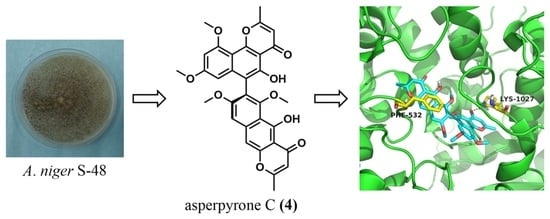
Naphtho-Gamma-Pyrones (NγPs) with Obvious Cholesterol Absorption Inhibitory Activity from the Marine-Derived Fungus Aspergillus niger S-48
Abstract
:1. Introduction
2. Experimental Section
2.1. General Experimental Procedures
2.2. Strain and Culturing Conditions
2.3. Extraction and Purification
2.4. Structural Modification
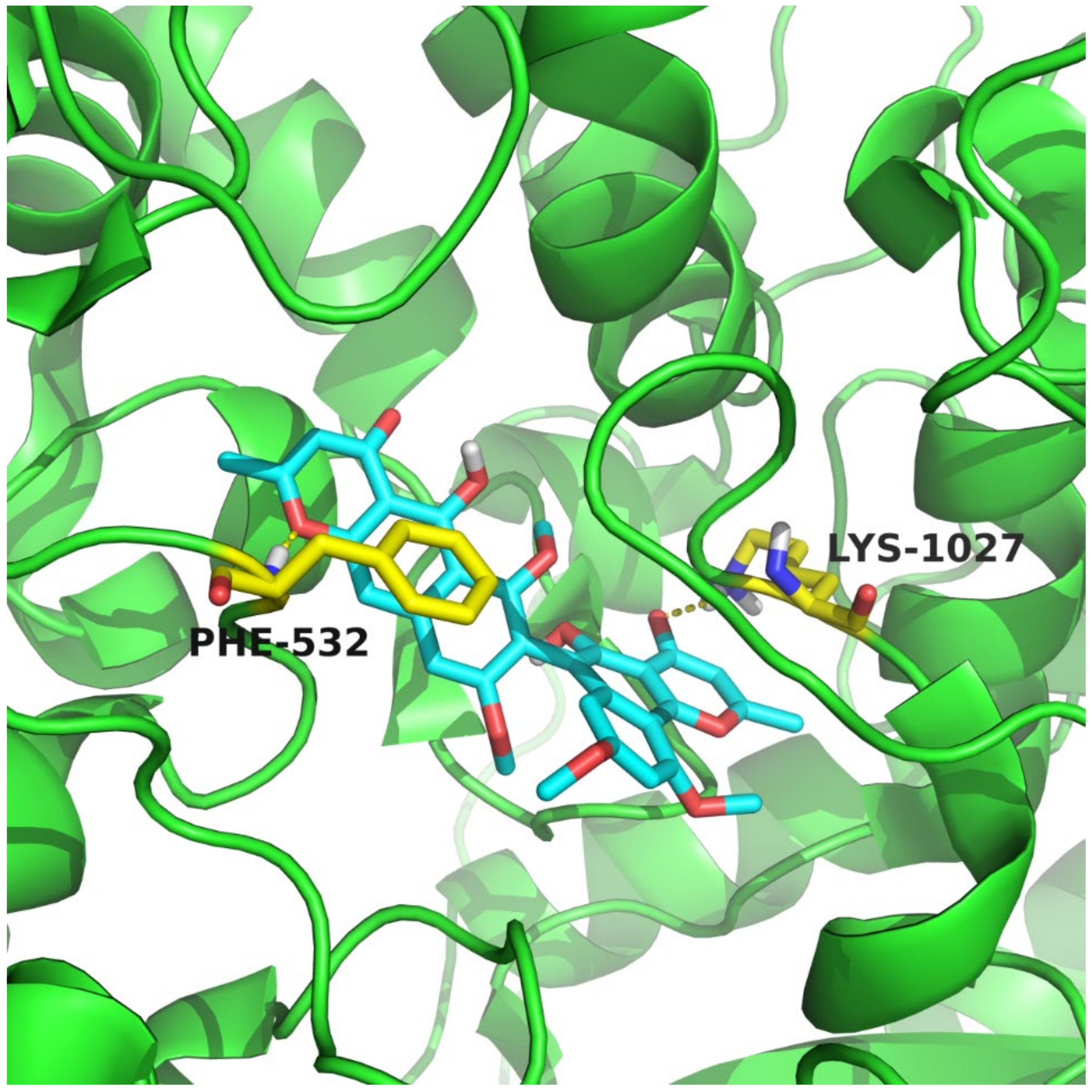
2.5. Molecular Docking Methods
2.6. Inhibition of Cholesterol Absorption
2.7. Antimicrobial Activities
3. Results and Discussion
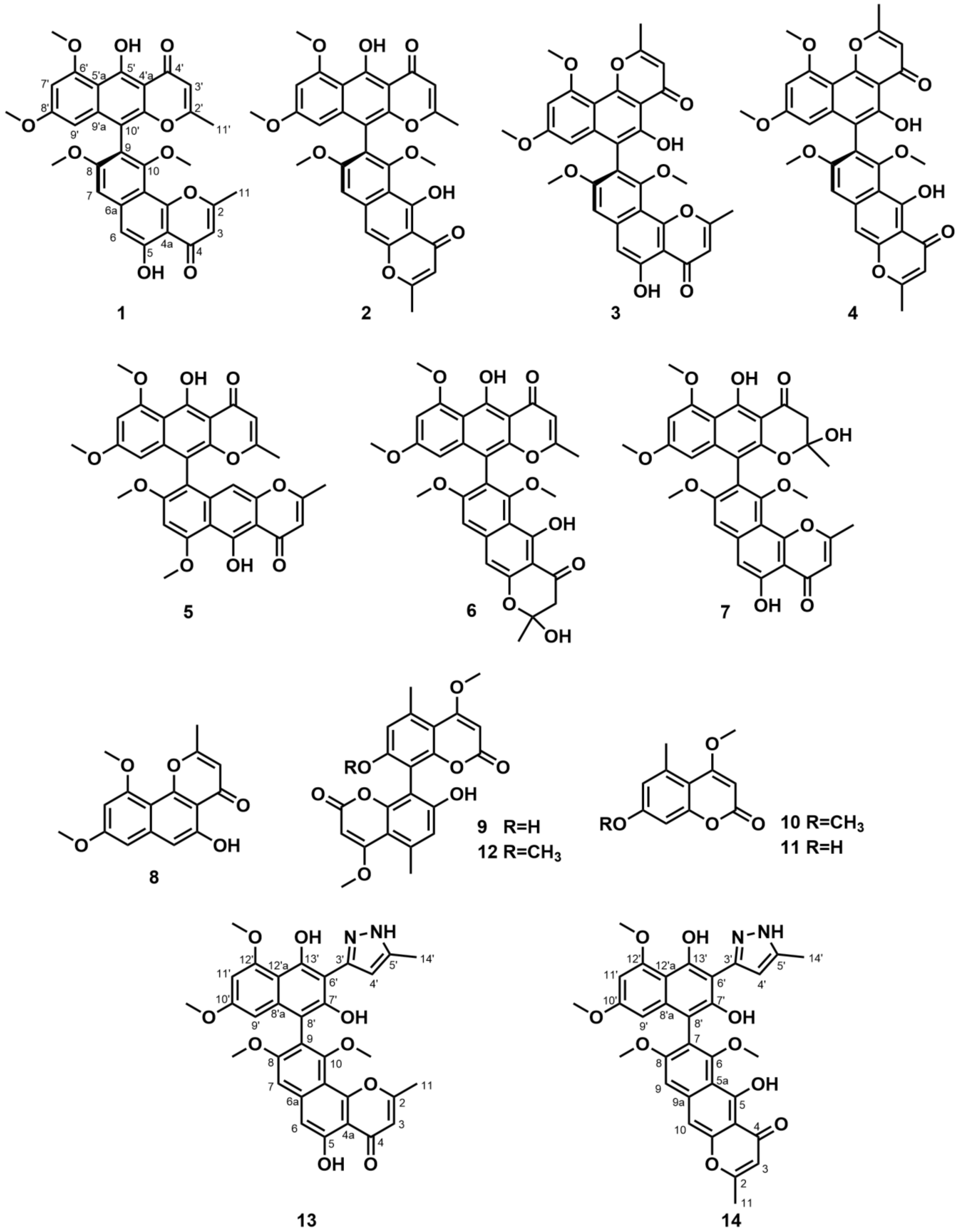
3.1. Identification of Metabolites
3.2. Modification and Identification of Semisynthetic NγPs
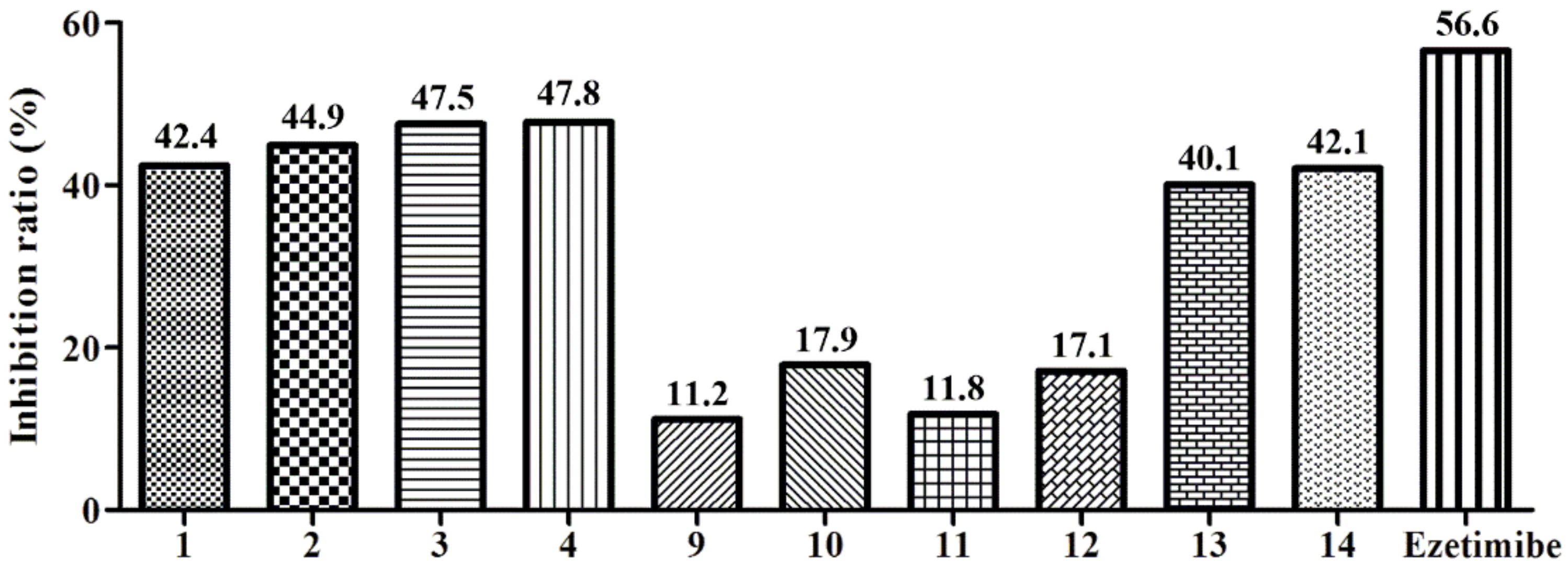
3.3. Biological Activities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sutak, R.; Camadro, J.M.; Lesuisse, E. Iron uptake mechanisms in marine phytoplankton. Front. Microbiol. 2020, 11, 566691. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.M.; Ju, C.X.; Li, G.; Sun, Y.; Peng, Y.; Li, Y.X.; Peng, X.P.; Lou, H.X. Dimeric 1,4-benzoquinone derivatives with cytotoxic activities from the marine-derived fungus Penicillium sp. L129. Mar. Drugs 2019, 17, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anh, C.V.; Kang, J.S.; Choi, B.-K.; Lee, H.-S.; Heo, C.-S.; Shin, H.J. Polyketides and meroterpenes from the marine-derived fungi Aspergillus unguis 158SC-067 and A. flocculosus 01NT-1.1.5 and their cytotoxic and antioxidant activities. Mar. Drugs 2021, 19, 415. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, M.J.; Li, H.; Zhang, P.; Bao, B.; Lee, K.J.; Jung, J.H. Marine-derived Aspergillus species as a source of bioactive secondary metabolites. Mar. Biotechnol. 2013, 15, 499–519. [Google Scholar] [CrossRef]
- Lund, N.A.; Robertson, A.; Whalley, W.B. The chemistry of fungi. Part XXI.* asperxanthone and a preliminary examination of aspergillin. J. Chem. Soc. 1953, 3, 2434–2439. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Mogensen, J.M.; Johansen, M.; Larsen, T.O.; Frisvad, J.C. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 2009, 395, 1225–1242. [Google Scholar] [CrossRef]
- Carboue, Q.; Maresca, M.; Herbette, G.; Roussos, S.; Hamrouni, R.; Bombarda, I. Naphtho-gamma-pyrones produced by Aspergillus tubingensis G131: New source of natural nontoxic antioxidants. Biomolecules 2020, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Yu, H.; Yu, Y.; Li, Q.; Chen, B.; Huang, Y.; Zou, X.; Huang, B.; Tang, J. Separation of five naphtho-gamma-pyrones from pleurotus ostreatus by high-speed countercurrent chromatography. J. Sep. Sci. 2018, 41, 4551–4558. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Gao, Y.Q.; Shi, X.W.; Gao, J.M. Antifungal and antibacterial metabolites from an endophytic Aspergillus sp. associated with Melia azedarach. Nat. Prod. Res. 2014, 28, 1388–1392. [Google Scholar] [CrossRef]
- Zhang, R.; Song, Z.; Wang, X.; Xue, J.; Xing, D. One-step modification to identify dual-inhibitors targeting both pancreatic triglyceride lipase and Niemann-Pick C1-like 1. Eur. J. Med. Chem. 2021, 216, 113358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, W.; Zeng, J.; Meng, J.; Jiang, H.; Wang, J.; Xing, D. Niemann-Pick C1-Like 1 inhibitors for reducing cholesterol absorption. Eur. J. Med. Chem. 2022, 230, 114111. [Google Scholar] [CrossRef] [PubMed]
- Altmann, S.W.; Davis, H.R.; Zhu, L.; Yao, X.; Hoos, L.M.; Tetzloff, G.; Iyer, S.N.; Maguire, M.; Golovko, A.; Zeng, M.; et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science 2004, 303, 1201–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wang, N.; Wang, X.; Hu, J.; Wang, S. Characterization of two anti-fungal lipopeptides produced by Bacillus amyloliquefaciens SH-B10. Bioresour. Technol. 2010, 101, 8822–8827. [Google Scholar] [CrossRef]
- Hua, Y.; Pan, R.; Bai, X.; Wei, B.; Chen, J.; Wang, H.; Zhang, H. Aromatic polyketides from a symbiotic strain Aspergillus fumigatus D and characterization of their biosynthetic gene D8.t287. Mar. Drugs 2020, 18, 324. [Google Scholar] [CrossRef]
- Han, J.; Yang, N.; Wei, S.; Jia, J.; Lin, R.; Li, J.; Bi, H.; Song, F.; Xu, X. Dimeric hexylitaconic acids from the marine-derived fungus Aspergillus welwitschiae CUGBMF180262. Nat. Prod. Res. 2020, 36, 578–585. [Google Scholar] [CrossRef]
- Huang, H.B.; Feng, X.J.; Liu, L.; Chen, B.; Lu, Y.J.; Ma, L.; She, Z.G.; Lin, Y.C. Three dimeric naphtho-γ-pyrones from the mangrove endophytic fungus Aspergillus tubingensis isolated from Pongamia pinnata. Planta Med. 2010, 76, 1888–1891. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Tian, J.; Chen, X.; Sun, W.; Zhu, H.; Li, Q.; Lei, L.; Yao, G.; Xue, Y.; Wang, J.; et al. Fungal naphtho-γ-pyrones: Potent antibiotics for drug-resistant microbial pathogens. Sci. Rep. 2016, 6, 24291. [Google Scholar] [CrossRef]
- Kouipou Toghueo, R.M.; Kemgne, E.A.M.; Sahal, D.; Yadav, M.; Kenou Kagho, D.U.; Yang, B.; Baker, B.J.; Boyom, F.F. Specialized antiplasmodial secondary metabolites from Aspergillus niger 58, an endophytic fungus from Terminalia catappa. J. Ethnopharmacol. 2021, 269, 113672. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.F.; Zhao, Y.L.; Liu, K.C.; Wang, X.M.; Yang, Y.P.; Li, X.L. Chemical constituents from Clematis delavayi var. spinescens. Molecules 2009, 14, 4433–4439. [Google Scholar] [CrossRef]
- Ur Rehman, N.; Halim, S.A.; Khan, M.; Hussain, H.; Yar Khan, H.; Khan, A.; Abbas, G.; Rafiq, K.; Al-Harrasi, A. Antiproliferative and carbonic anhydrase II inhibitory potential of chemical constituents from Lycium shawii and Aloe vera: Evidence from in silico target fishing and in vitro testing. Pharmaceuticals 2020, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, C.L.M.; Lacey, H.J.; Vuong, D.; Pitt, J.I.; Lange, L.; Lacey, E.; Pilgaard, B.; Chooi, Y.H.; Piggott, A.M. Comprehensive chemotaxonomic and genomic profiling of a biosynthetically talented Australian fungus, Aspergillus burnettii sp. nov. Fungal Genet. Biol. 2020, 143, 103435. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Tian, J.; Sun, W.; Meng, J.; Wang, X.; Fu, X.; Wang, A.; Lai, D.; Liu, Y.; Zhou, L. Bis-naphtho-γ-pyrones from fungi and their bioactivities. Molecules 2014, 19, 7169–7188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Wang, A.; Xu, D.; Wang, W.; Meng, J.; Dai, J.; Liu, Y.; Lai, D.; Zhou, L. New ustilaginoidins from rice false smut balls caused by Villosiclava virens and their phytotoxic and cytotoxic activities. J. Agric. Food Chem. 2017, 65, 5151–5160. [Google Scholar] [CrossRef]
- Solis, C.M.; Salazar, M.O.; Ramallo, I.A.; Garcia, P.; Furlan, R.L.E. A tyrosinase inhibitor from a nitrogen-enriched chemically engineered extract. ACS Comb. Sci. 2019, 21, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Thilavech, T.; Adisakwattana, S. Cyanidin-3-rutinoside acts as a natural inhibitor of intestinal lipid digestion and absorption. BMC Complem. Altern. Med. 2019, 19, 242. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-Z.; Peng, X.-P.; Li, G.; Wang, Q.; Lou, H.-X. Naphtho-Gamma-Pyrones (NγPs) with Obvious Cholesterol Absorption Inhibitory Activity from the Marine-Derived Fungus Aspergillus niger S-48. Molecules 2022, 27, 2514. https://doi.org/10.3390/molecules27082514
Wu C-Z, Peng X-P, Li G, Wang Q, Lou H-X. Naphtho-Gamma-Pyrones (NγPs) with Obvious Cholesterol Absorption Inhibitory Activity from the Marine-Derived Fungus Aspergillus niger S-48. Molecules. 2022; 27(8):2514. https://doi.org/10.3390/molecules27082514
Chicago/Turabian StyleWu, Chang-Zheng, Xiao-Ping Peng, Gang Li, Qi Wang, and Hong-Xiang Lou. 2022. "Naphtho-Gamma-Pyrones (NγPs) with Obvious Cholesterol Absorption Inhibitory Activity from the Marine-Derived Fungus Aspergillus niger S-48" Molecules 27, no. 8: 2514. https://doi.org/10.3390/molecules27082514