Responses of Issatchenkia terricola WJL-G4 upon Citric Acid Stress
Abstract
:1. Introduction
2. Results
2.1. Effect of Citric Acid on Cell Structure by TEM and SEM Observation
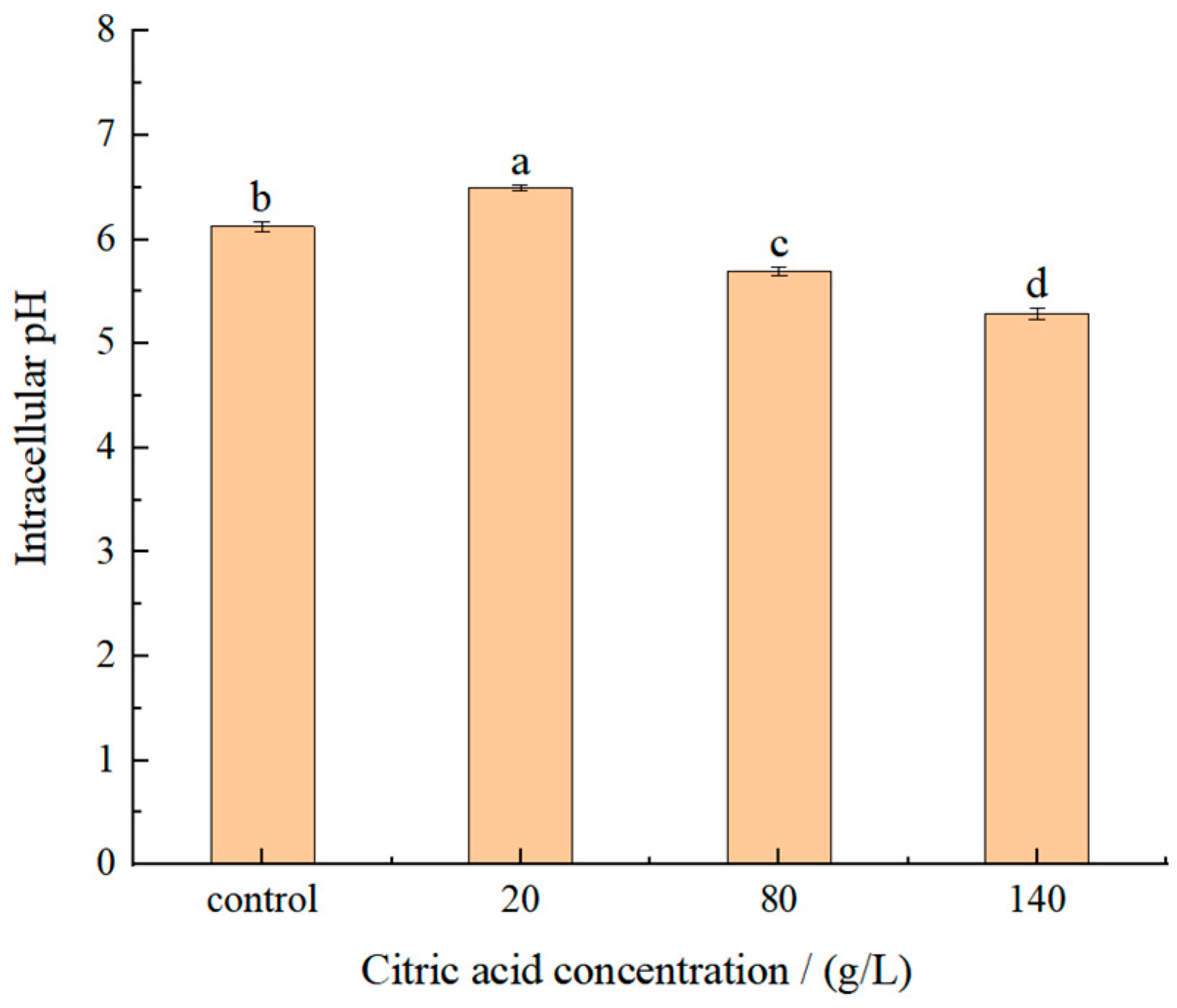
2.2. Effect of Citric Acid on Intracellular pH
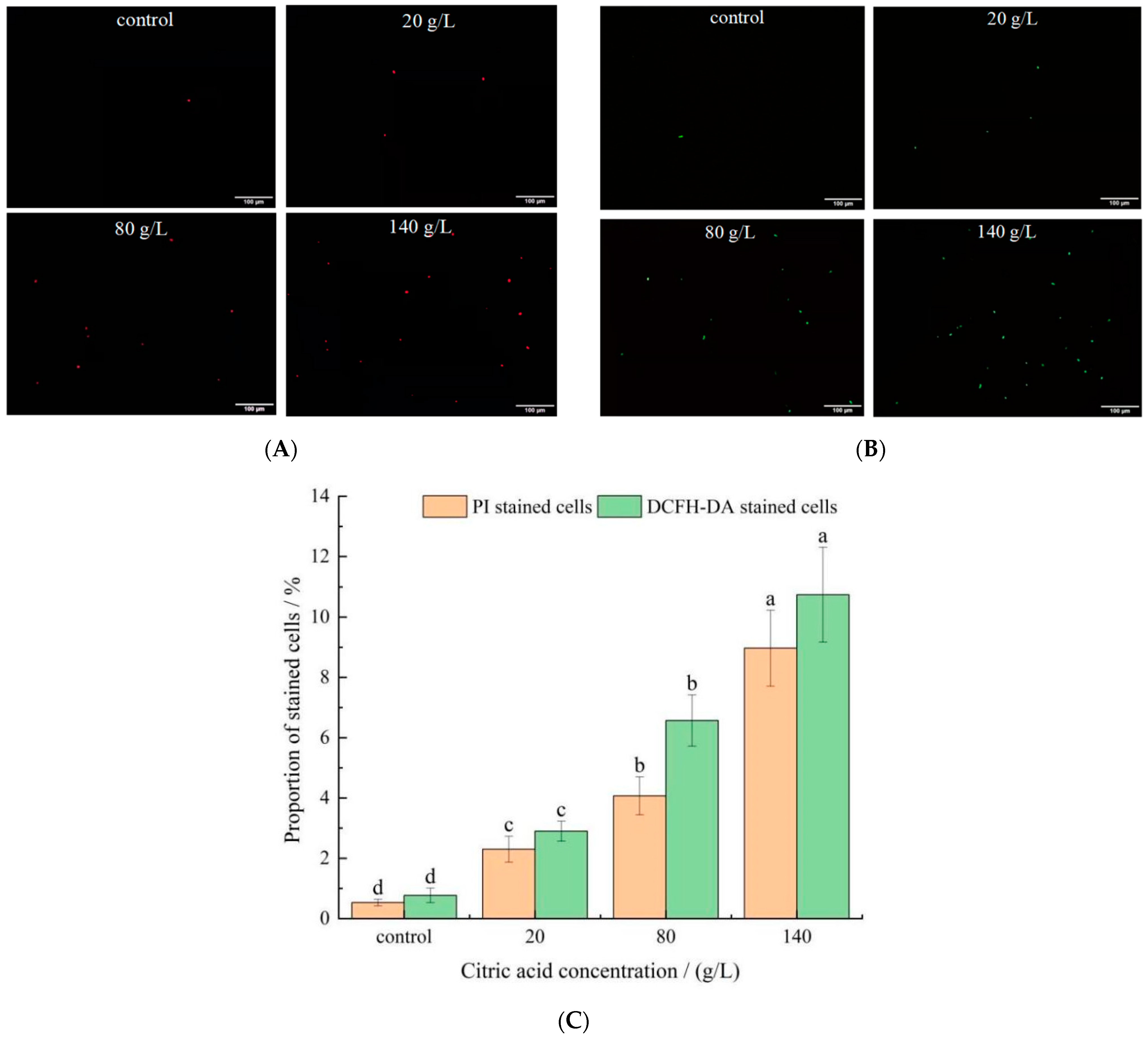
2.3. Effect of Citric Acid on Cell Membrane Permeability
2.4. Effect of Citric Acid on Membrane Fatty Acids Composition by GC-MS Analysis
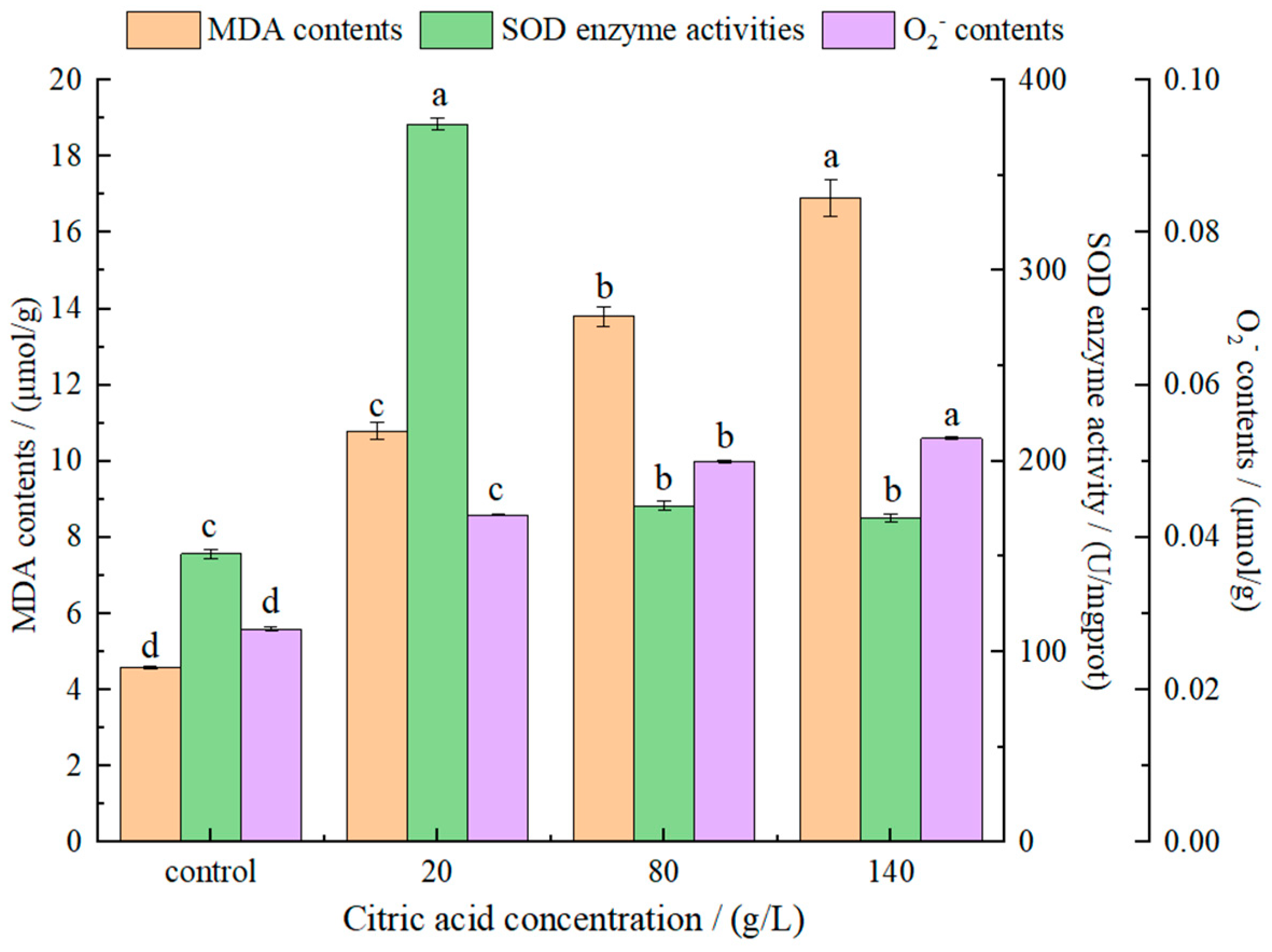
2.5. Effect of Citric Acid on Antioxidative Enzyme System
3. Discussion
3.1. Intracellular pH as Stress Homeostasis Indicator for Yeast
3.2. Impact of USFAs Levels on Stress Tolerance of Yeast
3.3. Yeast Stress Response Is Affected by SOD Levels
4. Materials and Methods
4.1. Yeast Cultivation
4.2. Transmission Electron Microscopy (TEM) Observation
4.3. Scanning Electron Microscope (SEM) Observation
4.4. Measurement of Intracellular pH
4.5. Measurement of Cell Membrane Permeability
4.5.1. PI Fluorescent Staining Method
4.5.2. DCFH-DA Fluorescent Staining Method
4.6. Membrane Fatty Acids Analysis
4.7. Antioxidant System Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carmelo, V.; Bogaerts, P.; Sa-Correia, I. Activity of plasma membrane H+-ATPase and expression of PMA1 and PMA2 genes in Saccharomyces cerevisiae cells grown at optimal and low pH. Arch. Microbiol. 1996, 166, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Lu, L.; Duan, C.Q.; Yan, G.L. The contribution of indigenous non-Saccharomyces wine yeast to improved aromatic quality of Cabernet Sauvignon wines by spontaneous fermentation. LWT 2016, 71, 356–363. [Google Scholar] [CrossRef]
- de Nadal, E.; Ammerer, G.; Posas, F. Controlling gene expression in response to stress. Nat. Rev. Genet. 2011, 12, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Piper, P.; Calderon, C.O.; Hatzixanthis, K.; Mollapour, M. Weak acid adaptation: The stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 2001, 147, 2635–2642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piper, P.W. Yeast superoxide dismutase mutants reveal a pro-oxidant action of weak organic acid food preservatives. Free Radic. Biol. Med. 1999, 27, 1219–1227. [Google Scholar] [CrossRef]
- Kren, A.; Mamnun, Y.M.; Bauer, B.E.; Schuller, C.; Wolfger, H.; Hatzixanthis, K.; Mollapour, M.; Gregori, C.; Piper, P.; Kuchler, K. War1p, a novel transcription factor controlling weak acid stress response in yeast. Mol. Cell. Biol. 2003, 23, 1775–1785. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Zhang, M.; Huang, J.; Zhou, R.; Jin, Y.; Wu, C. Zygosaccharomyces rouxii combats salt stress by maintaining cell membrane structure and functionality. J. Microbiol. Biotechnol. 2020, 30, 62–70. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, M.; Huang, J.; Zhou, R.; Jin, Y.; Zhao, D.; Zheng, J.; Wu, C. Heat preadaptation improved the ability of Zygosaccharomyces rouxii to salt stress: A combined physiological and transcriptomic analysis. Appl. Microbiol. Biotechnol. 2021, 105, 259–270. [Google Scholar] [CrossRef]
- Landolfo, S.; Politi, H.; Angelozzi, D.; Mannazzu, I. ROS accumulation and oxidative damage to cell structures in Saccharomyces cerevisiae wine strains during fermentation of high-sugar-containing medium. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 892–898. [Google Scholar] [CrossRef]
- Rodríguez-Vargas, S.; Sánchez-García, A.; Martínez-Rivas, J.M.; Prieto, J.A.; Randez-Gil, F. Fluidization of membrane lipids enhances the tolerance of Saccharomyces cerevisiae to freezing and salt stress. Appl. Environ. Microbiol. 2007, 73, 110–116. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.S.; Rose, A.H. Inhibitory effect of ethanol on growth and solute accumulation by Saccharomyces cerevisiae as affected by plasma-membrane lipid composition. Arch. Microbiol. 1979, 122, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Beniwal, A.; Kokkiligadda, A.; Vij, S. Response and tolerance of yeast to changing environmental stress during ethanol fermentation. Process Biochem. 2018, 72, 1–12. [Google Scholar] [CrossRef]
- Godinho, C.P.; Prata, C.S.; Pinto, S.N.; Cardoso, C.; Bandarra, N.M.; Fernandes, F.; Sa-Correia, I. Pdr18 is involved in yeast response to acetic acid stress counteracting the decrease of plasma membrane ergosterol content and order. Sci. Rep. 2018, 8, 7860. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, C.L.; Botting, C.H.; Antrobus, R.; Coote, P.J. Evidence of a new role for the high-osmolarity glycerol mitogen-activated protein kinase pathway in yeast: Regulating adaptation to citric acid stress. Mol. Cell. Biol. 2004, 24, 3307–3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, M.K.; Arneborg, N. The effect of citric acid and pH on growth and metabolism of anaerobic Saccharomyces cerevisiae and Zygosaccharomyces bailii cultures. Food Microbiol. 2007, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Omori, T.; Ogawa, K.; Shimoda, M. Effect of citric acid on glycerol formation by Saccharomyces cerevisiae in barley Shochu mash. J. Ferment. Bioeng. 1995, 73, 89–95. [Google Scholar] [CrossRef]
- Chen, S.R.; Tang, L.L.; Feng, J.; Sun, L.; Wang, J.L. Isolation and identification of yeast capable of efficiently degrading citric acid and its acid degradation characteristics in red raspberry juice. Food Sci. 2020, 41, 133–139. [Google Scholar] [CrossRef]
- Liu, B.; Yuan, D.X.; Li, Q.Y.; Zhou, X.; Wu, H.; Bao, Y.H.; Lu, H.Y.; Luo, T.; Wang, J.L. Changes in organic acids, phenolic compounds, and antioxidant activities of lemon juice fermented by Issatchenkia terricola. Molecules 2021, 26, 6712. [Google Scholar] [CrossRef]
- Uexküll, H.R.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Hallberg, K.B. New perspectives in acid mine drainage microbiology. Hydrometallurgy 2009, 104, 448–453. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Vitorino, M.V.; Godinho, C.P.; Bourbon-Melo, N.; Robalo, T.T.; Fernandes, F.; Rodrigues, M.S.; Sa-Correia, I. Yeast adaptive response to acetic acid stress involves structural alterations and increased stiffness of the cell wall. Sci. Rep. 2021, 11, 12652. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Lei, J.; Su, S.; Chen, F.; Duan, Z.; Chen, Y.; Gong, X.; Li, H.; Jin, J. Absence of Yps7p, a putative glycosylphosphatidylinositol-linked aspartyl protease in Pichia pastoris, results in aberrant cell wall composition and increased osmotic stress resistance. FEMS Yeast Res. 2012, 12, 969–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Zhang, J.; Wang, M.; Du, G.; Chen, J. Lactobacillus casei combats acid stress by maintaining cell membrane functionality. J. Ind. Microbiol. Biotechnol. 2012, 39, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Fei, Y.; Yu, J.; Liu, R.; Gao, S.; Bai, W. Nutrient utilization of Zygosaccharomyces mellis and its metabolic characteristics in response to high-glucose stress. Food Sci. 2019, 40, 166–171. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Wang, T.; Sun, J.; Zhang, Y.; Ji, J.; Sun, X. Effects of acid, alkaline, cold, and heat environmental stresses on the antibiotic resistance of the Salmonella enterica serovar Typhimurium. Food Res. Int. 2021, 144, 110359. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Huang, J.; Feng, P.; An, J.; Si, Z.; Long, X.; Wu, S.; Yi, Y. Cytotoxicity of formic acid to Saccharomyces cerevisiae. Food Sci. 2022, 43, 125–131. [Google Scholar]
- Chen, M.; Zou, X.P.; Luo, H.S.; Cao, J.; Zhang, X.P.; Zhang, B.; Liu, W.J. Effects and mechanisms of proton pump inhibitors as a novel chemosensitizer on human gastric adenocarcinoma (SGC7901) cells. Cell. Biol. Int. 2009, 33, 1008–1019. [Google Scholar] [CrossRef]
- Lambert, R.J.; Stratford, M. Weak-acid preservatives: Modelling microbial inhibition and response. J. Appl. Microbiol. 1999, 86, 157–164. [Google Scholar] [CrossRef]
- Shu, Q.; Lou, H.; Wei, T.; Liu, X.; Chen, Q. Contributions of glycolipid biosurfactants and glycolipid-modified materials to antimicrobial strategy: A review. Pharmaceutics 2021, 13, 227. [Google Scholar] [CrossRef]
- Câmara, A.D.A.; Maréchal, P.; Tourdot-Maréchal, R.; Husson, F. Oxidative stress resistance during dehydration of three non-Saccharomyces wine yeast strains. Food Res. Int. 2019, 123, 364–372. [Google Scholar] [CrossRef]
- Franca, M.B.; Panek, A.D.; Eleutherio, E.C. The role of cytoplasmic catalase in dehydration tolerance of Saccharomyces cerevisiae. Cell Stress Chaperones 2005, 10, 167–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auesukaree, C. Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation. J. Biosci. Bioeng. 2017, 124, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wei, T.; Lou, H.; Shu, X.; Chen, Q. A critical review on communication mechanism within plant-endophytic fungi interactions to cope with biotic and abiotic stresses. J. Fungi 2021, 7, 719. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Ji, Z.; Zhang, Y.; Tian, J.; Wang, F.; Song, M.; Li, H. Oxidative stress and cytotoxicity induced by tetrachlorobisphenol A in Saccharomyces cerevisiae cells. Ecotoxicol. Environ. Saf. 2018, 161, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Wong, C.K.C.; Oger, C.; Durand, T.; Galano, J.M.; Lee, J.C.Y. Prenatal exposure to the contaminant perfluorooctane sulfonate elevates lipid peroxidation during mouse fetal development but not in the pregnant dam. Free Radic. Res. 2015, 49, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Han, P.; Zhang, R.; Li, H. Ultrastructural changes of Saccharomyces cerevisiae in response to ethanol stress. Can. J. Microbiol. 2013, 59, 589–597. [Google Scholar] [CrossRef]
- Chi, M.; Li, G.; Liu, Y.; Liu, G.; Li, M.; Zhang, X.; Sun, Z.; Sui, Y.; Liu, J. Increase in antioxidant enzyme activity, stress tolerance and biocontrol efficacy of Pichia kudriavzevii with the transition from a yeast-like to biofilm morphology. Biol. Control 2015, 90, 113–119. [Google Scholar] [CrossRef]
- Jie, Z.; Liu, J.; Shu, M.; Ying, Y.; Yang, H. Detection strategies for superoxide anion: A review. Talanta 2022, 236, 122892. [Google Scholar] [CrossRef]
- Orij, R.; Brul, S.; Smits, G.J. Intracellular pH is a tightly controlled signal in yeast. Biochim. Biophys. Acta 2011, 1810, 933–944. [Google Scholar] [CrossRef]
- Cláudia, P.G.; Sá-Correia, I. Yeasts in Biotechnology and Human Health; Springer: New York, NY, USA, 2009; pp. 37–60. [Google Scholar]
- Abrat, O.B.; Semchyshyn, H.M.; Lushchak, V.I. Acid stress in yeast Saccharomyces cerevisiae. Ukr. Biokhim. Zhurnal 2008, 80, 19–31. [Google Scholar]
- Xu, Z.; Ren, A.; Guo, J.; Liu, X.; Huang, S.; Feng, J. A theoretical investigation of two typical two-photon pH fluorescent probes. Photochem. Photobiol. 2013, 89, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, J.; Chen, W.; Wang, M.; Du, G.; Chen, J.A. combined physiological and proteomic approach to reveal lactic-acid-induced alterations in Lactobacillus casei Zhang and its mutant with enhanced lactic acid tolerance. Appl. Microbiol. Biotechnol. 2012, 93, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Cholewa, J.M.; Guimarães-Ferreira, L.; Zanchi, N.E. Effects of betaine on performance and body composition: A review of recent findings and potential mechanisms. Amino Acids 2014, 46, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Turk, M.; Montiel, V.; Žigon, D.; Plemenitaš, A.; Ramos, J. Plasma membrane composition of Debaryomyces hansenii adapts to changes in pH and external salinity. Microbiology 2007, 153, 3586–3592. [Google Scholar] [CrossRef] [Green Version]
- Nasution, O.; Lee, Y.M.; Kim, E.; Lee, Y.; Kim, W.; Choi, W. Overexpression of OLE1 enhances stress tolerance and constitutively activates the MAPK HOG pathway in Saccharomyces cerevisiae. Biotechnol. Bioeng. 2017, 114, 620–631. [Google Scholar] [CrossRef]
- Steels, E.L.; Learmonth, R.P.; Watson, K. Stress tolerance and membrane lipid unsaturation in Saccharomyces cerevisiae grown aerobically or anaerobically. Microbiology 1994, 140, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Sajbidor, J.; Grego, J. Fatty acid alterations in Saccharomyces cerevisiae exposed to ethanol stress. FEMS Microbiol. Lett. 1992, 72, 13–16. [Google Scholar] [CrossRef]
- Chi, Z.; Arneborg, N. Relationship between lipid composition, frequency of ethanol-induced respiratory deficient mutants, and ethanol tolerance in Saccharomyces cerevisiae. J. Appl. Microbiol. 1999, 86, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.Q.; Liu, T.Z.; Chen, J.; Zhang, K.; Li, O.; Zhu, L.; Zhao, Y.H.; Wu, X.C.; Wang, P.M. Comparative functional genomics to reveal the molecular basis of phenotypic diversities and guide the genetic breeding of industrial yeast strains. Appl. Microbiol. Biotechnol. 2013, 97, 2067–2076. [Google Scholar] [CrossRef]
- Lindberg, L.; Santos, A.X.; Riezman, H.; Olsson, L.; Bettiga, M. Lipidomic profiling of Saccharomyces cerevisiae and Zygosaccharomyces bailii reveals critical changes in lipid composition in response to acetic acid stress. PLoS ONE 2013, 8, e73936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Britton, L.; Malinowski, D.P.; Fridovich, I. Superoxide dismutase and oxygen metabolism in Streptococcus faecalis and comparisons with other organisms. J. Bacteriol. 1978, 134, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; He, C.; Zhang, Z.; Zou, Z.; Wang, H. Changes of antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCl stress. Colloids Surf. B Biointerfaces 2007, 59, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, Y.; Jiang, W.; Lv, X.; Dong, X. Effect of sodium chloride and cadmium on the growth, oxidative stress and antioxidant enzyme activities of Zygosaccharomyces rouxii. J. Ocean Univ. China 2014, 13, 460–466. [Google Scholar] [CrossRef]
- Lee, J.; Kwon, E.; Kim, D.; Cha, J.; Roe, J. Regulation and the role of Cu, Zn-containing superoxide dismutase in cell cycle progression of Schizosaccharomyces pombe. Biochem. Biophys. Res. Commun. 2002, 297, 854–862. [Google Scholar] [CrossRef]
- Breeuwer, P.; Abee, T. Assessment of the intracellular pH of immobilized and continuously perfused yeast cells employing fluorescence ratio imaging analysis. J. Microbiol. Methods 2000, 39, 253–264. [Google Scholar] [CrossRef]
- Câmara Jr, A.A.; Maréchal, P.A.; Tourdot-Maréchal, R.; Husson, F. Dehydration stress responses of yeasts Torulaspora delbrueckii, Metschnikowia pulcherrima and Lachancea thermotolerans: Effects of glutathione and trehalose biosynthesis. Food Microb. 2019, 79, 137–146. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial gold nanoclusters. ACS Nano 2017, 11, 6904–6910. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Howlett, N.G.; Avery, S.V. Induction of lipid peroxidation during heavy metal stress in Saccharomyces cerevisiae and influence of plasma membrane fatty acid unsaturation. Appl. Environ. Microbiol. 1997, 63, 2971–2976. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Du, G.; Zhang, Y.; Liao, X.; Wang, M.; Li, Y.; Chen, J. Glutathione protects Lactobacillus sanfranciscensis against freeze-thawing, freeze-drying, and cold treatment. Appl. Environ. Microbiol. 2010, 76, 2989–2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigogno, C.; Khozin-Goldberg, I.; Boussiba, S.; Vonshak, A.; Cohen, Z. Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 2002, 60, 497–503. [Google Scholar] [CrossRef]
- Saharan, R.K.; Kanwal, S.; Sharma, S.C. Role of glutathione in ethanol stress tolerance in yeast Pachysolen tannophilus. Biochem. Biophys. Res. Commun. 2010, 397, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Beyer, W.J.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Ke, D.; Wang, A.; Sun, G.; Dong, L. The effect of active oxygen on the activity of ACC synthase induced by exogenous IAA. Acta Bot. Sin. 2002, 44, 551–556. [Google Scholar] [CrossRef]

| Fatty Acid Types | Contents of Cell Membrane/(mg/g DW) | |||
|---|---|---|---|---|
| Control | 20 g/L | 80 g/L | 140 g/L | |
| Myristic acid | 0.09 ± 0.01 | - | - | - |
| Pentadecanoic acid | 0.10 ± 0.02 | - | - | - |
| (Z)-hexadec-9-enoate acid | 0.43 ± 0.06 | - | - | - |
| (9E)-hexadec-9-enoate acid | 1.01 ± 0.42 b | 1.41 ± 0.17 b | 3.67 ± 0.63 a | 3.45 ± 0.11 a |
| Palmitic acid | 14.28 ± 1.68 c | 14.81 ± 0.34 bc | 18.72 ± 0.99 ab | 22.26 ± 0.08 a |
| 7,12-Octadecadienoate acid | 0.19 ± 0.02 | - | - | - |
| Linoleic acid | 9.08 ± 1.09 b | 19.29 ± 0.46 a | 20.28 ± 1.73 a | 18.46 ± 0.72 a |
| (Z)-9-Octadecenoic acid | 16.61 ± 0.54 d | 32.10 ± 1.56 c | 49.53 ± 0.63 b | 58.17 ± 0.60 a |
| 11-Octadecenoic acid | 8.00 ± 1.19 a | 3.86 ± 0.16 b | 3.24 ± 0.54 b | 2.64 ± 0.05 b |
| Stearic acid | 1.51 ± 0.09 a | 2.03 ± 0.08 a | 1.98 ± 0.64 a | 2.50 ± 0.42 a |
| 9-Octadecynoate acid | 0.33 ± 0.04 a | 0.30 ± 0.19 a | - | - |
| Oleic acid | 0.41 ± 0.04 b | 0.74 ± 0.05 a | - | - |
| 10-Hydroxy-octadecanoic acid | 4.70 ± 0.41 a | 2.91 ± 0.09 b | 0.34 ± 0.07 c | 0.20 ± 0.03 c |
| 17-Methoxy-octadecanoic acid | 0.28 ± 0.03 | - | - | - |
| (Z)-11-Eicosenoic acid | - | 0.46 ± 0.01 b | 0.86 ± 0.07 a | 0.30 ± 0.02 b |
| 5,9-Octadecadienoate acid | - | - | 0.44 ± 0.08 | - |
| 6,10-Octadecadienoate acid | - | - | 0.51 ± 0.19 | - |
| Total | 57.02 ± 1.01 d | 77.91 ± 2.84 c | 99.57 ± 2.42 b | 107.98 ± 1.6 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Tang, Y.; Ning, W.; Bao, Y.; Luo, T.; Wang, J. Responses of Issatchenkia terricola WJL-G4 upon Citric Acid Stress. Molecules 2022, 27, 2664. https://doi.org/10.3390/molecules27092664
Liu X, Tang Y, Ning W, Bao Y, Luo T, Wang J. Responses of Issatchenkia terricola WJL-G4 upon Citric Acid Stress. Molecules. 2022; 27(9):2664. https://doi.org/10.3390/molecules27092664
Chicago/Turabian StyleLiu, Xinyi, Ying Tang, Weiyu Ning, Yihong Bao, Ting Luo, and Jinling Wang. 2022. "Responses of Issatchenkia terricola WJL-G4 upon Citric Acid Stress" Molecules 27, no. 9: 2664. https://doi.org/10.3390/molecules27092664






