A Waterborne Epoxy Composite Coating with Smart Corrosion Resistance Based on 2-Phenylbenzimidazole-5-sulfonic Acid/Layered Double Hydroxide Composite
Abstract
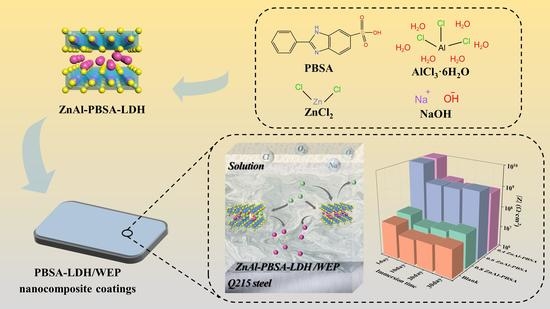
:1. Introduction
2. Results and Discussion
2.1. Characterization of MgAl-CO32−-LDH, ZnAl-NO3−-LDH, and ZnAl-PBSA-LDH
2.2. Characterization of Nanocomposite Coatings
2.3. Electrochemical Measurements
3. Materials and Methods
3.1. Materials
3.2. Preparation of MgAl-CO32−-LDH
3.3. Preparation of ZnAl-NO3−-LDH
3.4. Preparation of ZnAl-LDHs Loaded with PBSA
3.5. Coating Preparation
3.6. Characterization
3.7. Electrochemical Measures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, B.; Wang, Y. A Novel Design for Water-Based Modified Epoxy Coating with Anti-Corrosive Application Properties. Prog. Org. Coat. 2014, 77, 219–224. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Y.; Zhang, L.; Sheng, X. Construction of a High-Performance Anti-Corrosion and Anti-Wear Coating Based on the MXene@PTA-Zn(II): Electrochemical/Tribological Investigations. Prog. Org. Coat. 2023, 182, 107706. [Google Scholar] [CrossRef]
- Tian, H.; Li, W.; Liu, A.; Gao, X.; Han, P.; Ding, R.; Yang, C.; Wang, D. Controlled Delivery of Multi-Substituted Triazole by Metal-Organic Framework for Efficient Inhibition of Mild Steel Corrosion in Neutral Chloride Solution. Corros. Sci. 2018, 131, 1–16. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, W.; Li, D.; Sun, Y.; Wang, Z.; Hou, C.; Chen, L.; Cao, Y.; Liu, Y. Mechanical and Anticorrosive Properties of Graphene/Epoxy Resin Composites Coating Prepared by in-Situ Method. Int. J. Mol. Sci. 2015, 16, 2239–2251. [Google Scholar] [CrossRef] [Green Version]
- Hayatgheib, Y.; Ramezanzadeh, B.; Kardar, P.; Mahdavian, M. A Comparative Study on Fabrication of a Highly Effective Corrosion Protective System Based on Graphene Oxide-Polyaniline Nanofibers/Epoxy Composite. Corros. Sci. 2018, 133, 358–373. [Google Scholar] [CrossRef]
- Cui, M.; Ren, S.; Chen, J.; Liu, S.; Zhang, G.; Zhao, H.; Wang, L.; Xue, Q. Anticorrosive Performance of Waterborne Epoxy Coatings Containing Water-Dispersible Hexagonal Boron Nitride (h-BN) Nanosheets. Appl. Surf. Sci. 2017, 397, 77–86. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Tian, Y.; Xie, Y.; Sheng, X.; Jiang, X.; Zhang, X. Exfoliation and Functionalization of α-Zirconium Phosphate in One Pot for Waterborne Epoxy Coatings with Enhanced Anticorrosion Performance. Prog. Org. Coat. 2020, 138, 105390. [Google Scholar] [CrossRef]
- Sjåstad, A.O.; Andersen, N.H.; Vajeeston, P.; Karthikeyan, J.; Arstad, B.; Karlsson, A.; Fjellvåg, H. On the Thermal Stability and Structures of Layered Double Hydroxides Mg1−XAlx(OH)2(NO3)X·mH2O (0.18 ≤ x ≤ 0.38). Eur. J. Inorg. Chem. 2015, 2015, 1775–1788. [Google Scholar] [CrossRef]
- Nguyen Thuy, D.; To Thi Xuan, H.; Nicolay, A.; Paint, Y.; Olivier, M.-G. Corrosion Protection of Carbon Steel by Solvent Free Epoxy Coating Containing Hydrotalcites Intercalated with Different Organic Corrosion Inhibitors. Prog. Org. Coat. 2016, 101, 331–341. [Google Scholar] [CrossRef]
- Neves, C.S.; Bastos, A.C.; Salak, A.N.; Starykevich, M.; Rocha, D.; Zheludkevich, M.L.; Cunha, A.; Almeida, A.; Tedim, J.; Ferreira, M.G.S. Layered Double Hydroxide Clusters as Precursors of Novel Multifunctional Layers: A Bottom-Up Approach. Coatings 2019, 9, 328. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Zhang, C.; Song, L.; Zeng, R.; Liu, Z.; Cui, H. Corrosion of In-Situ Grown MgAl-LDH Coating on Aluminum Alloy. Trans. Nonferrous Met. Soc. China 2015, 25, 3498–3504. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.-G.; Zeng, R.-C.; Li, S.-Q.; Cui, H.-Z.; Song, L.; Han, E.-H. Corrosion Resistance of Mg–Al-LDH Coating on Magnesium Alloy AZ31. Surf. Coat. Technol. 2014, 258, 1152–1158. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent Progress in Layered Double Hydroxides (LDH)-Containing Hybrids as Adsorbents for Water Remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Q.; Li, B.; Xue, H.; Pang, H.; Chen, C. Recent Progress in Layered Double Hydroxide Based Materials for Electrochemical Capacitors: Design, Synthesis and Performance. Nanoscale 2017, 9, 15206–15225. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Qiu, S.; Yang, D.; Liu, S.; Zhao, H.; Wang, L.; Xue, Q. Active Anti-Corrosion of Epoxy Coating by Nitrite Ions Intercalated MgAl LDH. J. Hazard. Mater. 2020, 391, 122215. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, R.G.; Guan, H.; Mahajanam, S.; Wong, F. Active Corrosion Protection and Corrosion Sensing in Chromate-Free Organic Coatings. Prog. Org. Coat. 2003, 47, 174–182. [Google Scholar] [CrossRef]
- Salleh, S.Z.; Yusoff, A.H.; Zakaria, S.K.; Taib, M.A.A.; Abu Seman, A.; Masri, M.N.; Mohamad, M.; Mamat, S.; Ahmad Sobri, S.; Ali, A.; et al. Plant Extracts as Green Corrosion Inhibitor for Ferrous Metal Alloys: A Review. J. Clean. Prod. 2021, 304, 127030. [Google Scholar] [CrossRef]
- Mohsin, S.M.N.; Hussein, M.Z.; Sarijo, S.H.; Fakurazi, S.; Arulselvan, P.; Taufiq-Yap, Y.H. Nanolayered Composite with Enhanced Ultraviolet Ray Absorption Properties from Simultaneous Intercalation of Sunscreen Molecules. Int. J. Nanomed. 2018, 13, 6359. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Zhou, L.; Ferronato, C.; Salvador, A.; Yang, X.; Chovelon, J.-M. Degradation of Sunscreen Agent 2-Phenylbenzimidazole-5-Sulfonic Acid by TiO2 Photocatalysis: Kinetics, Photoproducts and Comparison to Structurally Related Compounds. Appl. Catal. B Environ. 2013, 140–141, 457–467. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, L.; Zhang, Y.; Ferronato, C.; Brigante, M.; Mailhot, G.; Yang, X.; Chovelon, J.-M. Photochemical Degradation of Sunscreen Agent 2-Phenylbenzimidazole-5-Sulfonic Acid in Different Water Matrices. Water Res. 2013, 47, 5865–5875. [Google Scholar] [CrossRef]
- Javidparvar, A.A.; Naderi, R.; Ramezanzadeh, B. Manipulating Graphene Oxide Nanocontainer with Benzimidazole and Cerium Ions: Application in Epoxy-Based Nanocomposite for Active Corrosion Protection. Corros. Sci. 2020, 165, 108379. [Google Scholar] [CrossRef]
- Aljourani, J.; Golozar, M.A.; Raeissi, K. The Inhibition of Carbon Steel Corrosion in Hydrochloric and Sulfuric Acid Media Using Some Benzimidazole Derivatives. Mater. Chem. Phys. 2010, 121, 320–325. [Google Scholar] [CrossRef]
- Ferrari, I.V.; Narducci, R.; Prestopino, G.; Costantino, F.; Mattoccia, A.; Di Giamberardino, L.; Nocchetti, M.; Di Vona, M.L.; Paolone, A.; Bini, M.; et al. Layered Double Hydroxides as a Drug Delivery Vehicle for S-Allyl-Mercapto-Cysteine (SAMC). Processes 2021, 9, 1819. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, S.; Zheng, D.; Wang, J.; Zhang, X.; Du, R.; Song, G.; Lin, C. Multifunctional Inhibition Based on Layered Double Hydroxides to Comprehensively Control Corrosion of Carbon Steel in Concrete. Corros. Sci. 2017, 126, 166–179. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of Two Dimensional Layered Double Hydroxide Nanosheets and Their Applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef]
- Smalenskaite, A.; Vieira, D.E.L.; Salak, A.N.; Ferreira, M.G.S.; Katelnikovas, A.; Kareiva, A. A Comparative Study of Co-Precipitation and Sol-Gel Synthetic Approaches to Fabricate Cerium-Substituted MgAl Layered Double Hydroxides with Luminescence Properties. Appl. Clay Sci. 2017, 143, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, G.; Yang, J. Preparation and Characterization of Poly(Vinyl Chloride)/Layered Double Hydroxide Nanocomposites with Enhanced Thermal Stability. Polymer 2008, 49, 3923–3927. [Google Scholar] [CrossRef]
- Alibakhshi, E.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B. A Comparative Study on Corrosion Inhibitive Effect of Nitrate and Phosphate Intercalated Zn-Al- Layered Double Hydroxides (LDHs) Nanocontainers Incorporated into a Hybrid Silane Layer and Their Effect on Cathodic Delamination of Epoxy Topcoat. Corros. Sci. 2017, 115, 159–174. [Google Scholar] [CrossRef]
- Pushparaj, S.S.C.; Forano, C.; Prevot, V.; Lipton, A.S.; Rees, G.J.; Hanna, J.V.; Nielsen, U.G. How the Method of Synthesis Governs the Local and Global Structure of Zinc Aluminum Layered Double Hydroxides. J. Phys. Chem. C 2015, 119, 27695–27707. [Google Scholar] [CrossRef]
- Guo, X.; Xu, S.; Zhao, L.; Lu, W.; Zhang, F.; Evans, D.G.; Duan, X. One-Step Hydrothermal Crystallization of a Layered Double Hydroxide/Alumina Bilayer Film on Aluminum and Its Corrosion Resistance Properties. Langmuir 2009, 25, 9894–9897. [Google Scholar] [CrossRef]
- Serdechnova, M.; Salak, A.N.; Barbosa, F.S.; Vieira, D.E.L.; Tedim, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Interlayer Intercalation and Arrangement of 2-Mercaptobenzothiazolate and 1,2,3-Benzotriazolate Anions in Layered Double Hydroxides: In Situ X-ray Diffraction Study. J. Solid State Chem. 2016, 233, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Liu, A.; Tian, H.; Wang, D. Controlled Release of Nitrate and Molybdate Intercalated in Zn-Al-Layered Double Hydroxide Nanocontainers towards Marine Anticorrosion Applications. Colloid Interface Sci. Commun. 2018, 24, 18–23. [Google Scholar] [CrossRef]
- Xu, Z.P.; Braterman, P.S. Synthesis, Structure and Morphology of Organic Layered Double Hydroxide (LDH) Hybrids: Comparison between Aliphatic Anions and Their Oxygenated Analogs. Appl. Clay Sci. 2010, 48, 235–242. [Google Scholar] [CrossRef]
- Liu, A.; Tian, H.; Li, W.; Wang, W.; Gao, X.; Han, P.; Ding, R. Delamination and Self-Assembly of Layered Double Hydroxides for Enhanced Loading Capacity and Corrosion Protection Performance. Appl. Surf. Sci. 2018, 462, 175–186. [Google Scholar] [CrossRef]
- Hu, J.; Gan, M.; Ma, L.; Zhang, J.; Xie, S.; Xu, F.; Shen, J.Z.X.; Yin, H. Preparation and Enhanced Properties of Polyaniline/Grafted Intercalated ZnAl-LDH Nanocomposites. Appl. Surf. Sci. 2015, 328, 325–334. [Google Scholar] [CrossRef]
- Kaghazchi, L.; Naderi, R.; Ramezanzadeh, B. Improvement of the Dual Barrier/Active Corrosion Inhibition Function of the Epoxy Composite Filled with Zinc Doped-Phytic Acid-Modified Graphene Oxide Nanosheets. Prog. Org. Coat. 2022, 168, 106884. [Google Scholar] [CrossRef]
- He, X.; Wu, J.; Li, S.; Chen, Y.; Zhang, L.; Sheng, X. In Situ Growth of Aminated Silica on MXene Nanosheets: A Novel 0D/2D Hybrid Structure for Multifunctional Waterborne Epoxy Composite Coatings. Prog. Org. Coat. 2022, 171, 107042. [Google Scholar] [CrossRef]
- Deip, A.R.; Leal, D.A.; Sakae, G.H.; Maia, F.; Berton, M.A.C.; Ferreira, M.G.S.; Marino, C.E.B. Performance of Commercial LDH Traps for Chloride Ion in a Commercial Corrosion Protection Primer for Petrochemical Industry. Corros. Eng. Sci. Technol. 2020, 55, 66–74. [Google Scholar] [CrossRef]
- Hirschorn, B.; Orazem, M.E.; Tribollet, B.; Vivier, V.; Frateur, I.; Musiani, M. Determination of Effective Capacitance and Film Thickness from Constant-Phase-Element Parameters. Electrochim. Acta 2010, 55, 6218–6227. [Google Scholar] [CrossRef]
- Feng, Y.; Li, H.; Zhang, M.; Jin, J.; Zhang, B.; Wang, Y.; Li, Z. Preparation of BTA@PDA/PANI Microcapsules and Anti-Corrosion Performance of Self-Healing Epoxy Coatings on Low Carbon Steel. Colloids Surf. Physicochem. Eng. Asp. 2022, 649, 129481. [Google Scholar] [CrossRef]
- Huang, H.; Huang, X.; Xie, Y.; Tian, Y.; Jiang, X.; Zhang, X. Fabrication of H-BN-RGO@PDA Nanohybrids for Composite Coatings with Enhanced Anticorrosion Performance. Prog. Org. Coat. 2019, 130, 124–131. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, M.; Xie, D.; Zhong, L.; Zhang, X. A Fast, Low Temperature Zinc Phosphate Coating on Steel Accelerated by Graphene Oxide. Corros. Sci. 2017, 128, 1–8. [Google Scholar] [CrossRef]
- Feng, Z.; Frankel, G.S. Evaluation of Coated Al Alloy Using the Breakpoint Frequency Method. Electrochim. Acta 2016, 187, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Huang, H.; Chen, F.; He, X.; Ma, Y.; Zhang, L.; Sheng, X.; Chen, Y.; Shchukina, E.; Shchukin, D. Reinforced Anticorrosion Performance of Waterborne Epoxy Coating with Eco-Friendly L-Cysteine Modified Ti3C2Tx MXene Nanosheets. Prog. Org. Coat. 2021, 161, 106478. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, D.; Lin, C. Effect of Physical Barrier and Anion-Exchange Process of Nitrate-Intercalated ZnAl Layered Double Hydroxide Films Grown on Al on Corrosion Protection. Surf. Coat. Technol. 2021, 421, 127436. [Google Scholar] [CrossRef]
- Tedim, J.; Kuznetsova, A.; Salak, A.N.; Montemor, F.; Snihirova, D.; Pilz, M.; Zheludkevich, M.L.; Ferreira, M.G.S. Zn–Al Layered Double Hydroxides as Chloride Nanotraps in Active Protective Coatings. Corros. Sci. 2012, 55, 1–4. [Google Scholar] [CrossRef]
- Ning, Y.; Jian, D.; Liu, S.; Chen, F.; Song, Y.; Li, S.; Liu, B. Designing a Ti3C2Tx MXene with Long-Term Antioxidant Stability for High-Performance Anti-Corrosion Coatings. Carbon 2023, 202, 20–30. [Google Scholar] [CrossRef]
- Zhang, S.; Hou, L.; Wei, Y.; Du, H.; Wei, H.; Liu, B.; Chen, X. Dual Functions of Chloride Ions on Corrosion Behavior of Mild Steel in CO2 Saturated Aqueous Solutions. Mater. Corros. 2019, 70, 888–896. [Google Scholar] [CrossRef]










| Sample | Ecorr (mV) | Icorr (A/cm2) | Rp (Ω·cm2) | ba (mV/dec) | bc (mV/dec) | CR (mpy) |
|---|---|---|---|---|---|---|
| Blank WEP | −3340 | 3.225 × 10−9 | 1.29 × 107 | 209.12 | −176.55 | 4.72 × 10−4 |
| 4-ZPL/WEP | −4010 | 1.986 × 10−10 | 1.93 × 108 | 175.60 | −177.56 | 2.90 × 10−5 |
| 6-ZPL/WEP | −2440 | 8.012 × 10−11 | 5.49 × 108 | 205.55 | −198.93 | 1.17 × 10−5 |
| 8-ZPL/WEP | −2960 | 2.286 × 10−9 | 1.91 × 107 | 199.40 | −203.42 | 3.34 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, C.; Wu, J.; Liu, Y.; Sheng, X.; Cheng, X.; Xiong, X.; Zhao, W. A Waterborne Epoxy Composite Coating with Smart Corrosion Resistance Based on 2-Phenylbenzimidazole-5-sulfonic Acid/Layered Double Hydroxide Composite. Molecules 2023, 28, 5199. https://doi.org/10.3390/molecules28135199
Ding C, Wu J, Liu Y, Sheng X, Cheng X, Xiong X, Zhao W. A Waterborne Epoxy Composite Coating with Smart Corrosion Resistance Based on 2-Phenylbenzimidazole-5-sulfonic Acid/Layered Double Hydroxide Composite. Molecules. 2023; 28(13):5199. https://doi.org/10.3390/molecules28135199
Chicago/Turabian StyleDing, Caiyou, Jiongxin Wu, Yuan Liu, Xinxin Sheng, Xiaoling Cheng, Xiaoyan Xiong, and Wenlin Zhao. 2023. "A Waterborne Epoxy Composite Coating with Smart Corrosion Resistance Based on 2-Phenylbenzimidazole-5-sulfonic Acid/Layered Double Hydroxide Composite" Molecules 28, no. 13: 5199. https://doi.org/10.3390/molecules28135199