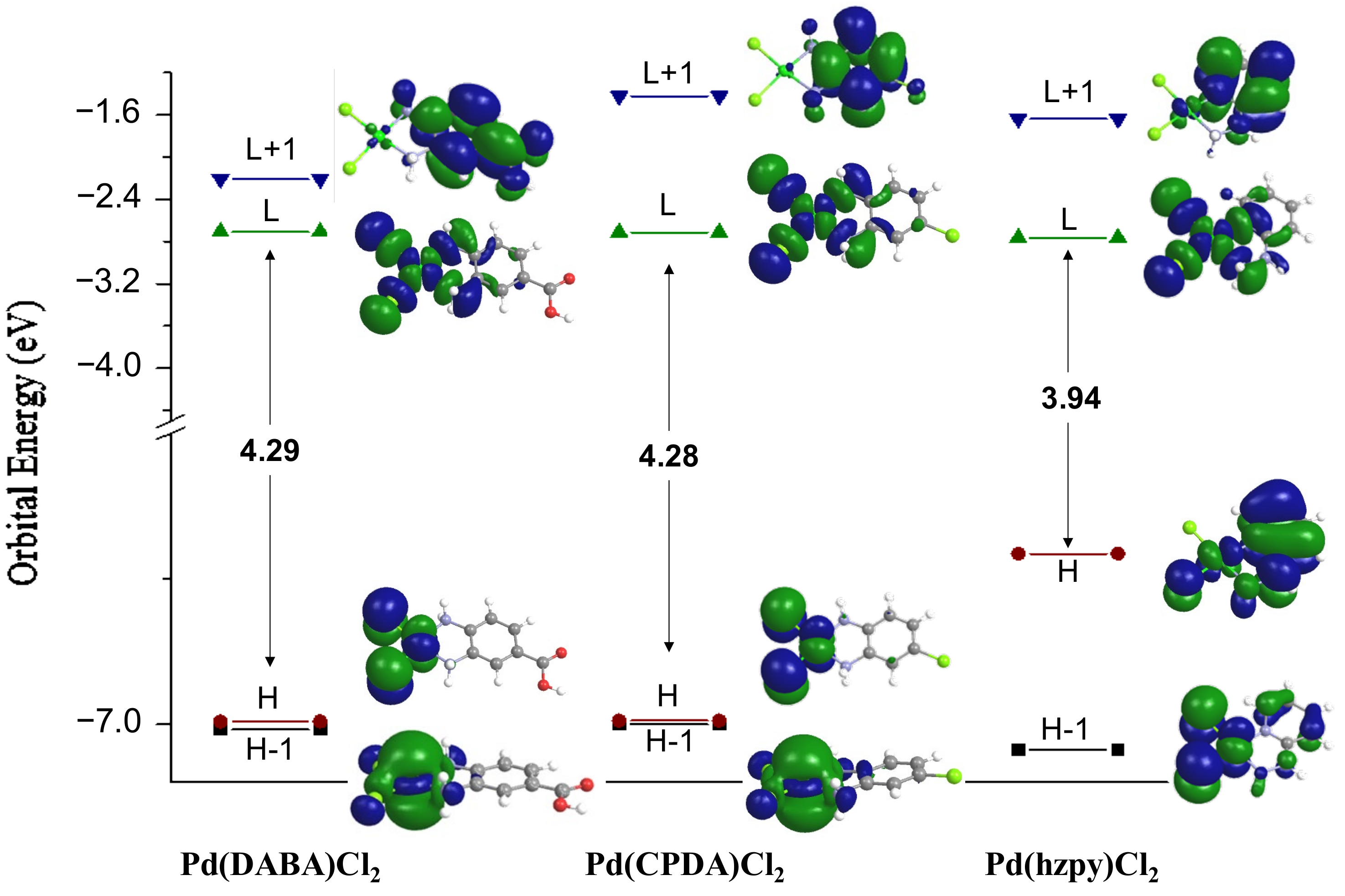
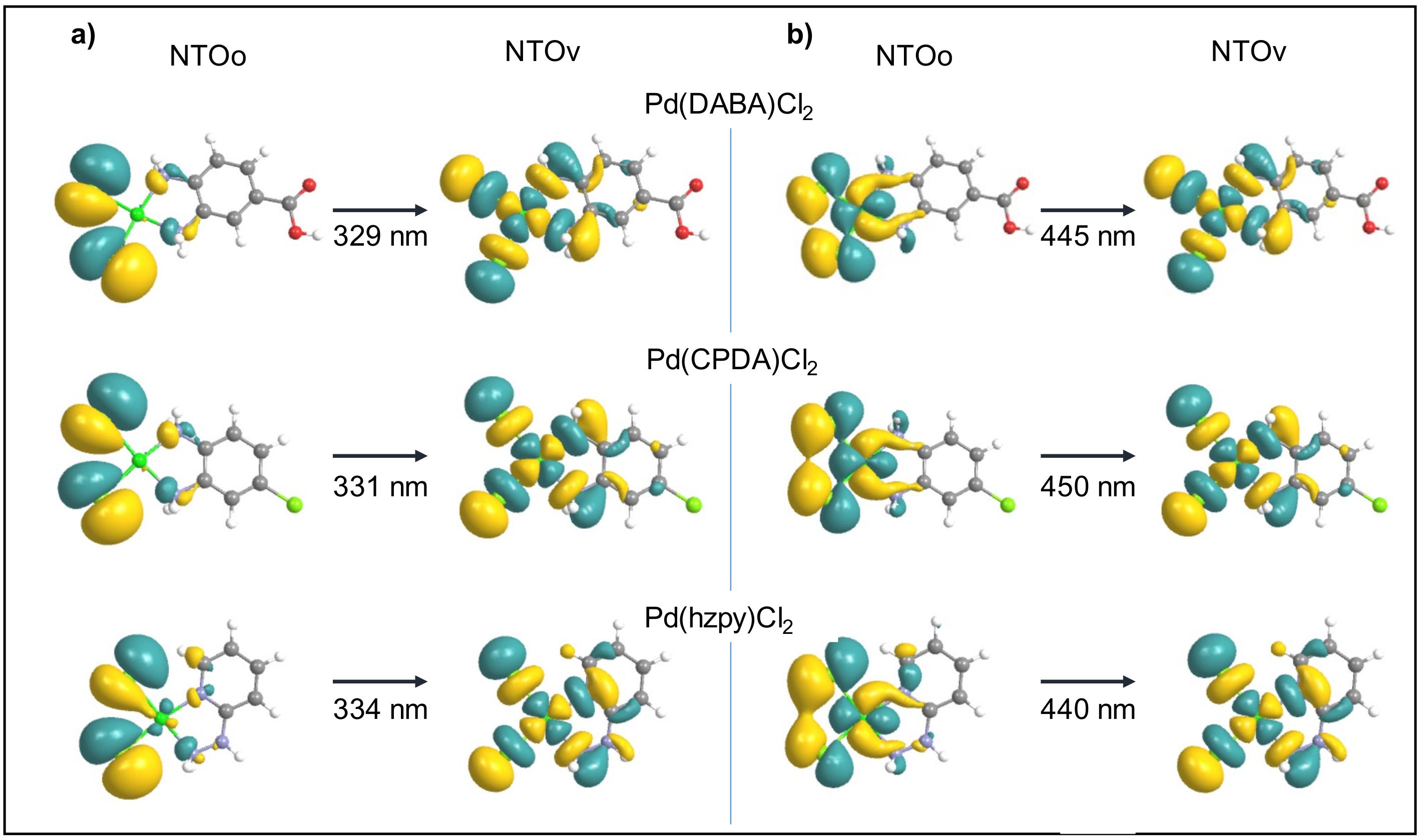
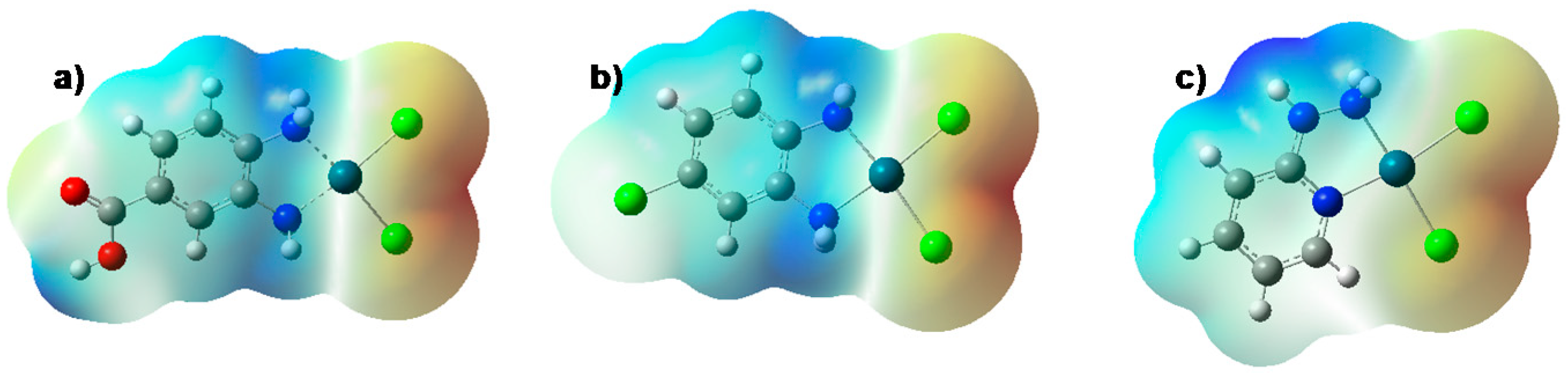
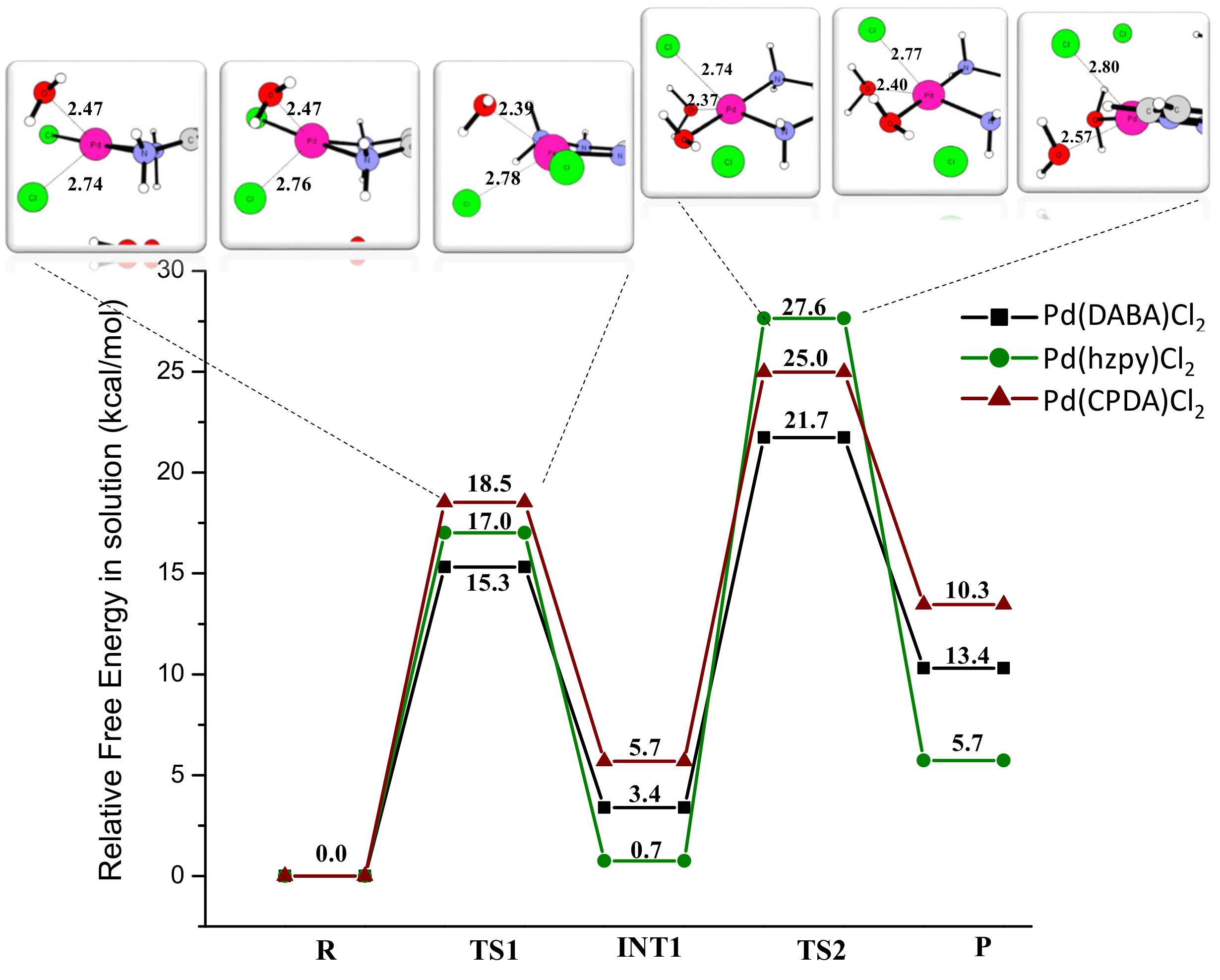
2.3. Theoretical Studies
2.4. The Antitumor Activities of the Pd Complexes
3. Materials and Methods
3.1. Materials
3.2. Physical Measurements
3.3. Computational Details
3.4. Antitumor Activity
3.5. Synthesis of the Palladium Complexes
3.5.1. Synthesis of [Pd(DABA)Cl2] Complex
3.5.2. Synthesis of [Pd(CPDA)Cl2] Complex
3.5.3. Synthesis of [Pd(hzpy)Cl2] Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sun, Y.-G.; Sun, D.; Yu, W.; Zhu, M.-C.; Ding, F.; Liu, Y.-N.; Gao, E.-J.; Wang, S.-J.; Xiong, G.; Dragutan, I.; et al. Synthesis, characterization, interaction with DNA and cytotoxicity of Pd(ii) and Pt(ii) complexes containing pyridine carboxylic acid ligands. Dalton Trans. 2013, 42, 3957–3967. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.L.; Bruice, P.Y.; Szabó, I.E.; Bruice, T.C. Incorporation of Positively Charged Linkages into DNA and RNA Backbones: A Novel Strategy for Antigene and Antisense Agents. Chem. Rev. 2012, 112, 1284–1309. [Google Scholar] [CrossRef] [PubMed]
- Eryazici, I.; Moorefield, C.N.; Newkome, G.R. Square-Planar Pd(II), Pt(II), and Au(III) Terpyridine Complexes: Their Syntheses, Physical Properties, Supramolecular Constructs, and Biomedical Activities. Chem. Rev. 2008, 108, 1834–1895. [Google Scholar] [CrossRef] [PubMed]
- Sigel, H. Metal Ions in Biological Systems: Volume 42: Metal Complexes in Tumor Diagnosis and as Anticancer Agents, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 143–177. [Google Scholar]
- Soliman, A.A.; Amin, M.A.; Sayed, A.M.; Abou-Hussein, A.A.A.; Linert, W. Cobalt and copper complexes with formamidine ligands: Synthesis, crystal X-ray study, DFT calculations and cytotoxicity. Polyhedron 2019, 161, 213–221. [Google Scholar] [CrossRef]
- Roque, J.A., III; Barrett, P.C.; Cole, H.D.; Lifshits, L.M.; Bradner, E.; Shi, G.; Von Dohlen, D.; Kim, S.; Russo, N.; Deep, G.; et al. Os(II) Oligothienyl Complexes as a Hypoxia-Active Photosensitizer Class for Photodynamic Therapy. Inorg. Chem. 2020, 59, 16341–16360. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.E.; Russo, N.; Adamo, C. Synergistic Effects of Metals in a Promising RuIIPtII Assembly for a Combined Anticancer Approach: Theoretical Exploration of the Photophysical Properties. Chem. Eur. J. 2016, 22, 9162–9168. [Google Scholar] [CrossRef] [Green Version]
- Roque, J.A., III; Barrett, P.C.; Cole, H.D.; Lifshits, L.M.; Shi, G.; Monro, S.; Von Dohlen, D.; Kim, S.; Russo, N.; Deep, G.; et al. Breaking the Barrier: An Osmium Photosensitizer with Unprecedented Hypoxic Phototoxicity for Real World Photodynamic Therapy. Chem. Sci. 2020, 11, 9784–9806. [Google Scholar] [CrossRef]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef]
- Jamieson, E.R.; Lippard, S.J. Structure, Recognition, and Processing of Cisplatin−DNA Adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef] [Green Version]
- Bruijnincx, P.C.; Sadler, P.J. New trends for metal complexes with anticancer activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carneiro, T.J.; Martins, A.S.; Marques, M.P.M.; Gil, A.M. Metabolic Aspects of Palladium(II) Potential Anti-Cancer Drugs. Front. Oncol. 2020, 10, 2218. [Google Scholar] [CrossRef] [PubMed]
- Abu-Surrah, A.S.; Kettunen, M. Platinum group antitumor chemistry: Design and development of new anticancer drugs complementary to cisplatin. Curr. Med. Chem. 2006, 13, 1337–1357. [Google Scholar] [CrossRef] [PubMed]
- Abu-Safieh, K.A.; Abu-Surrah, A.S.; Tabba, H.D.; AlMasri, H.A.; Bawadi, R.M.; Boudjelal, F.M.; Tahtamouni, L.H. Novel Palladium(II) and Platinum(II) Complexes with a Fluoropiperazinyl Based Ligand Exhibiting High Cytotoxicity and Anticancer Activity In Vitro. J. Chem. 2016, 2016, 7508724. [Google Scholar] [CrossRef] [Green Version]
- Lüköová, A.; Drweesh, E.A.; Volarevic, V.; Miloradovic, D.; Simovic Markovic, B.; Smolková, R.; Samoľová, E.; Kuchár, J.; Vilková, M.; Potočňák, I. Low-dimensional compounds containing bioactive ligands. Part XIII: Square planar anti-cancer Pd(II) complexes with halogenderivatives of 8-quinolinol and dimethylamine. Polyhedron 2020, 184, 114535. [Google Scholar] [CrossRef]
- Omondi, R.O.; Bellam, R.; Ojwach, S.O.; Jaganyi, D.; Fatokun, A.A. Palladium(II) complexes of tridentate bis(benzazole) ligands: Structural, substitution kinetics, DNA interactions and cytotoxicity studies. J. Inorg. Biochem. 2020, 210, 111156. [Google Scholar] [CrossRef] [PubMed]
- Małecki, J.G.; Maroń, A. Spectroscopic, structure and DFT studies of palladium(II) complexes with pyridine-type ligands. Transit. Met. Chem. 2011, 36, 297–305. [Google Scholar] [CrossRef] [Green Version]
- Najajreh, Y.; Perez, J.M.; Navarro-Ranninger, C.; Gibson, D. Novel Soluble Cationic trans-Diaminedichloroplatinum(II) Complexes that Are Active against Cisplatin Resistant Ovarian Cancer Cell Lines. J. Med. Chem. 2002, 45, 5189–5195. [Google Scholar] [CrossRef]
- Garoufis, A.; Hadjikakou, S.K.; Hadjiliadis, N. 46Pd The Use of Palladium Complexes in Medicine. In Metallotherapeutic Drugs and Metal-Based Diagnostic Agents; Wiley & Sons: Chichester, UK, 2005; pp. 399–419. [Google Scholar]
- Shoukry, A.; Rau, T.; Shoukry, M.; van Eldik, R. Kinetics and mechanisms of the ligand substitution reactions of bis(amine)(cyclobutane-1,1-dicarboxylato)palladium(II). J. Chem. Soc. Dalton Trans. 1998, 18, 3105–3112. [Google Scholar] [CrossRef]
- González, M.L.; Tercero, J.M.; Matilla, A.; Niclós-Gutiérrez, J.; Fernández, M.T.; López, M.C.; Alonso, C.; González, S. cis-Dichloro(α,ω-diamino carboxylate ethyl ester)palladium(II) as Palladium(II) versus Platinum(II) Model Anticancer Drugs: Synthesis, Solution Equilibria of Their Aqua, Hydroxo, and/or Chloro Species, and in Vitro/in Vivo DNA-Binding Properties. Inorg. Chem. 1997, 36, 1806–1812. [Google Scholar] [CrossRef]
- Soliman, A.A.; Khattab, M.M.; Linert, W. Kinetic and characterization studies of iron(II) and iron(III) complex formation reactions with hydrazinopyridine. J. Coord. Chem. 2008, 61, 2017–2031. [Google Scholar] [CrossRef]
- Nakamoto, K. Applications in Inorganic Chemistry. In Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 149–354. [Google Scholar]
- Samota, M.K.; Seth, G. Synthesis, characterization, and antimicrobial activi25 of palladium(II) and platinum(II) complexes with 2-substituted benzoxazole ligands. Heteroat. Chem. 2010, 21, 44–50. [Google Scholar]
- Horowitz, H.H.; Metzger, G. A New Analysis of Thermogravimetric Traces. Anal. Chem. 1963, 35, 1464–1468. [Google Scholar] [CrossRef]
- Nath, M.; Arora, P. Spectral and Thermal Studies of Cobalt(II), Nickel(II) and Copper(II) Complexes of Schiff Bases Obtained from o-Hydroxyacetophenone and Amino Acids. Synth. React. Inorg. Met.-Org. Chem. 1993, 23, 1523–1546. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Soliman, A.A.; El-Medani, S.M.; Ali, O.A.M. Thermalstudy of chromium and molybdenum complexes with some nitrogen and nitrogen-oxygendonors ligands. J. Therm. Anal. Calorim. 2006, 83, 385–392. [Google Scholar] [CrossRef]
- Soliman, A.A.; Taha, A.; Linert, W. Spectral and thermal study on the adduct formation between square planar nickel(II) chelates and some bidentate ligands. Spectrochim. Acta Part A 2006, 64, 1058–1064. [Google Scholar] [CrossRef]
- Dikmen, G.; Hür, D. Palladium (II) complex: Synthesis, spectroscopic studies and DFT calculations. Chem. Phys. Lett. 2019, 716, 49–60. [Google Scholar] [CrossRef]
- Alberto, M.E.; Butera, V.; Russo, N. Which One among the Pt-Containing Anticancer Drugs More Easily Forms Monoadducts with G and A DNA Bases? A Comparative Study among Oxaliplatin, Nedaplatin, and Carboplatin. Inorg. Chem. 2011, 50, 6965–6971. [Google Scholar] [CrossRef]
- Alberto, M.E.; Cosentino, C.; Russo, N. Hydrolysis mechanism of anticancer Pd(II) complexes with coumarin derivatives: A theoretical investigation. Struct. Chem. 2012, 23, 831–839. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Z.; You, X.-Z. Hydrolysis Theory for Cisplatin and Its Analogues Based on Density Functional Studies. J. Am. Chem. Soc. 2001, 123, 9378–9387. [Google Scholar] [CrossRef] [PubMed]
- Ponte, F.; Alberto, M.E.; De Simone, B.C.; Russo, N.; Sicilia, E. Photophysical Exploration of Dual-Approach PtII–BODIPY Conjugates: Theoretical Insights. Inorg. Chem. 2019, 58, 9882–9889. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Zhu, C.; Eriksson, L.A. Activation of anti-cancer drug cisplatin—Is the activated complex fully aquated? Mol. Phys. 2004, 102, 2537–2544. [Google Scholar] [CrossRef]
- Pavelka, M.; Lucas, M.F.A.; Russo, N. On the Hydrolysis Mechanism of the Second-Generation Anticancer Drug Carboplatin. Chem. Eur. J. 2007, 13, 10108–10116. [Google Scholar] [CrossRef]
- Alberto, M.E.; Lucas, M.F.A.; Pavelka, M.; Russo, N. The Second-Generation Anticancer Drug Nedaplatin: A Theoretical Investigation on the Hydrolysis Mechanism. J. Phys. Chem. B 2009, 113, 14473–14479. [Google Scholar] [CrossRef]
- Lucas, M.F.A.; Pavelka, M.; Alberto, M.E.; Russo, N. Neutral and Acidic Hydrolysis Reactions of the Third Generation Anticancer Drug Oxaliplatin. J. Phys. Chem. B 2009, 113, 831–838. [Google Scholar] [CrossRef]
- Alberto, M.E.; Adamo, C. Synergistic Effects in PtII–Porphyrinoid Dyes as Candidates for a Dual-Action Anticancer Therapy: A Theoretical Exploration. Chem. Eur. J. 2017, 23, 15124–15132. [Google Scholar] [CrossRef] [Green Version]
- Alberto, M.E.; Mazzone, G.; Regina, C.; Russo, N.; Sicilia, E. Two-components RuII-Porphirin dyes as promising assemblies for a combined antitumor effect: Theoretical elucidation of the photophysical properties. Dalton Trans. 2020, 49, 12653–12661. [Google Scholar] [CrossRef]
- Casida, M.E. Time-Dependent Density Functional Response Theory of Molecular Systems: Theory, Computational Methods, and Functionals. In Theoretical and Computational Chemistry; Seminario, J.M., Ed.; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1996; pp. 155–192. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V. Solvent effect on vertical electronic transitions by the polarizable continuum model. J. Chem. Phys. 2000, 112, 2427–2435. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Gomha, S.M.; Mahmmoud, E.A.; Elaasser, M.M. ChemInform Abstract: Synthesis and Anticancer Activities of Thiazoles, 1,3-Thiazines, and Thiazolidine Using Chitosan-Grafted-Poly(vinylpyridine) as Basic Catalyst. Heterocycles 2015, 46, 1227–1243. [Google Scholar] [CrossRef]




| Complex | Obs | Assignment | Complex | Obs | Assignment | Complex | Obs | Assignment |
|---|---|---|---|---|---|---|---|---|
| [Pd(DABA)Cl2] | 3213 | υ(NH2) | [Pd(CPDA)Cl2] | 3213 | υ(NH2) | [Pd(hzpy)Cl2] | 3274 | υ(NH2) |
| 1720 | υ(C=O) | 3143 | υ(NH2) | 3159 | υ(NH2) | |||
| 1242 | (ρt NH2) | 1265 | (ρt NH2) | 1284 | (ρt NH2) | |||
| 1172 | (ρwNH2) | 1145 | (ρwNH2) | 1188 | (ρwNH2) | |||
| 771 | (ρrNH2) | 779 | (ρrNH2) | 763 | (ρrNH2) | |||
| 428 | υ(M-N) | 428 | υ(M-N) | 466 | υ(M-N) |
| Complex | Molar Mass | m/z Values |
|---|---|---|
| [Pd(DABA)Cl2] | 329.48 | 328, 192, 150, 147, 109, 107, 106 |
| [Pd(CPDA)Cl2] | 319.91 | 320, 180, 141,110, 109, 106 |
| [Pd(hzpy)Cl2] | 286.46 | 286, 142, 109, 110, 109, 106, 104 |
| Complexes | TG Range (K) | DTAmax (K) | Mass Loss Found (calc. %) | Assignment of the Removed Species | Metallic Residue Found (calc.%) |
|---|---|---|---|---|---|
| [Pd(DABA)Cl2] | 477–642 | 631 | 59.8, (57.1) | C7H8N2O2, 1/2Cl2 | Pd |
| 708–1201 | 751 | 9.0, (10.7) | 1/2Cl2 | 31.2, (32.5) | |
| [Pd(CPDA)Cl2] | 550–682 | 572 | 55.21, (55.6) | (C6H7N2Cl), 1/2Cl2 | Pd |
| 1057–1224 | 1056 | 12.74, (11.76) | 1/2 Cl2 | 33.06, (33.28) | |
| [Pd(hzpy)Cl2] | 483–618 | 488 | 51.2, (53.9) | C5H7N3+1/2Cl2 | Pd |
| 620–1179 | 1141 | 10.6, (13.2) | Cl | 38.2, (38.4) |
| Complex | Decomposition Temperature (K) | ∆E/ KJ mol−1 | R2 | ∆S/ J K−1 mol−1 | ∆H/ KJ mol−1 | ∆G/ KJ mol−1 |
|---|---|---|---|---|---|---|
| [Pd(DABA)Cl2] | 477–642 708–1201 | 55 62 | 0.74 0.62 | −170 −228 | −51 −52 | 140 313 |
| 107 | −398 | 103 | 453 | |||
| [Pd(CPDA)Cl2] | 550–682 1057–1224 | 217 356 | 0.98 0.98 | 76 38.5 | 211 346 | 162 303 |
| 573 | 114.5 | 557 | 465 | |||
| [Pd(hzpy)Cl2] | 483–617 620–1179 | 43 537 | 0.86 0.98 | −177 599 | 39 531 | 128 153 |
| 580 | 422 | 570 | 281 |
| Complexes | PC3 | MCF7 | HEPG2 |
|---|---|---|---|
| Inhibition% | Inhibition% | Inhibition% | |
| [Pd(DABA)]Cl2 | 71 | 70 | 68 |
| [Pd(CPDA)Cl2] | 56 | 55 | 53 |
| [Pd(hzpy)Cl2] | 44 | 49 | 62 |
| Vinblastine Sulfate | 82 | 81 | 88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majeed, S.R.; Amin, M.A.; Attaby, F.A.; Alberto, M.E.; Soliman, A.A. Synthesis, Characterization, Thermal Analysis, DFT, and Cytotoxicity of Palladium Complexes with Nitrogen-Donor Ligands. Molecules 2022, 27, 964. https://doi.org/10.3390/molecules27030964
Majeed SR, Amin MA, Attaby FA, Alberto ME, Soliman AA. Synthesis, Characterization, Thermal Analysis, DFT, and Cytotoxicity of Palladium Complexes with Nitrogen-Donor Ligands. Molecules. 2022; 27(3):964. https://doi.org/10.3390/molecules27030964
Chicago/Turabian StyleMajeed, Sattar R., Mina A. Amin, Fawzy A. Attaby, Marta E. Alberto, and Ahmed A. Soliman. 2022. "Synthesis, Characterization, Thermal Analysis, DFT, and Cytotoxicity of Palladium Complexes with Nitrogen-Donor Ligands" Molecules 27, no. 3: 964. https://doi.org/10.3390/molecules27030964







