Bio-Inspired Iron-Loaded Polydopamine Functionalized Montmorillonite as an Environmentally Friendly Flame Retardant for Epoxy Resin
Abstract
:1. Introduction
2. Results and Discussion
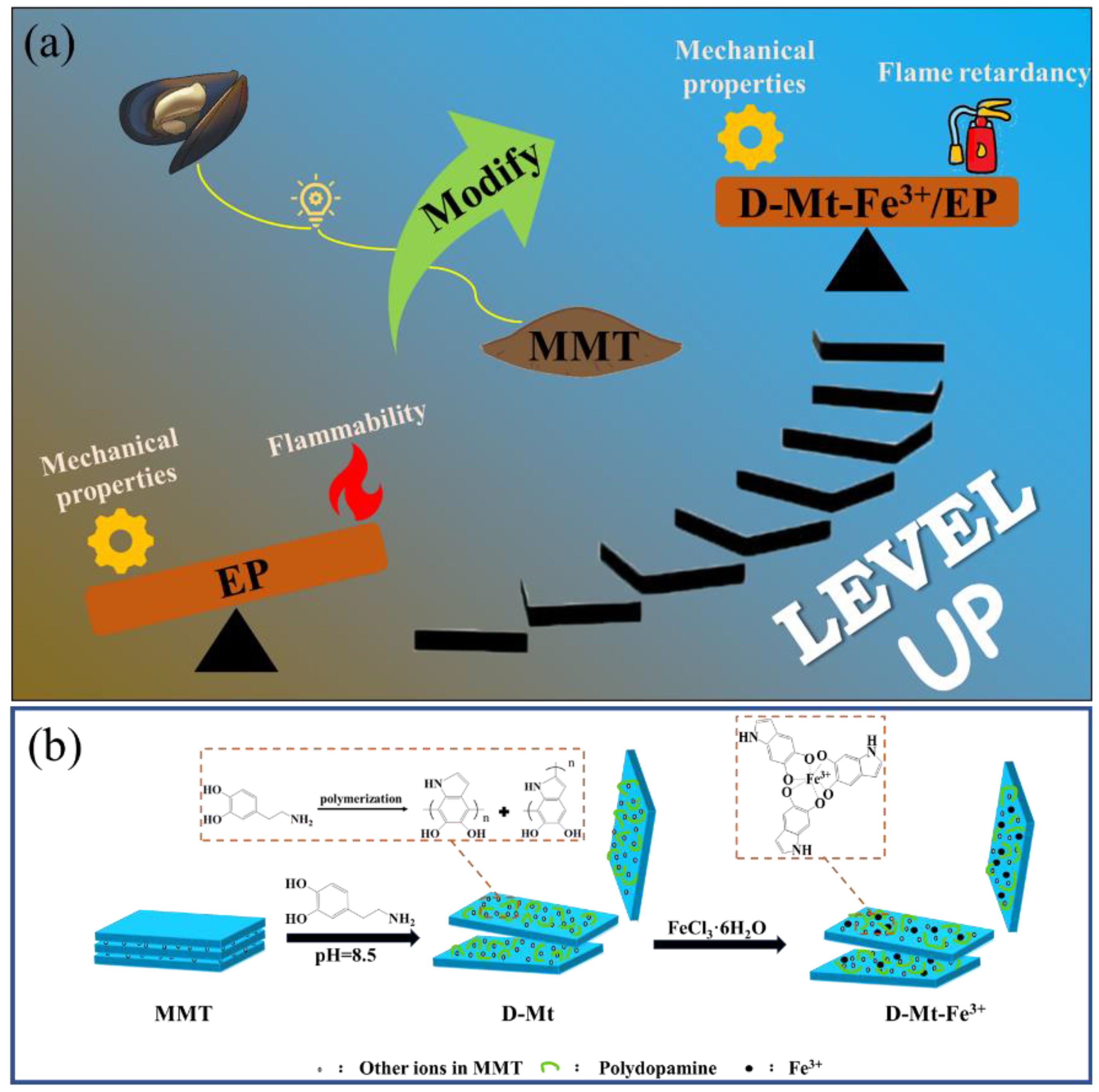
2.1. Structural Analysis of D-Mt-Fe3+
2.2. Thermal Stability
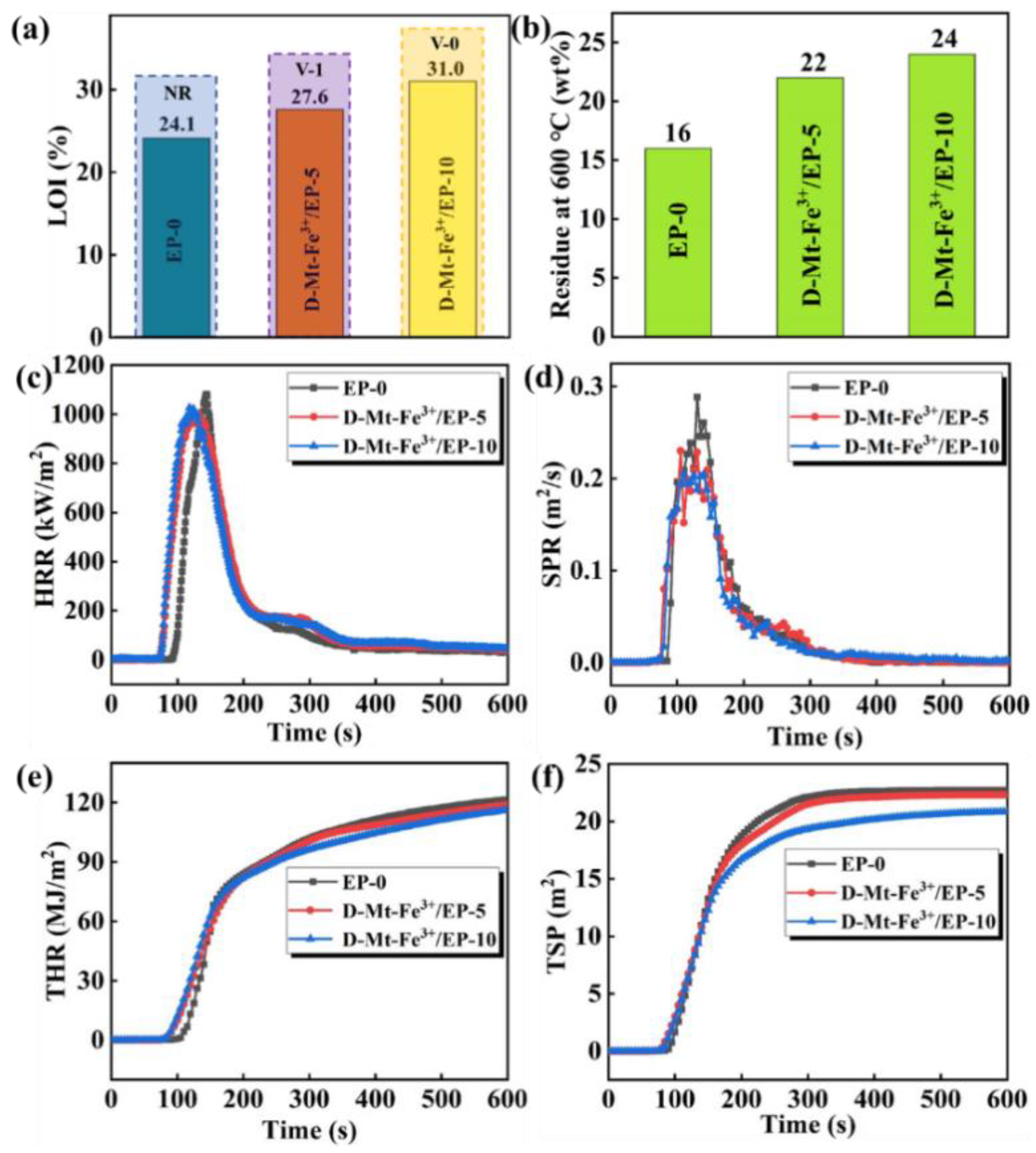
2.3. Flame Retardancy
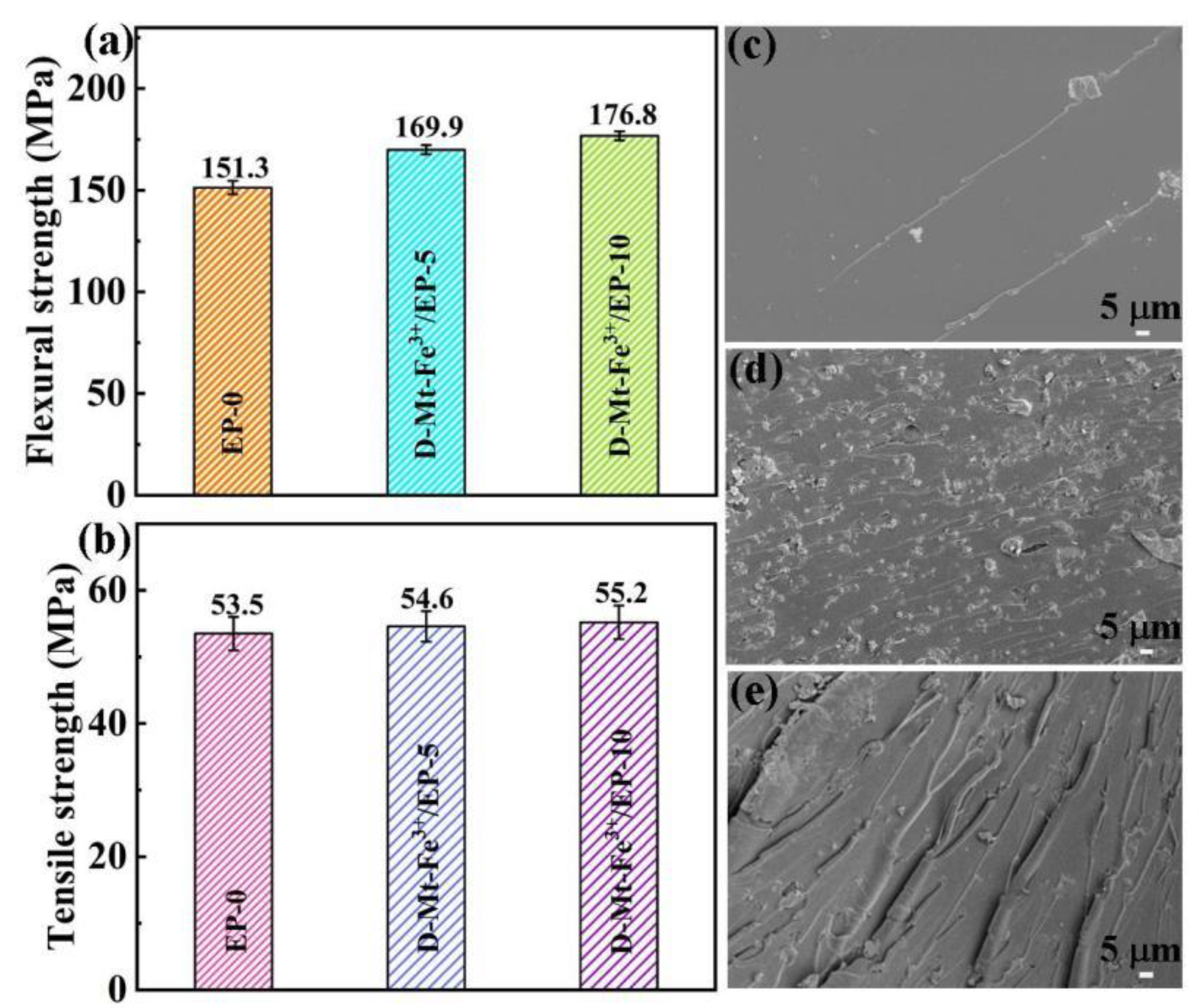
2.4. Mechanical Properties of D-Mt-Fe3+/EP
3. Experimental Procedure
3.1. Materials
3.2. Preparation of Iron-Loaded Polydopamine Functionalized Montmorillonite (D-Mt-Fe3+)
3.3. Preparation of Iron-Loaded Polydopamine Functionalized Montmorillonite/Epoxy Resin Composites (D-Mt-Fe3+/EP)
3.4. Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wicklein, B.; Kocjan, A.; Salazar-Alvarez, G.; Carosio, F.; Camino, G.; Antonietti, M.; Bergström, L. Thermally insulating and fire-retardant lightweight anisotropic foams based on nanocellulose and graphene oxide. Nat. Nanotechnol. 2015, 10, 277. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An overview of multifunctional epoxy nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Mi, X.; Liang, N.; Xu, H.; Wu, J.; Jiang, Y.; Nie, B.; Zhang, D. Toughness and its mechanisms in epoxy resins. Prog. Mater. Sci. 2022, 130, 100977. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Yin, G.-Z.; Wang, D.-Y. Construction of a novel three-in-one biomass based intumescent fire retardant through phosphorus functionalized metal-organic framework and β-cyclodextrin hybrids in achieving fire safe epoxy. Compos. Commun. 2021, 23, 100594. [Google Scholar] [CrossRef]
- Liu, S.; Chevali, V.S.; Xu, Z.; Hui, D.; Wang, H. A review of extending performance of epoxy resins using carbon nanomaterials. Compos. Part B-Eng. 2018, 136, 197–214. [Google Scholar] [CrossRef]
- Liu, Y.L. Flame-retardant epoxy resins from novel phosphorus-containing novolac. Polymer 2001, 42, 3445–3454. [Google Scholar] [CrossRef]
- Fahami, A.; Lee, J.; Lazar, S.; Grunlan, J.C. Mica-based multilayer nanocoating as a highly effective flame retardant and smoke suppressant. ACS Appl. Mater. Inter. 2020, 12, 19938–19943. [Google Scholar] [CrossRef]
- Wan, L.; Deng, C.; Chen, H.; Zhao, Z.-Y.; Huang, S.-C.; Wei, W.-C.; Yang, A.-H.; Zhao, H.-B.; Wang, Y.-Z. Flame-retarded thermoplastic polyurethane elastomer: From organic materials to nanocomposites and new prospects. Chem. Eng. J. 2021, 417, 129314. [Google Scholar] [CrossRef]
- Qiao, H.; Chen, M.; Chen, B.; Zhang, H.; Zheng, B.T. Understanding the curing kinetics of boron-based hyperbranched polysiloxane reinforced and toughened epoxy resin by rheology. Chem. Eng. J. 2023, 467, 143542. [Google Scholar] [CrossRef]
- Cheng, L.; Wu, W.; Meng, W.; Xu, S.; Han, H.; Yu, Y.; Qu, H.; Xu, J. Application of metallic phytates to poly(vinyl chloride) as efficient biobased phosphorous flame retardants. J. Appl. Polym. Sci. 2018, 135, 46601. [Google Scholar] [CrossRef]
- Duan, H.; Chen, Y.; Ji, S.; Hu, R.; Ma, H. A novel phosphorus/nitrogen-containing polycarboxylic acid endowing epoxy resin with excellent flame retardance and mechanical properties. Chem. Eng. J. 2019, 375, 121916. [Google Scholar] [CrossRef]
- Chen, M.; Lin, X.; Liu, C.; Zhang, H. An effective strategy to enhance the flame retardancy and mechanical properties of epoxy resin by using hyperbranched flame retardant. J. Mater. Sci. 2021, 56, 5956–5974. [Google Scholar] [CrossRef]
- Qi, X.-L.; Zhou, D.-D.; Zhang, J.; Hu, S.; Haranczyk, M.; Wang, D.-Y. Simultaneous improvement of mechanical and fire-safety properties of polymer composites with phosphonate-loaded MOF additives. ACS Appl. Mater. Inter. 2019, 11, 20325–20332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.-F.; Zhou, Z.-L.; Wu, T.; Li, Y.-Q.; Fu, S.-Y. Enhancements in LOX compatibility and fracture toughness of carbon fiber/epoxy composite for liquid oxygen cryotank by simultaneously introducing Al(OH)3 and phosphorous-nitrogen flame retardants. Compos. Commun. 2023, 39, 101562. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Wang, T.-C.; Lin, X.-B.; Song, X.; Cui, J.; Zhang, S.; Zhu, L. Facile construction of inorganic phosphorus/boron-layered double hydroxide complexes for highly efficient fire-safety epoxy resin. ACS Appl. Polym. Mater. 2023, 5, 3768–3776. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Wang, Y. Advanced flame-retardant methods for polymeric materials. Adv. Mater. 2021, 34, 2107905. [Google Scholar] [CrossRef]
- Sathish Kumar, P.; Senthil, S.M.; Pal, S.K.; Rajasekar, R. Organic/Montmorillonite Nanocomposite Membranes. Org.-Inorg. Compos. Polym. Electrolyte Membr. 2017, 133–164. [Google Scholar] [CrossRef]
- Tang, S.; Wachtendorf, V.; Klack, P.; Qian, L.; Dong, Y.; Schartel, B. Enhanced flame-retardant effect of a montmorillonite/phosphaphenanthrene compound in an epoxy thermoset. RSC Adv. 2017, 7, 720–728. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Han, J. Comparative performance of carbon nanotubes and nanoclays as flame retardants for epoxy composites. Results Phys. 2019, 14, 102481. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, Y.; Qian, L.; Chen, Y.; Xu, B. Strengthen flame retardancy of epoxy thermoset by montmorillonite particles adhering phosphorus-containing fragments. J. Appl. Polym. Sci. 2020, 137, 47500. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, W.; Yang, R. Synthesis of novel phosphonium bromide-montmorillonite nanocompound and its performance in flame retardancy and dielectric properties of epoxy resins. Polym. Compos. 2021, 42, 362–374. [Google Scholar] [CrossRef]
- He, X.D.; Zhang, W.C.; Yang, R.J. The characterization of DOPO/MMT nanocompound and its effect on flame retardancy of epoxy resin. Compos. Part A-Appl. S. 2017, 98, 124–135. [Google Scholar] [CrossRef]
- Yue, X.P.; Cao, P.P.; Yang, M.X.; Li, C.F.; Wang, Z.W. Improving flame retardant and smoke suppression efficiency for PBS by adding a tannin surface and interfacial modified IFR/MMT synergist. Eur. Polym. J. 2022, 181, 111662. [Google Scholar] [CrossRef]
- Natarajan, S.; Rathanasamy, R.; Palaniappan, S.K.; Velayudham, S.; Subburamamurthy, H.B.; Pal, K. Comparison of MA-g-PP effectiveness through mechanical performance of functionalised graphene reinforced polypropylene. Polimeros 2020, 30, e2020035. [Google Scholar] [CrossRef]
- Guo, Y.X.; Liu, J.H.; Gates, W.P.; Zhou, C.H. Organo-modification of montmorillonite. Clay Clay Miner. 2020, 68, 601–622. [Google Scholar] [CrossRef]
- Vo, V.-S.; Mahouche-Chergui, S.; Nguyen, V.-H.; Naili, S.; Carbonnier, B. Crucial role of covalent surface functionalization of clay nanofillers on improvement of the mechanical properties of bioepoxy resin. ACS Sustain. Chem. Eng. 2019, 7, 15211–15220. [Google Scholar] [CrossRef]
- Kim, D.; Mittal, G.; Kim, M.; Kim, S.H.; Rhee, K.Y. Surface modification of MMT and its effect on fatigue and fracture behavior of basalt/epoxy-based composites in a seawater environment. Appl. Surf. Sci. 2019, 473, 55–58. [Google Scholar] [CrossRef]
- Batool, S.; Gill, R.; Arshad, M.; Siddiqi, H.M.; Qureshi, S.S. Layer-by-layer fabrication of nacre inspired epoxy/MMT multilayered composites. J. Appl. Polym. Sci. 2018, 135, 46079. [Google Scholar] [CrossRef]
- Hua, Q.; Jing, B.; He, M.; Sun, P.; Zhao, Q.; Su, S.; Hu, G.; Ping, D.; Li, S. Preparation of modified montmorillonite/graphene oxide composites to enhance the anticorrosive performance of epoxy coatings. J. Coat. Technol. Res. 2023, 20, 1111–1119. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry—A decade of discovery. ACS Appl. Mater. Inter. 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.C.; He, F.; Peng, S.; Li, Y.; Shao, L.; Darling, S.B. Mussel-inspired surface engineering for water-remediation materials. Matter 2019, 1, 115–155. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Yuan, L.; Zhou, K.; Zeng, J.; Zhang, K.; Zheng, G.; Fu, Q.; Xia, Z.; Fu, Q. Mixed-solvent-mediated strategy for enhancing light absorption of polydopamine and adhesion Persistence of dopamine solutions. ACS Appl. Mater. Inter. 2023, 15, 22493–22505. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.W.; Qi, X.; Li, C.G.; Xian, G.J. Mussel-inspired fabrication of an environment-friendly and self-adhesive superhydrophobic polydopamine coating with excellent mechanical durability, anti-icing and self-cleaning performances. ACS Appl. Mater. Inter. 2023, 15, 26000–26015. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Razzak, A.; Deng, H.; Bai, H.H.; Zhu, J.F. Mussel-inspired ferric ion-polydopamine complex as a facile, green and efficient platform to functionalize carbon fiber for improving interfacial adhesion of composites. Surf. Interfaces 2023, 37, 102742. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Guo, J.R.; Chen, Y.T.; Yi, Y.H.; Zhu, G.B. Electrochemical sensing of phenolics based on copper/cobalt/nitrogen co-doped hollow nanocarbon spheres. J. Electroanal. Chem. 2021, 892, 115263. [Google Scholar] [CrossRef]
- Wang, J.; Ren, P.; Chen, Z.; Wu, T.; Wang, F.; You, C. Enhanced thermal conductivity of epoxy composites reinforced with oriented polydopamine-graphene foam complexed by metal ions. Appl. Surf. Sci. 2023, 610, 155309. [Google Scholar] [CrossRef]
- Li, X.Y.; Gao, P.; Tan, J.Y.; Xiong, K.Q.; Maitz, M.F.; Pan, C.J.; Wu, H.K.; Chen, Y.; Yang, Z.L.; Huang, N. Assembly of metal-phenolic/catecholamine networks for synergistically anti-inflammatory, antimicrobial and anticoagulant coatings. ACS Appl. Mater. Inter. 2018, 10, 40844–40853. [Google Scholar] [CrossRef]
- Zhang, J.H.; Kong, Q.H.; Wang, D.-Y. Simultaneously improving the fire safety and mechanical properties of epoxy resin with Fe-CNTs via large-scale preparation. J. Mater. Chem. A 2018, 6, 6376–6386. [Google Scholar] [CrossRef]
- Yang, W.M.; Wu, S.Y.; Yang, W.; Yuen, A.C.-Y.; Zhou, Y.; Yeoh, G.; Boyer, C.; Wang, C.H. Nanoparticles of polydopamine for improving mechanical and flame-retardant properties of an epoxy resin. Compos. Part B-Eng. 2020, 186, 107828. [Google Scholar] [CrossRef]
- Wang, M.; Yin, G.-Z.; Yang, Y.; Fu, W.; Palencia, J.L.D.; Zhao, J.; Wang, N.; Jiang, Y.; Wang, D.-Y. Bio-based flame retardants to polymers: A review. Adv. Ind. Eng. Polym. Res. 2023, 6, 132–155. [Google Scholar] [CrossRef]
- Bi, Q.; Yao, D.; Yin, G.Z.; You, J.; Liu, X.Q.; Wang, N.; Wang, D.Y. Surface engineering of magnesium hydroxide via bioinspired iron-loaded polydopamine as green and efficient strategy to epoxy composites with improved flame retardancy and reduced smoke release. React. Funct. Polym. 2020, 155, 104690. [Google Scholar] [CrossRef]
- Gong, J.; Chen, X.; Tang, T. Recent progress in controlled carbonization of (waste) polymers. Prog. Polym. Sci. 2019, 94, 1–32. [Google Scholar] [CrossRef]
- Shao, Z.-B.; Cui, J.; Li, X.-L.; Palencia, J.L.D.; Wang, D.-Y. Chemically inorganic modified ammonium polyphosphate as eco-friendly flame retardant and its high fire safety for epoxy resin. Compos. Commun. 2021, 28, 100959. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, K.-X.; Wang, X.; Chen, M.-J.; Xin, F.; Liu, Z.-G. Synergistic effects and flame-retardant mechanism of aluminum diethyl phosphinate in combination with melamine polyphosphate and aluminum oxide in epoxy resin. J. Therm. Anal. Calorim. 2018, 134, 1637–1646. [Google Scholar] [CrossRef]
- Sai, T.; Ran, S.; Guo, Z.; Song, P.; Fang, Z. Recent advances in fire-retardant carbon-based polymeric nanocomposites through fighting free radicals. SusMat 2022, 2, 411–434. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Jian, R.-K.; Wang, D.-Y. Wang, Bioinspired iron-loaded polydopamine nanospheres as green flame retardants for epoxy resin via free radical scavenging and catalytic charring. J. Mater. Chem. A 2020, 8, 2529–2538. [Google Scholar] [CrossRef]
- Shi, C.; Qian, X.; Jing, J. Phosphorylated cellulose/Fe3+ complex: A novel flame retardant for epoxy resins. Polym. Advan. Technol. 2020, 32, 183–189. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, X.; Luo, Y.; Shi, X.; Asiri, A.M.; Li, T.; Sun, X. Ambient NH3 synthesis via electrochemical reduction of N2 over cubic sub-micron SnO2 particles. Chem. Commun. 2018, 54, 12966–12969. [Google Scholar] [CrossRef]
- Yuan, B.H.; Hu, Y.; Chen, X.F.; Shi, Y.Q.; Niu, Y.; Zhang, Y.; He, S.; Dai, H.M. Dual modification of graphene by polymeric flame retardant and Ni(OH)2 nanosheets for improving flame retardancy of polypropylene. Compos Part A-Appl. S. 2017, 100, 106–117. [Google Scholar] [CrossRef]
- Wang, L.C.; Tawiah, B.; Shi, Y.Q.; Cai, S.C.; Rao, X.H.; Liu, C.; Yang, Y.; Yang, F.Q.; Yu, B.; Liang, Y.T.; et al. Highly effective flame-retardant rigid polyurethane foams: Fabrication and applications in inhibition of coal combustion. Polymers 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- Chi, C.; He, S.Y.; Peng, C.H.; Zeng, B.R.; Xia, L.; Miao, Z.X.; Xu, H.; Wang, S.C.; Chen, G.R.; Dai, L.Z. LDH@boronate polymer core–Shell nanoparticles: Nanostructure design for synergistically enhancing the flame retardancy of epoxy resin. Polymers 2023, 15, 2198. [Google Scholar] [CrossRef]
- Xu, W.; Li, W.; Wang, X.; Zhang, X.; Cheng, Z. Effect of different ionic layered compounds decorated with zinc hydroxystannate on flame retardancy and smoke performance of epoxy resin. Polym. Advan. Technol. 2020, 31, 731–740. [Google Scholar] [CrossRef]
- Tan, Y.; Shao, Z.-B.; Chen, X.-F.; Long, J.-W.; Chen, L.; Wang, Y.-Z. Novel multifunctional organic–inorganic hybrid curing agent with high flame-retardant efficiency for epoxy resin. ACS Appl. Mater. Inter. 2015, 7, 17919–17928. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, X.; Guo, Y.; Liu, X.; Zhao, X.; Zhou, H.; Zhang, S.; Zhao, T. Synergistic effects of ladder and cage structured phosphorus-containing POSS with tetrabutyl titanate on flame retardancy of vinyl epoxy resins. Polymers 2021, 13, 1363. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Luo, W.; Lv, J.; Lin, S.; Zheng, B.; Zhang, H.; Chen, M. A universal strategy toward flame retardant epoxy resin with ultra-tough and transparent properties. Polym. Degrad. Stab. 2022, 205, 110132. [Google Scholar] [CrossRef]

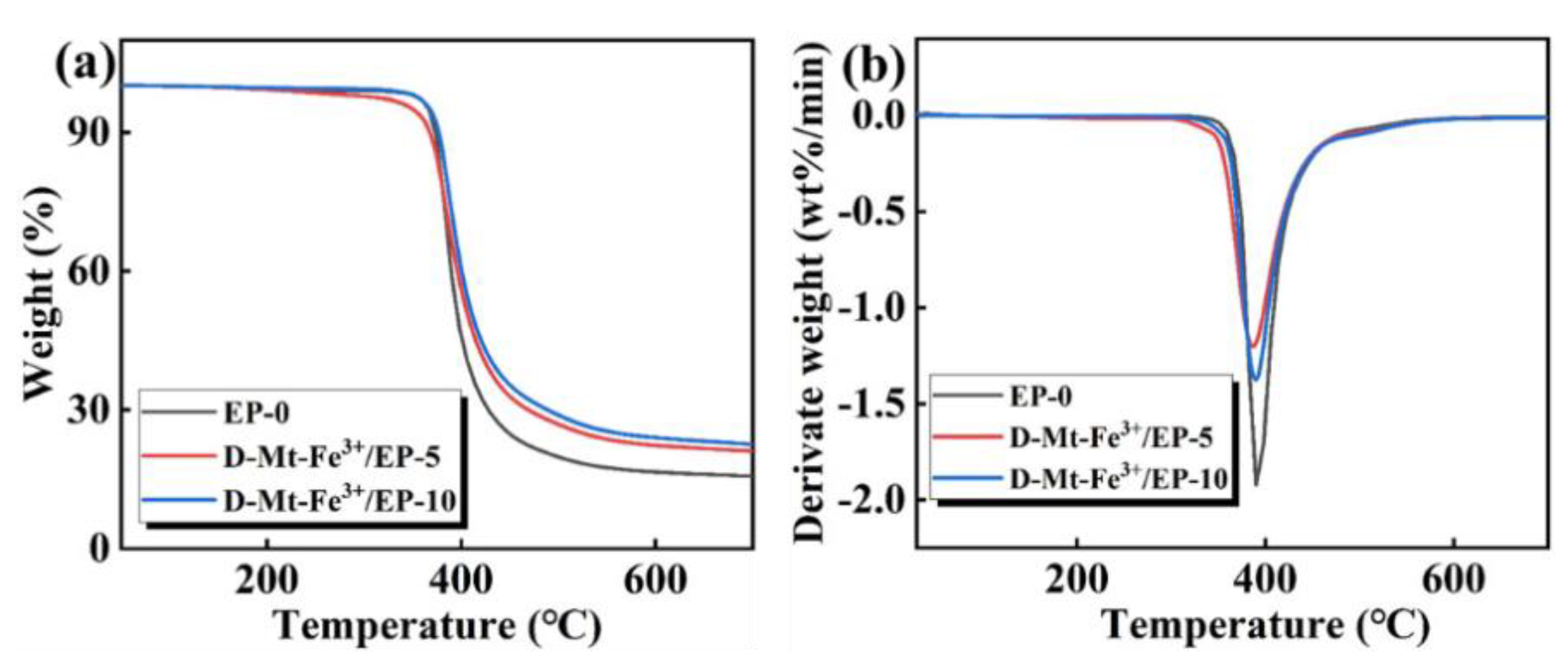


| Sample | T5% (°C) | Tmax (°C) | RC600 (wt%) |
|---|---|---|---|
| EP-0 | 370 | 390 | 16 |
| D-Mt-Fe3+/EP-5 | 350 | 386 | 22 |
| D-Mt-Fe3+/EP-10 | 367 | 389 | 24 |
| Sample | TTI (s) | Av-EHC (MJ/kg) | MLR (g/s) | TSP (m2) | Av-COY (kg/kg) | Av-CO2Y (kg/kg) |
|---|---|---|---|---|---|---|
| EP-0 | 87 | 26 | 0.053 | 22.7 | 0.16 | 8.9 |
| D-Mt-Fe3+/EP-5 | 74 | 25 | 0.050 | 22.3 | 0.46 | 4.2 |
| D-Mt-Fe3+/EP-10 | 74 | 23 | 0.049 | 20.8 | 0.42 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, J.; Li, D.; Zhong, W.; Luo, W.; Zhang, H.; Chen, M. Bio-Inspired Iron-Loaded Polydopamine Functionalized Montmorillonite as an Environmentally Friendly Flame Retardant for Epoxy Resin. Molecules 2023, 28, 5354. https://doi.org/10.3390/molecules28145354
Lan J, Li D, Zhong W, Luo W, Zhang H, Chen M. Bio-Inspired Iron-Loaded Polydopamine Functionalized Montmorillonite as an Environmentally Friendly Flame Retardant for Epoxy Resin. Molecules. 2023; 28(14):5354. https://doi.org/10.3390/molecules28145354
Chicago/Turabian StyleLan, Jiashui, Dingsi Li, Wei Zhong, Wenhui Luo, Huagui Zhang, and Mingfeng Chen. 2023. "Bio-Inspired Iron-Loaded Polydopamine Functionalized Montmorillonite as an Environmentally Friendly Flame Retardant for Epoxy Resin" Molecules 28, no. 14: 5354. https://doi.org/10.3390/molecules28145354




