Analytical Methods for the Characterization of Vegetable Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Apparatus and Reagents
2.3. Procedures
2.3.1. Profile of Inorganic Component
2.3.2. Reductive Oxide Profile
2.4. Chemometric Treatment
3. Results and Discussion
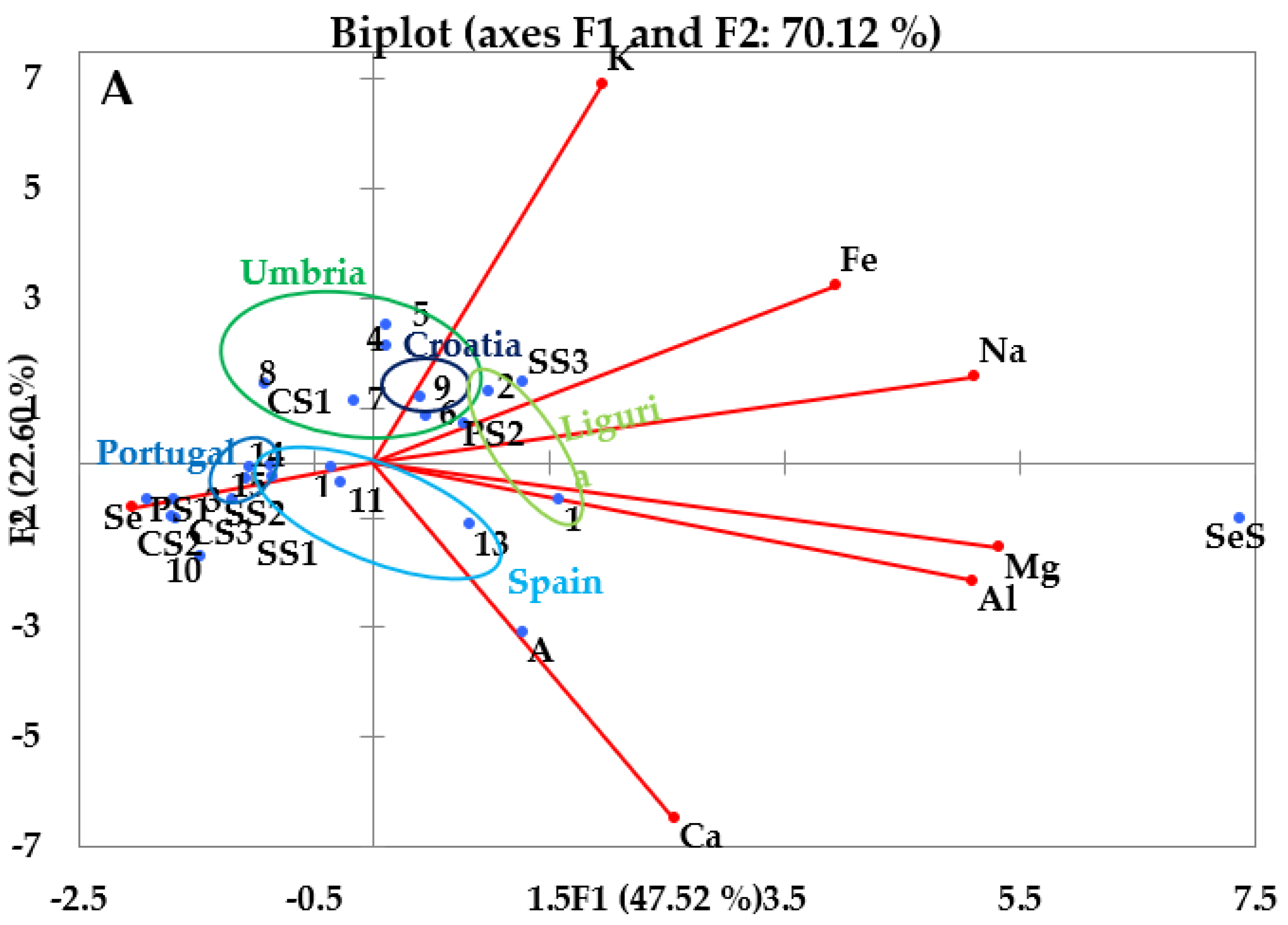
3.1. Inorganic Component
3.1.1. Reference Material
3.1.2. EVOOs and other Vegetable Oil Samples
3.2. Redox Profiles
3.2.1. Method Development with Commercial EVOO
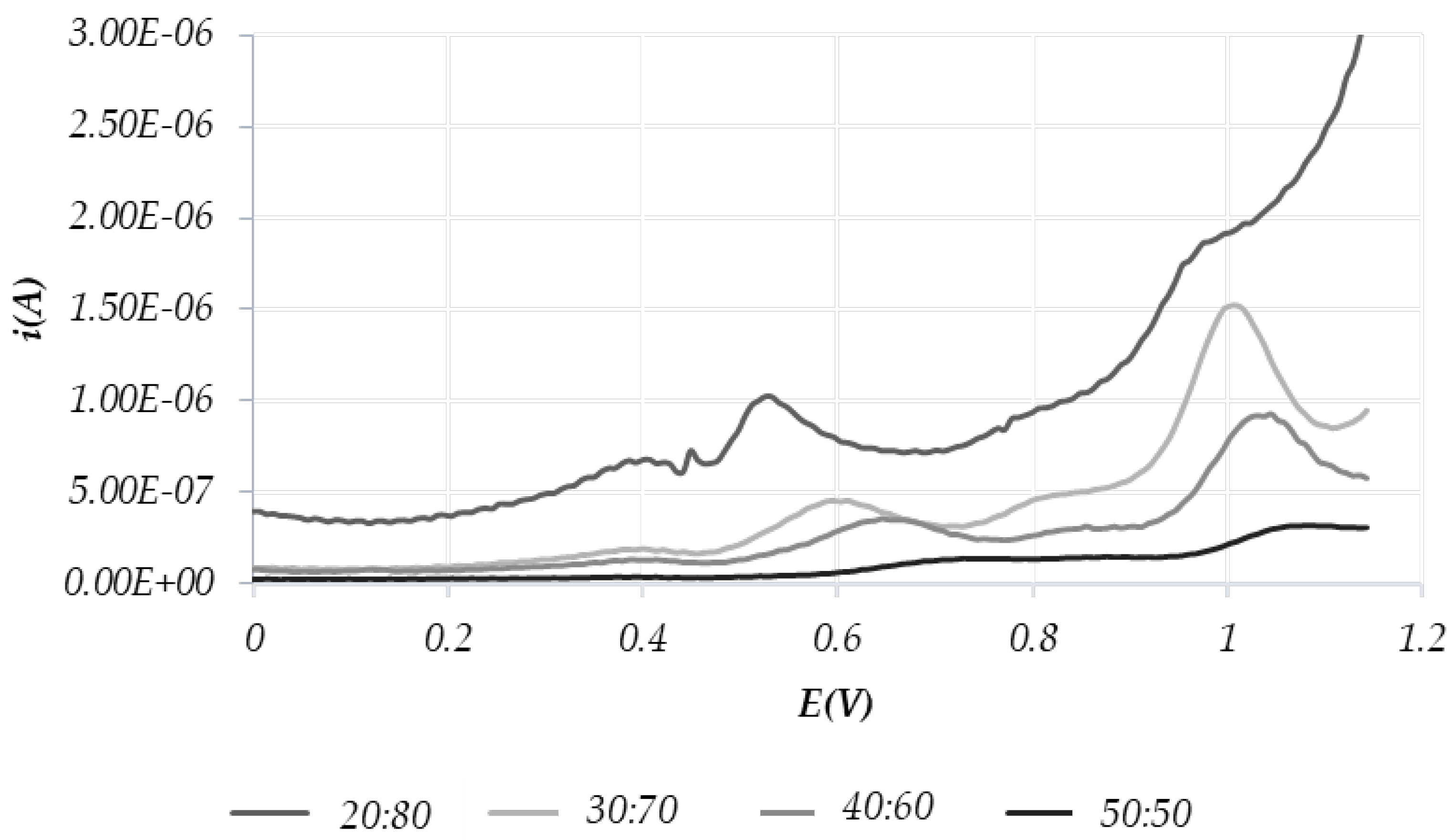
- -
- peaks around the potentials of 0.4 V and 0.6 V correspond to polyphenols
- -
- the peaks around the 1.1 V potential are relative to tocopherols.
3.2.2. EVOO and VO Samples
3.2.3. Applications of Redox Profiles
Identification of Possible Adulterations with Other Oils
Assessment of the Conservation Status
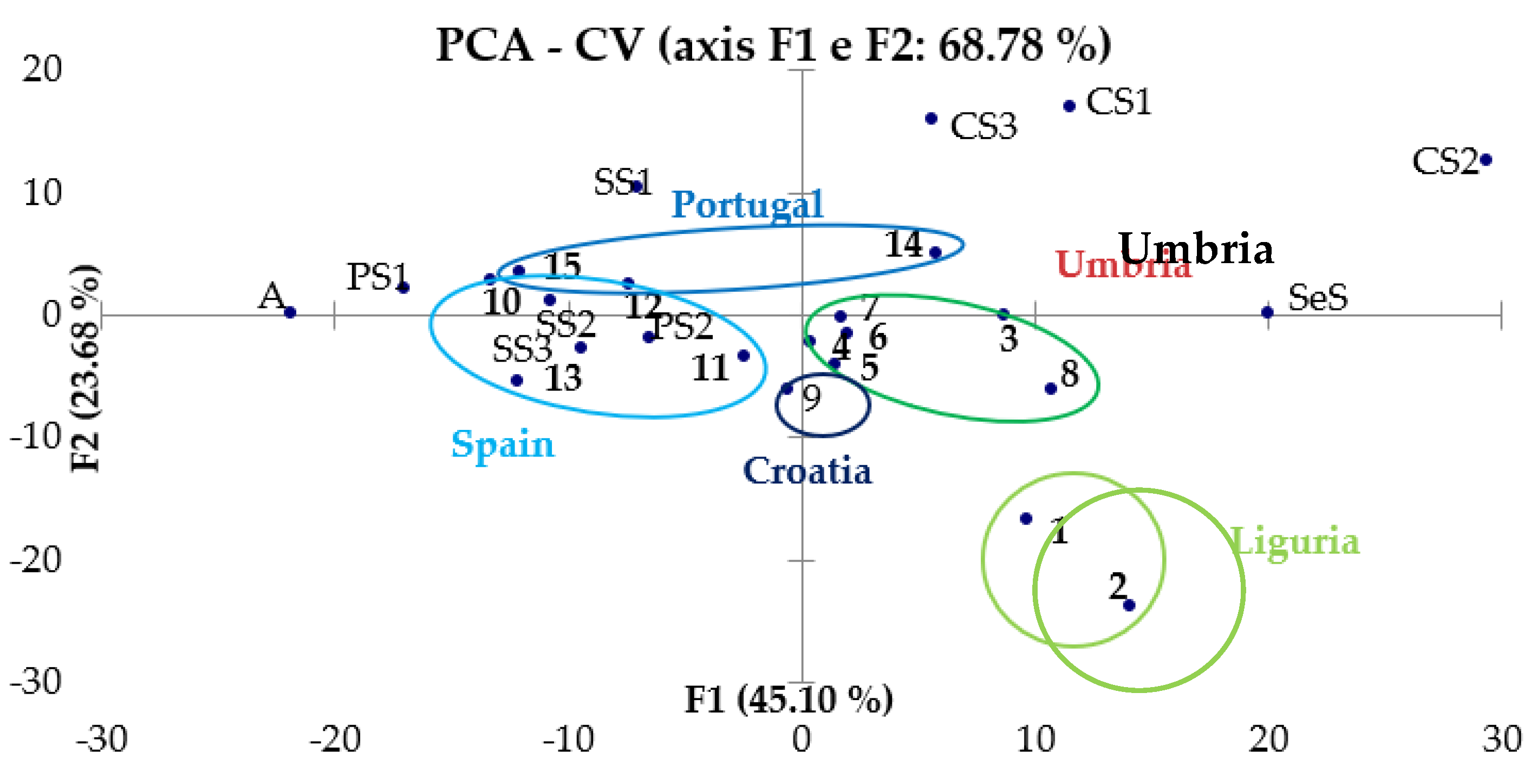
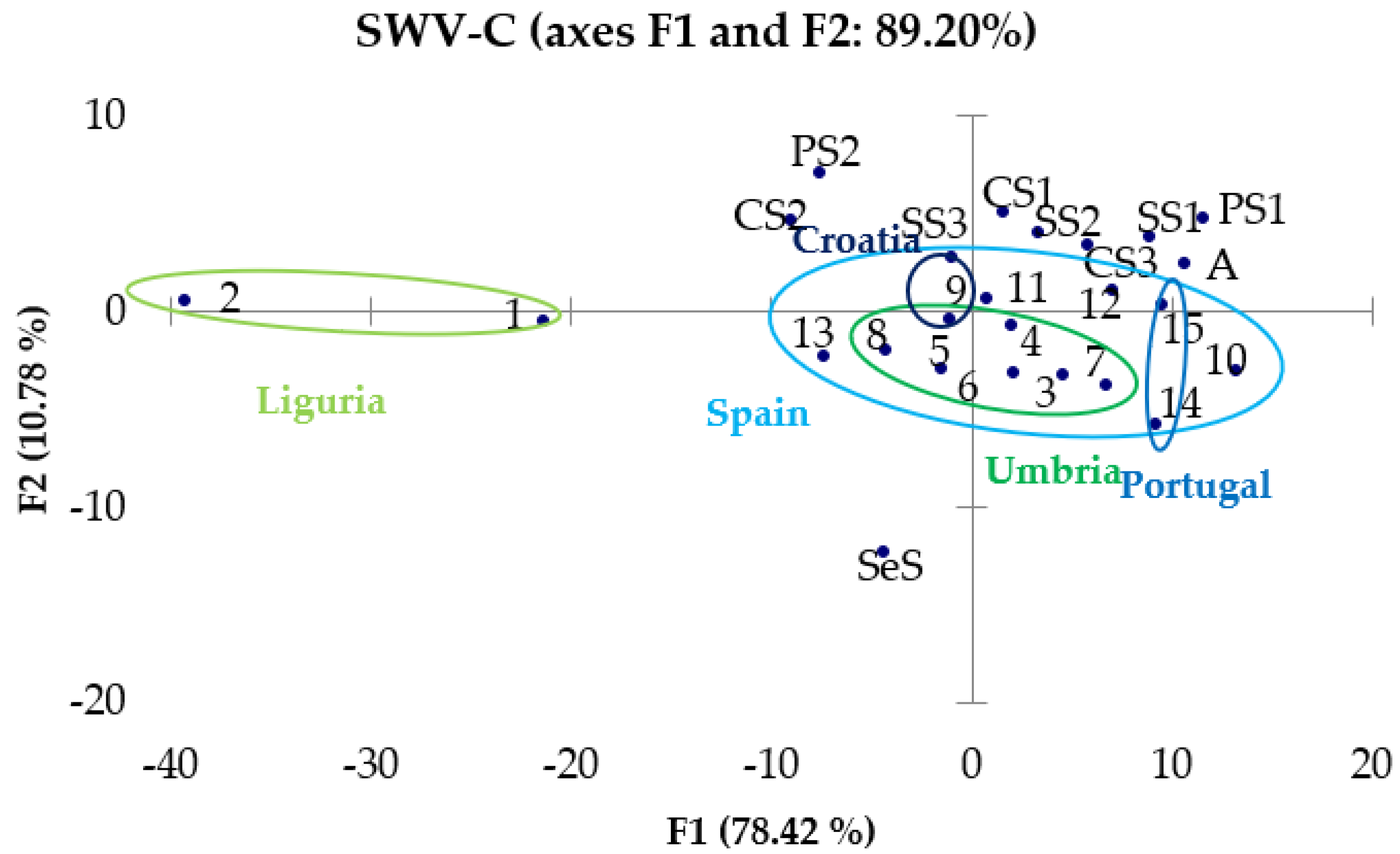
3.3. Chemometric Treatment
3.3.1. PCA of the Inorganic Components
3.3.2. PCA of CV and SWV Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. WHO Codex Commitee on Food Import and Export Inspection and Certification Systems; Food and Agriculture Organization: Rome, Italy, 2018. [Google Scholar]
- Dugo, G.; Lapera, L.; Latorre, G.; Giuffrida, D. Determination of Cd(II), Cu(II), Pb(II), and Zn(II) Content in Commercial Vegetable Oils Using Derivative Potentiometric Stripping Analysis. Food Chem. 2004, 87, 639–645. [Google Scholar] [CrossRef]
- Charlebois, S.; Schwab, A.; Henn, R.; Huck, C.W. Food Fraud: An Exploratory Study for Measuring Consumer Perception towards Mislabeled Food Products and Influence on Self-Authentication Intentions. Trends Food Sci. Technol. 2016, 50, 211–218. [Google Scholar] [CrossRef]
- Portarena, S.; Leonardi, L.; Scartazza, A.; Lauteri, M.; Baldacchini, C.; Farinelli, D.; Famiani, F.; Ciolfi, M.; Brugnoli, E. Combining Analysis of Fatty Acid Composition and Δ13C in Extra-Virgin Olive Oils as Affected by Harvest Period and Cultivar: Possible Use in Traceability Studies. Food Control 2019, 105, 151–158. [Google Scholar] [CrossRef]
- Aued-Pimentel, S.; Takemoto, E.; Antoniassi, R.; Gastaldo Badolato, E.S. Composition of Tocopherols in Sesame Seed Oil: An Indicative of Adulteration. Grasas Aceites 2006, 57, 205–210. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; García-González, D.L.; Lobo-Prieto, A.; Aparicio, R. Andalusian Protected Designations of Origin of Virgin Olive Oil: The Role of Chemical Composition in Their Authentication. Eur. J. Lipid Sci. Technol. 2019, 121, 1800133. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Available online: https://ec.europa.eu/info/index_en (accessed on 15 November 2022).
- Di Serio, M.G.; Di Giacinto, L.; Di Loreto, G.; Giansante, L.; Pellegrino, M.; Vito, R.; Perri, E. Chemical and Sensory Characteristics of Italian Virgin Olive Oils from Grossa Di Gerace Cv: Grossa Di Gerace’ Characterization. Eur. J. Lipid Sci. Technol. 2016, 118, 288–298. [Google Scholar] [CrossRef]
- Tura, D.; Failla, O.; Bassi, D.; Pedò, S.; Serraiocco, A. Environmental and Seasonal Influence on Virgin Olive (Olea Europaea L.) Oil Volatiles in Northern Italy. Sci. Hortic. 2009, 122, 385–392. [Google Scholar] [CrossRef]
- Morelló, J.-R.; Romero, M.-P.; Motilva, M.-J. Effect of the Maturation Process of the Olive Fruit on the Phenolic Fraction of Drupes and Oils from Arbequina, Farga, and Morrut Cultivars. J. Agric. Food Chem. 2004, 52, 6002–6009. [Google Scholar] [CrossRef]
- Morelló, J.-R.; Motilva, M.-J.; Tovar, M.-J.; Romero, M.-P. Changes in Commercial Virgin Olive Oil (Cv Arbequina) during Storage, with Special Emphasis on the Phenolic Fraction. Food Chem. 2004, 85, 357–364. [Google Scholar] [CrossRef]
- Lercker, G.; Bendini, A.; Cerretani, L. Quality, Composition and Production Process of Virgin Olive Oils. Prog. Nutr. 2007, 9, 134–148. [Google Scholar]
- Fasciotti, M.; Pereira Netto, A.D. Optimization and Application of Methods of Triacylglycerol Evaluation for Characterization of Olive Oil Adulteration by Soybean Oil with HPLC–APCI-MS–MS. Talanta 2010, 81, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Dais, P.; Hatzakis, E. Quality Assessment and Authentication of Virgin Olive Oil by NMR Spectroscopy: A Critical Review. Anal. Chim. Acta 2013, 765, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, J.; Wardencki, W. Trends in edible vegetable oils analysis part b application of different analytical techniques. Pol. J. Food Nutr. Sci. 2011, 61, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Yildiz Tiryaki, G.; Ayvaz, H. Quantification of Soybean Oil Adulteration in Extra Virgin Olive Oil Using Portable Raman Spectroscopy. J. Food Meas. Charact. 2017, 11, 523–529. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M.Z. Perspective of Vibrational Spectroscopy Analytical Methods in On-Field/Official Control of Olives and Virgin Olive Oil: Vibrational Spectroscopy in Virgin Olive Oil Analysis. Eur. J. Lipid Sci. Technol. 2017, 119, 1600148. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Sánchez-Rodríguez, M.I.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Caridad, J.M.; Moalem, M. Chemometric Study of Andalusian Extra Virgin Olive Oils Raman Spectra: Qualitative and Quantitative Information. Talanta 2016, 156–157, 180–190. [Google Scholar] [CrossRef]
- Guzmán, E.; Baeten, V.; Pierna, J.A.F.; García-Mesa, J.A. Determination of the Olive Maturity Index of Intact Fruits Using Image Analysis. J. Food Sci. Technol. 2015, 52, 1462–1470. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Sun, Y.; Liu, S.; Li, S.; Zhang, W. A Feasibility Study of the Rapid Evaluation of Oil Oxidation Using Synchronous Fluorescence Spectroscopy. Food Anal. Methods 2018, 11, 3464–3470. [Google Scholar] [CrossRef]
- Lia, F.; Castellano, A.M.; Zammit-Mangion, M.; Farrugia, C. Application of Fluorescence Spectroscopy and Chemometric Models for the Detection of Vegetable Oil Adulterants in Maltese Virgin Olive Oils. J. Food Sci. Technol. 2018, 55, 2143–2151. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.B.C. Application of Fourier Transform Infrared Spectroscopy for Authentication of Functional Food Oils. Appl. Spectrosc. Rev. 2012, 47, 1–13. [Google Scholar] [CrossRef]
- Vasconcelos, M.; Coelho, L.; Barros, A.; de Almeida, J.M.M.M. Study of Adulteration of Extra Virgin Olive Oil with Peanut Oil Using FTIR Spectroscopy and Chemometrics. Cogent Food Agric. 2015, 1, 1018695. [Google Scholar] [CrossRef]
- Torrecilla, J.S.; Rojo, E.; Domínguez, J.C.; Rodríguez, F. A Novel Method to Quantify the Adulteration of Extra Virgin Olive Oil with Low-Grade Olive Oils by UV−Vis. J. Agric. Food Chem. 2010, 58, 1679–1684. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zheng, H.; Lu, H. Application of UV Spectrometry and Chemometric Models for Detecting Olive Oil-Vegetable Oil Blends Adulteration. J. Food Sci. Technol. 2015, 52, 479–485. [Google Scholar] [CrossRef]
- Zhu, F.; Fan, W.; Wang, X.; Qu, L.; Yao, S. Health Risk Assessment of Eight Heavy Metals in Nine Varieties of Edible Vegetable Oils Consumed in China. Food Chem. Toxicol. 2011, 49, 3081–3085. [Google Scholar] [CrossRef] [PubMed]
- Zeiner, M.; Steffan, I.; Cindric, I.J. Determination of Trace Elements in Olive Oil by ICP-AES and ETA-AAS: A Pilot Study on the Geographical Characterization. Microchem. J. 2005, 81, 171–176. [Google Scholar] [CrossRef]
- Pereira, J.S.F.; Moraes, D.P.; Antes, F.G.; Diehl, L.O.; Santos, M.F.P.; Guimarães, R.C.L.; Fonseca, T.C.O.; Dressler, V.L.; Flores, É.M.M. Determination of Metals and Metalloids in Light and Heavy Crude Oil by ICP-MS after Digestion by Microwave-Induced Combustion. Microchem. J. 2010, 96, 4–11. [Google Scholar] [CrossRef]
- Zeiner, M.; Juranovic-Cindric, I.; Škevin, D. Characterization of Extra Virgin Olive Oils Derived from the Croatian Cultivar Oblica. Eur. J. Lipid Sci. Technol. 2010, 112, 1248–1252. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Fernández-de Córdova, M.L.; Domínguez-Vidal, A.; Ruiz-Medina, A. Investigation by ICP-MS of Trace Element Levels in Vegetable Edible Oils Produced in Spain. Food Chem. 2011, 127, 1257–1262. [Google Scholar] [CrossRef]
- Bajoub, A.; Medina-Rodríguez, S.; Gómez-Romero, M.; Ajal, E.A.; Bagur-González, M.G.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Assessing the Varietal Origin of Extra-Virgin Olive Oil Using Liquid Chromatography Fingerprints of Phenolic Compound, Data Fusion and Chemometrics. Food Chem. 2017, 215, 245–255. [Google Scholar] [CrossRef]
- Frankel, E.N. Chemistry of Extra Virgin Olive Oil: Adulteration, Oxidative Stability, and Antioxidants. J. Agric. Food Chem. 2010, 58, 5991–6006. [Google Scholar] [CrossRef]
- Jabeur, H.; Drira, M.; Rebai, A.; Bouaziz, M. Putative Markers of Adulteration of Higher-Grade Olive Oil with Less Expensive Pomace Olive Oil Identified by Gas Chromatography Combined with Chemometrics. J. Agric. Food Chem. 2017, 65, 5375–5383. [Google Scholar] [CrossRef] [PubMed]
- Salghi, R.; Armbruster, W.; Schwack, W. Detection of Argan Oil Adulteration with Vegetable Oils by High-Performance Liquid Chromatography–Evaporative Light Scattering Detection. Food Chem. 2014, 153, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Melucci, D.; Bendini, A.; Tesini, F.; Barbieri, S.; Zappi, A.; Vichi, S.; Conte, L.; Toschi, T.G. Rapid Direct Analysis to Discriminate Geographic Origin of Extra Virgin Olive Oils by Flash Gas Chromatography Electronic Nose and Chemometrics. Food Chem. 2016, 204, 263–273. [Google Scholar] [CrossRef] [Green Version]
- López Ortíz, C.M.; Prats Moya, M.S.; Berenguer Navarro, V. A Rapid Chromatographic Method for Simultaneous Determination of β-Sitosterol and Tocopherol Homologues in Vegetable Oils. J. Food Compos. Anal. 2006, 19, 141–149. [Google Scholar] [CrossRef]
- Stilo, F.; Liberto, E.; Reichenbach, S.E.; Tao, Q.; Bicchi, C.; Cordero, C. Untargeted and Targeted Fingerprinting of Extra Virgin Olive Oil Volatiles by Comprehensive Two-Dimensional Gas Chromatography with Mass Spectrometry: Challenges in Long-Term Studies. J. Agric. Food Chem. 2019, 67, 5289–5302. [Google Scholar] [CrossRef]
- Valli, E.; Bendini, A.; Berardinelli, A.; Ragni, L.; Riccò, B.; Grossi, M.; Gallina Toschi, T. Rapid and Innovative Instrumental Approaches for Quality and Authenticity of Olive Oils: Innovative Approaches for Quality of Virgin Olive Oils. Eur. J. Lipid Sci. Technol. 2016, 118, 1601–1619. [Google Scholar] [CrossRef] [Green Version]
- Zappi, D.; Gabriele, S.; Gontrani, L.; Dini, D.; Sadun, C.; Marini, F.; Antonelli, M.L. Biologically Friendly Room Temperature Ionic Liquids and Nanomaterials for the Development of Innovative Enzymatic Biosensors: Part II. Talanta 2019, 194, 26–31. [Google Scholar] [CrossRef]
- Zappi, D.; Sadun, C.; Gontrani, L.; Dini, D.; Antonelli, M.L. A New Electrochemical Sensor for Extra-Virgin Olive Oils Classification. Food Control 2020, 109, 106903. [Google Scholar] [CrossRef]
- Jamali, M.K.; Kazi, T.G.; Arain, M.B.; Afridi, H.I.; Jalbani, N.; Sarfraz, R.A.; Baig, J.A. A Multivariate Study: Variation in Uptake of Trace and Toxic Elements by Various Varieties of Sorghum Bicolor L. J. Hazard. Mater. 2008, 158, 644–651. [Google Scholar] [CrossRef]
- Mourhat, Z.; Touzara, S.; Maallah, R.; Mbarki, M.; Chtaini, A. Electrochemical Evaluation of the Antioxidant Capacity of Phenolic Compounds in Virgin Olive Oil. J. Biosens. Bioelectron. 2017, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Cimato, A.; Dello Monaco, D.; Distante, C.; Epifani, M.; Siciliano, P.; Taurino, A.M.; Zuppa, M.; Sani, G. Analysis of Single-Cultivar Extra Virgin Olive Oils by Means of an Electronic Nose and HS-SPME/GC/MS Methods. Sens. Actuators B Chem. 2006, 114, 674–680. [Google Scholar] [CrossRef]
- Inaudi, P.; Giacomino, A.; Malandrino, M.; La Gioia, C.; Conca, E.; Karak, T.; Abollino, O. The Inorganic Component as a Possible Marker for Quality and for Authentication of the Hazelnut’s Origin. Int. J. Environ. Res. Public Health 2020, 17, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaggero, E.; Malandrino, M.; Fabbri, D.; Bordiglia, G.; Fusconi, A.; Mucciarelli, M.; Inaudi, P.; Calza, P. Uptake of Potentially Toxic Elements by Four Plant Species Suitable for Phytoremediation of Turin Urban Soils. Appl. Sci. 2020, 10, 3948. [Google Scholar] [CrossRef]
- Barabasz, W.; Albi, D.; Jaśkowska, M.; Lipiec, J. Ecotoxicology of Aluminium. Pol. J. Environ. Stud. 2002, 11, 199–203. [Google Scholar]
- Rodríguez-Méndez, M.L.; Apetrei, C.; de Saja, J.A. Evaluation of the Polyphenolic Content of Extra Virgin Olive Oils Using an Array of Voltammetric Sensors. Electrochim. Acta 2008, 53, 5867–5872. [Google Scholar] [CrossRef]






| Code | Region | Classification |
|---|---|---|
| EVOO1 | Liguria | MV |
| EVOO2 | Liguria | MV |
| EVOO3 | Umbria | PDO |
| EVOO4 | Umbria | PDO |
| EVOO5 | Umbria | Blend |
| EVOO6 | Umbria | Blend |
| EVOO7 | Umbria | Blend |
| EVOO8 | Umbria | Blend |
| EVOO9 | Croatian | MV |
| EVOO10 | Spain | PDO |
| EVOO11 | Spain | MV |
| EVOO12 | Spain | MV |
| EVOO13 | Spain | MV |
| EVOO14 | Portugal | PDO |
| EVOO15 | Portugal | PDO |
| Code | Classification |
| SS1 | Sunflower Seed Oil (Brand A) |
| SS2 | Sunflower Seed Oil (Brand B) |
| SS3 | Sunflower Seed Oil (Brand C) |
| CS1 | Corn Seed Oil (Brand A) |
| CS2 | Corn Seed Oil (Brand B) |
| CS3 | Corn Seed Oil (Brand D) |
| PS1 | Peanut Seed Oil (Brand A) |
| PS2 | Peanut Seed Oil (Brand E) |
| A | Almond Oil (Brand F) |
| SeS | Sesame Seed Oil (Brand G) |
| Element | Certified Value | Experimental Result | Recovery (%) |
|---|---|---|---|
| Al | 598 ± 12 | 451 ± 15 | 75.5 |
| B | 33.3 ± 0.7 | 27.5 ± 0.3 | 82.7 |
| Ca | 50,500 ± 900 | 45,072 ± 1604 | 89.2 |
| Cd | 1.52 ± 0.04 | 1.32 ± 0.01 | 87.1 |
| Co | 0.57 ± 0.02 | 0.64 ± 0.01 | 112 |
| Cr | 1.99 ± 0.06 | 1.87 ± 0.02 | 94.2 |
| Cu | 4.70 ± 0.14 | 4.05 ± 0.09 | 86.2 |
| Fe | 368 ± 7 | 284 ± 4 | 77.2 |
| K | 27,000 ± 500 | 24,455 ± 916 | 90.6 |
| Mg | 12000 * | 9706 ± 307 | 80.9 |
| Mn | 246 ± 8 | 197 ± 5 | 80.1 |
| P | 2160 ± 40 | 2192 ± 28 | 101.5 |
| Sr | 85 * | 67.1 ± 0.8 | 79.0 |
| Zn | 30.9 ± 0.7 | 27.0 ± 0.4 | 87.4 |
| EVOO | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Al | Ca | Fe | K | Mg | Na | Se |
| EVOO 1 | 1.22 ± 0.03 | 11.4 ± 0.3 | 0.59 ± 0.02 | 0.43 ± 0.06 | 2.90 ± 0.04 | 2.59 ± 0.09 | 1.42 ± 0.03 |
| EVOO 2 | 0.79 ± 0.02 | 9.32 ± 0.30 | 0.99 ± 0.02 | 9.97 ± 0.72 | 2.08 ± 0.02 | 2.80 ± 0.07 | 1.47 ± 0.02 |
| EVOO 3 | 0.65 ± 0.02 | 16.2 ± 0.4 | 0.94 ± 0.02 | 5.53 ± 0.15 | 1.86 ± 0.02 | 1.07 ± 0.08 | 4.17 ± 0.02 |
| EVOO 4 | 0.68 ± 0.02 | 5.59 ± 0.18 | 0.56 ± 0.03 | 15.9 ± 0.02 | 2.11 ± 0.11 | 1.69 ± 0.10 | 1.25 ± 0.08 |
| EVOO 5 | 0.67 ± 0.02 | 4.72 ± 0.19 | 0.70 ± 0.02 | 17.4 ± 0.5 | 1.56 ± 0.02 | 1.75 ± 0.07 | 0.95 ± 0.06 |
| EVOO 6 | 0.94 ± 0.02 | 8.36 ± 0.61 | 0.47 ± 0.02 | 9.91 ± 0.13 | 2.26 ± 0.03 | 1.44 ± 0.11 | 1.10 ± 0.02 |
| EVOO 7 | 0.83 ± 0.02 | 6.99 ± 0.10 | 0.49 ± 0.02 | 11.5 ± 0.2 | 2.17 ± 0.05 | 1.00 ± 0.02 | 1.78 ± 0.11 |
| EVOO 8 | 0.60 ± 0.02 | 4.02 ± 0.18 | 0.89 ± 0.02 | 8.55 ± 0.22 | 1.47 ± 0.02 | 1.15 ± 0.06 | 2.21 ± 0.05 |
| EVOO 9 | 0.75 ± 0.02 | 7.74 ± 0.60 | 1.18 ± 0.02 | 8.33 ± 0.29 | 2.07 ± 0.07 | 1.91 ± 0.03 | 1.43 ± 0.03 |
| EVOO 10 | 0.60 ± 0.02 | 14.6 ± 0.3 | <0.02 | 0.06 ± 0.06 | 1.54 ± 0.02 | 0.90 ± 0.15 | 3.61 ± 0.10 |
| EVOO 11 | 0.70 ± 0.02 | 9.78 ± 0.13 | <0.02 | 2.02 ± 0.05 | 1.90 ± 0.06 | 1.94 ± 0.02 | 1.16 ± 0.08 |
| EVOO 12 | 0.72 ± 0.02 | 11.1 ± 0.3 | <0.02 | 1.07 ± 0.03 | 2.03 ± 0.02 | 1.86 ± 0.17 | 1.09 ± 0.11 |
| EVOO 13 | 1.21 ± 0.03 | 13.3 ± 0.7 | <0.02 | 0.89 ± 0.06 | 2.75 ± 0.02 | 1.43 ± 0.04 | 1.08 ± 0.29 |
| EVOO 14 | 0.63 ± 0.02 | 8.66 ± 0.30 | <0.02 | 0.35 ± 0.02 | 1.82 ± 0.03 | 1.38 ± 0.28 | 1.90 ± 0.17 |
| EVOO 15 | 0.64 ± 0.02 | 5.98 ± 0.21 | <0.02 | 0.42 ± 0.02 | 1.95 ± 0.02 | 1.40 ± 0.08 | 1.79 ± 0.02 |
| MIN | 0.60 | 4.02 | <0.02 | 0.06 | 1.47 | 0.90 | 0.95 |
| MAX | 1.22 | 16.2 | 1.18 | 17.4 | 2.90 | 2.80 | 4.17 |
| MEAN | 0.77 | 9.19 | 0.46 | 6.15 | 2.03 | 1.62 | 1.76 |
| VO | |||||||
| Sample | Al | Ca | Fe | K | Mg | Na | Se |
| CS1 | 0.60 ± 0.02 | 6.73 ± 0.18 | <0.02 | 0.71 ± 0.02 | 2.22 ± 0.02 | 1.61 ± 0.19 | 1.58 ± 0.03 |
| CS2 | 0.61 ± 0.03 | 13.9 ± 0.5 | <0.02 | <0.02 | 1.39 ± 0.02 | 0.24 ± 0.04 | 2.00 ± 0.08 |
| CS3 | 0.66 ± 0.02 | 9.91 ± 0.81 | <0.02 | <0.02 | 1.15 ± 0.02 | <0.02 | 1.61 ± 0.08 |
| SS1 | 0.55 ± 0.02 | 12.3 ± 0.1 | <0.02 | 0.03 ± 0.02 | 1.55 ± 0.07 | 0.24 ± 0.18 | 1.36 ± 0.02 |
| SS2 | 0.64 ± 0.02 | 12.1 ± 0.5 | <0.02 | <0.02 | 1.56 ± 0.05 | 0.79 ± 0.08 | 1.17 ± 0.13 |
| SS3 | 0.89 ± 0.02 | 8.25 ± 0.41 | 2.72 ± 0.03 | 8.26 ± 0.02 | 2.30 ± 0.03 | 1.91 ± 0.13 | 2.36 ± 0.05 |
| PS1 | 0.68 ± 0.02 | 20.3 ± 0.7 | < 0.02 | <0.02 | 1.77 ± 0.02 | 0.45 ± 0.08 | 3.44 ± 0.10 |
| PS2 | 0.84 ± 0.02 | 11.7 ± 0.6 | 2.45 ± 0.02 | 5.17 ± 0.09 | 2.15 ± 0.05 | 1.38 ± 0.24 | 2.13 ± 0.04 |
| A | 1.09 ± 0.02 | 37.0 ± 1.0 | <0.02 | 0.49 ± 0.06 | 2.67 ± 0.05 | 1.23 ± 0.02 | 0.83 ± 0.10 |
| SeS | 1.54 ± 0.02 | 29.0 ± 0.6 | 2.67 ± 0.00 | 8.00 ± 0.30 | 9.32 ± 0.20 | 4.67 ± 0.05 | 1.07 ± 0.02 |
| MIN | 0.55 | 6.73 | <0.02 | < 0.02 | 1.15 | <0.02 | 0.83 |
| MAX | 1.54 | 37.0 | 2.72 | 8.26 | 9.32 | 4.67 | 3.44 |
| MEAN | 0.81 | 16.12 | 0.79 | 2.12 | 2.61 | 1.25 | 1.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacomino, A.; Inaudi, P.; Silletta, G.; Diana, A.; Bertinetti, S.; Gaggero, E.; Malandrino, M.; Stilo, F.; Abollino, O. Analytical Methods for the Characterization of Vegetable Oils. Molecules 2023, 28, 153. https://doi.org/10.3390/molecules28010153
Giacomino A, Inaudi P, Silletta G, Diana A, Bertinetti S, Gaggero E, Malandrino M, Stilo F, Abollino O. Analytical Methods for the Characterization of Vegetable Oils. Molecules. 2023; 28(1):153. https://doi.org/10.3390/molecules28010153
Chicago/Turabian StyleGiacomino, Agnese, Paolo Inaudi, Gessica Silletta, Aleandro Diana, Stefano Bertinetti, Elisa Gaggero, Mery Malandrino, Federico Stilo, and Ornella Abollino. 2023. "Analytical Methods for the Characterization of Vegetable Oils" Molecules 28, no. 1: 153. https://doi.org/10.3390/molecules28010153







