Simultaneous Enhancement of Flame Resistance and Antimicrobial Activity in Epoxy Nanocomposites Containing Phosphorus and Silver-Based Additives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of BPH
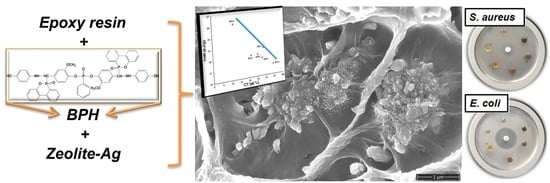
2.2. Structural and Morphological Characterization of Epoxy-Based Nanocomposites
2.3. Thermal Characterization of EPZ Nanocomposites
2.4. Microscale Combustion Calorimetry (MCC) Tests
2.5. Antimicrobial Activity
3. Materials and Methods
3.1. Materials
3.2. Synthesis of bis(4-(((4-Hidroxyphenyl)amino)(6-oxido-6H-dibenzo[c,e][1,2]oxaphosphinin-6-yl)methyl)-2-methoxyphenyl) Phenylphosphonate (BPH)
- 1H NMR (600.13 MHz, DMSO-d6, ppm): 3.53–3.56 (7.3H, dd, J = 6.3 Hz, 6.1 Hz, OCH3), 4.95–5.39 (1.8H, m, CH both isomers), 5.72–6.13 (1.8H, m, NH both isomers), 6.41–6.58 (7.3H, dd, JH-P = 9.1 Hz, 9.1 Hz, H14, H15, H17, H18 both isomers), 6.88–7.02 (4.7H, m, H2 isomer a, H24, H21), 7.09–7.13 (2H, m, H20), 7.18–7.22 (1H, m, H2 isomer b), 7.29 (1.9H, t, J = 6.91 Hz, H4), 7.42 (3H, t, J = 7.9Hz, H3), 7.57–7.61 (2.8H, m, H27, H10), 7.70–7.74 (2.2H, m, H28), 7.78 (1.2H, t, J = 7.6 Hz, H9), 7.89–7.92 (1.7H, m, H26), 8.5 (1H, m, H11), 8.12–8.21 (3.5H, m, H5, H8), 8.53–8.56 (1.7H, s, OH).
- 13C (100.61 MHz, DMSO-d6, ppm): 55.58 (s, OCH3), 55.8–56.8 (m, CH), 113.5 (s, C20), 115.3 (s, C14, C15, C17, C18), 119.8–120.1 (m, C2), 120.6 (C24, C21), 121.5 (dd, JC-P = 9.8 Hz, JC-P = 10.4 Hz, C6), 122.5 (d, JC-P = 47.6 Hz, C7), 123.4–123.9 (m, C8), 124.6 (d, JC-P = 11 Hz, C4), 125.6 (d, JC-P = 25.2 Hz, C5), 128.1–128.6 (m, C3a (isomer a), C27, C10), 130.3–130.4 (m, C3b (isomer b)), 130.6 (d, JC-P = 16.4 Hz, C28), 131.8 (d, JC-P = 10.6 Hz, C26, C11), 133.2–133.7 (m, C9, C25), 135.2–135.4 (m, C12), 138.2–138.3 (m, C19), 13.0–139.4 (m, C13), 148.7–148.9 (m, C1, C22), 149.4 (s, C16), 149.9 (C23).
3.3. Preparation of Epoxy Resin Nanocomposites
3.4. Measurements
3.4.1. FTIR Analysis
3.4.2. NMR Analysis
3.4.3. Scanning Electron Microscopy (SEM)
3.4.4. TGA Measurements
3.4.5. Differential Scanning Calorimetry (DSC) Measurements
3.4.6. Microscale Combustion Calorimetry (MCC)
3.5. Antimicrobial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Peerzada, M.; Abbasi, S.; Lau, K.T.; Hameed, N. Additive Manufacturing of Epoxy Resins: Materials, Methods, and Latest Trends. Ind. Eng. Chem. Res. 2020, 59, 6375–6390. [Google Scholar] [CrossRef]
- Frketic, J.; Dickens, T.; Ramakrishnan, S. Automated manufacturing and processing of fiber-reinforced polymer (FRP) composites: An additive review of contemporary and modern techniques for advanced materials manufacturing. Addit. Manuf. 2017, 14, 69–86. [Google Scholar] [CrossRef] [Green Version]
- Jun, Z.; Hai, L.; Xiaojian, Z.; Bowen, L. Epoxy Resin Adhesives: Modification and Applications. In Epoxy-Based Composites; Samson Jerold Samuel, C., Ramesh, A., Meera, M.R., Eds.; IntechOpen: Rijeka, Croatia, 2022; Chapter 5. [Google Scholar]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Carja, I.-D.; Serbezeanu, D.; Vlad-Bubulac, T.; Hamciuc, C.; Coroaba, A.; Lisa, G.; López, C.G.; Soriano, M.F.; Pérez, V.F.; Romero Sánchez, M.D. A straightforward, eco-friendly and cost-effective approach towards flame retardant epoxy resins. J. Mater. Chem. A 2014, 2, 16230–16241. [Google Scholar] [CrossRef]
- Fang, M.; Qian, J.; Wang, X.; Chen, Z.; Guo, R.; Shi, Y. Synthesis of a Novel Flame Retardant Containing Phosphorus, Nitrogen, and Silicon and Its Application in Epoxy Resin. ACS Omega 2021, 6, 7094–7105. [Google Scholar] [CrossRef]
- Issa, C.A. Introduction to Multifunctional Epoxy Composites. In Multifunctional Epoxy Resins: Self-Healing, Thermally and Electrically Conductive Resins; Hameed, N., Capricho, J.C., Salim, N., Thomas, S., Eds.; Springer Nature: Singapore, 2023; pp. 1–13. [Google Scholar]
- Lee, S.-H.; Oh, S.-W.; Lee, Y.-H.; Kim, I.-J.; Lee, D.-J.; Lim, J.-C.; Park, C.-C.; Kim, H.-D. Preparation and properties of flame-retardant epoxy resins containing reactive phosphorus flame retardant. J. Eng. Fibers Fabr. 2020, 15, 1558925020901323. [Google Scholar] [CrossRef]
- Gu, L.; Chen, G.; Yao, Y. Two novel phosphorus–nitrogen-containing halogen-free flame retardants of high performance for epoxy resin. Polym. Degrad. Stab. 2014, 108, 68–75. [Google Scholar] [CrossRef]
- Varganici, C.D.; Rosu, L.; Lehner, S.; Hamciuc, C.; Jovic, M.; Rosu, D.; Mustata, F.; Gaan, S. Semi–interpenetrating networks based on epoxy resin and oligophosphonate: Comparative effect of three hardeners on the thermal and fire properties. Mater. Des. 2021, 212, 110237. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, H.; Xu, M.; Li, B.; Lai, T. Synthesis of a novel phosphonate flame retardant and its application in epoxy resins. J. Appl. Polym. Sci. 2015, 132, 42765. [Google Scholar] [CrossRef]
- Zheng, P.; Wang, R.; Wang, D.; Peng, X.; Zhao, Y.; Liu, Q. A phosphorus-containing hyperbranched phthalocyanine flame retardant for epoxy resins. Sci. Rep. 2021, 11, 17731. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cassia, R.; Valle, R.; Valle, J.; Bezerra, F.; Meng, X.; Lis, M.; Arias, L. Recent Progress of DOPO-Containing Compounds as Flame Retardants for Versatile Polymeric Materials: Review. World J. Text. Eng. Technol. 2020, 6, 89–103. [Google Scholar] [CrossRef]
- Liu, X.; Liang, B. Impact of a novel phosphorus-nitrogen flame retardant curing agent on the properties of epoxy resin. Mater. Res. Express 2017, 4, 125103. [Google Scholar] [CrossRef]
- You, G.; Cheng, Z.; Tang, Y.; He, H. Functional Group Effect on Char Formation, Flame Retardancy and Mechanical Properties of Phosphonate–Triazine-based Compound as Flame Retardant in Epoxy Resin. Ind. Eng. Chem. Res. 2015, 54, 7309–7319. [Google Scholar] [CrossRef]
- Ramezanpour, J.; Ataei, S.; Khorasani, S.N. Development of smart epoxy coating through click reaction using a vegetable oil. Prog. Org. Coat. 2022, 170, 106985. [Google Scholar] [CrossRef]
- Ren, J.; Piao, J.; Wang, Y.; Wang, Y.; Feng, T.; Liu, W.; Dong, H.; Chen, W.; Jiao, C.; Chen, X. Effect of functionalized oyster shell powder with ammonium polyphosphate on fire safety performance of epoxy resin. Prog. Org. Coat. 2022, 172, 107054. [Google Scholar] [CrossRef]
- Patil, D.M.; Phalak, G.A.; Mhaske, S.T. Novel phosphorus-containing epoxy resin from renewable resource for flame-retardant coating applications. J. Coat. Technol. Res. 2019, 16, 531–542. [Google Scholar] [CrossRef]
- Sag, J.; Goedderz, D.; Kukla, P.; Greiner, L.; Schönberger, F.; Döring, M. Phosphorus-Containing Flame Retardants from Biobased Chemicals and Their Application in Polyesters and Epoxy Resins. Molecules 2019, 24, 3746. [Google Scholar] [CrossRef] [Green Version]
- Darge, A.; Kahsay, A.G.; Hailekiros, H.; Niguse, S.; Abdulkader, M. Bacterial contamination and antimicrobial susceptibility patterns of intensive care units medical equipment and inanimate surfaces at Ayder Comprehensive Specialized Hospital, Mekelle, Northern Ethiopia. BMC Res. Notes 2019, 12, 621. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Yan, Y.; Wang, Y.; Sun, P.; Qi, R. Antibacterial epoxy composites with addition of natural Artemisia annua waste. e-Polymers 2020, 20, 262–271. [Google Scholar] [CrossRef]
- El-Fattah, M.A.; El Saeed, A.M.; Azzam, A.M.; Abdul-Raheim, A.-R.M.; Hefni, H.H.H. Improvement of corrosion resistance, antimicrobial activity, mechanical and chemical properties of epoxy coating by loading chitosan as a natural renewable resource. Prog. Org. Coat. 2016, 101, 288–296. [Google Scholar] [CrossRef]
- Modjinou, T.; Versace, D.-L.; Abbad-Andaloussi, S.; Langlois, V.; Renard, E. Antibacterial and antioxidant photoinitiated epoxy co-networks of resorcinol and eugenol derivatives. Mater. Today Commun. 2017, 12, 19–28. [Google Scholar] [CrossRef]
- Bertani, R.; Bartolozzi, A.; Pontefisso, A.; Quaresimin, M.; Zappalorto, M. Improving the Antimicrobial and Mechanical Properties of Epoxy Resins via Nanomodification: An Overview. Molecules 2021, 26, 5426. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Ashby, R.; Fan, X.; Moreau, R.A.; Strahan, G.D.; Nuñez, A.; Ngo, H. Phenolic fatty acid-based epoxy curing agent for antimicrobial epoxy polymers. Prog. Org. Coat. 2020, 141, 105536. [Google Scholar] [CrossRef]
- Islam, M.R.; Parimalam, M.; Sumdani, M.G.; Taher, M.A.; Asyadi, F.; Yenn, T.W. Rheological and antimicrobial properties of epoxy-based hybrid nanocoatings. Polym. Test. 2020, 81, 106202. [Google Scholar] [CrossRef]
- Li, R.; Yang, G.; Wang, Y.; Liu, L.; Wang, Q.; Wang, G.; Ouyang, X. Synthesis of antibacterial polyether biguanide curing agent and its cured antibacterial epoxy resin. Des. Monomers Polym. 2021, 24, 63–72. [Google Scholar] [CrossRef]
- Dutta, P.; Wang, B. Zeolite-supported silver as antimicrobial agents. Coord. Chem. Rev. 2019, 383, 1–29. [Google Scholar] [CrossRef]
- Matsumura, Y.; Yoshikata, K.; Kunisaki, S.; Tsuchido, T. Mode of bactericidal action of silver zeolite and its comparison with that of silver nitrate. Appl. Environ. Microbiol. 2003, 69, 4278–4281. [Google Scholar] [CrossRef] [Green Version]
- Saengmee-Anupharb, S.; Srikhirin, T.; Thaweboon, B.; Thaweboon, S.; Amornsakchai, T.; Dechkunakorn, S.; Suddhasthira, T. Antimicrobial effects of silver zeolite, silver zirconium phosphate silicate and silver zirconium phosphate against oral microorganisms. Asian Pac. J. Trop. Biomed. 2013, 3, 47–52. [Google Scholar] [CrossRef] [Green Version]
- Kwakye-Awuah, B.; Williams, C.; Kenward, M.A.; Radecka, I. Antimicrobial action and efficiency of silver-loaded zeolite X. J. Appl. Microbiol. 2008, 104, 1516–1524. [Google Scholar] [CrossRef]
- Hamciuc, C.; Hamciuc, E.; Popovici, D.; Danaila, A.I.; Butnaru, M.; Rimbu, C.; Carp-Carare, C.; Kalvachev, Y. Biocompatible poly(ether-ether-ketone)/Ag-zeolite L composite films with antimicrobial properties. Mater. Lett. 2018, 212, 339–342. [Google Scholar] [CrossRef]
- Hamciuc, C.; Vlad-Bubulac, T.; Bercea, M.; Suflet, D.M.; Doroftei, F.; Rîmbu, C.M.; Enache, A.A.; Kalvachev, Y.; Todorova, T.; Butnaru, M.; et al. Electrospun Copoly(ether imide) Nanofibers Doped with Silver-Loaded Zeolite as Materials for Biomedical Applications. ACS Appl. Polym. Mater. 2022, 4, 6080–6091. [Google Scholar] [CrossRef]
- Lin, C.H.; Hwang, T.Y.; Taso, Y.R.; Lin, T.L. Phosphorus-Containing Epoxy Curing Agents via Imine Linkage. Macromol. Chem. Phys. 2007, 208, 2628–2641. [Google Scholar] [CrossRef]
- Przystas, A.; Jovic, M.; Salmeia, K.A.; Rentsch, D.; Ferry, L.; Mispreuve, H.; Perler, H.; Gaan, S. Some Key Factors Influencing the Flame Retardancy of EDA-DOPO Containing Flexible Polyurethane Foams. Polymers 2018, 10, 1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weil, E.D. Phosphorus-Based Flame Retardants. In Flame-Retardant Polymeric Materials; Lewin, M., Atlas, S.M., Pearce, E.M., Eds.; Springer: Boston, MA, USA, 1978; Volume 2, pp. 103–131. [Google Scholar]
- Sinha Ray, S.; Kuruma, M. Types of Flame Retardants Used for the Synthesis of Flame-Retardant Polymers. In Halogen-Free Flame-Retardant Polymers: Next-Generation Fillers for Polymer Nanocomposite Applications; Sinha Ray, S., Kuruma, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 15–45. [Google Scholar]
- Zhao, P.; Rao, W.; Luo, H.; Wang, L.; Liu, Y.; Yu, C. Novel organophosphorus compound with amine groups towards self-extinguishing epoxy resins at low loading. Mater. Des. 2020, 193, 108838. [Google Scholar] [CrossRef]
- Hamciuc, C.; Vlad-Bubulac, T.; Serbezeanu, D.; Carja, I.-D.; Hamciuc, E.; Lisa, G.; Pérez, V.F. Environmentally friendly fire-resistant epoxy resins based on a new oligophosphonate with high flame retardant efficiency. RSC Adv. 2016, 6, 22764–22776. [Google Scholar] [CrossRef]
- Peng, W.; Xu, Y.-x.; Nie, S.-b.; Yang, W. A bio-based phosphaphenanthrene-containing derivative modified epoxy thermosets with good flame retardancy, high mechanical properties and transparency. RSC Adv. 2021, 11, 30943–30954. [Google Scholar] [CrossRef] [PubMed]
- Vlad-Bubulac, T.; Hamciuc, C. Synthesis and properties of new aromatic copolyesters containing phosphorous cyclic bulky groups. Polym. Eng. Sci. 2010, 50, 1028–1035. [Google Scholar] [CrossRef]
- Lyon, R.; Speitel, L.; Walters, R.; Crowley, S. Fire-resistant elastomers. Fire Mater. 2003, 27, 195–208. [Google Scholar] [CrossRef]
- Cogen, J.M.; Lin, T.S.; Lyon, R.E. Correlations between pyrolysis combustion flow calorimetry and conventional flammability tests with halogen-free flame retardant polyolefin compounds. Fire Mater. 2009, 33, 33–50. [Google Scholar] [CrossRef]
- Guerrero Correa, M.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469. [Google Scholar] [CrossRef] [PubMed]
- Crisan, C.M.; Mocan, T.; Manolea, M.; Lasca, L.I.; Tăbăran, F.-A.; Mocan, L. Review on Silver Nanoparticles as a Novel Class of Antibacterial Solutions. Appl. Sci. 2021, 11, 1120. [Google Scholar] [CrossRef]
- Santos, K.S.; Prata Silva, A.P.B.; Barbosa, A.M.; Mélo Silva, I.S.d.; Cardoso, J.C.; Dariva, C.; da Costa, L.P.; Pinheiro, M.S.; Padilha, F.F. Antibacterial evaluation silver of nanocomposite on Staphylococcus aureus and Escherichia coli. BMC Proc. 2014, 8, P8. [Google Scholar] [CrossRef]
- Borhan, O.; Muresan, A.; Radu, C.D.; Muresan, E.; Rîmbu, C.M.; Sandu, I.G. Silver Nanoparticles Used to Obtain Cellulosic Materials with Antibacterial Properties. Rev. Chim. 2015, 66, 1796–1801. [Google Scholar]
- Quaresimin, M.; Bertani, R.; Zappalorto, M.; Pontefisso, A.; Simionato, F.; Bartolozzi, A. Multifunctional polymer nanocomposites with enhanced mechanical and anti-microbial properties. Compos. Part B Eng. 2015, 80, 108–115. [Google Scholar] [CrossRef]
- Monsif, M.; Zerouale, A.; Kandri, N.I.; Bertani, R.; Bartolozzi, A.; Bresolin, B.M.; Zorzi, F.; Tateo, F.; Zappalorto, M.; Quaresimin, M.; et al. Multifunctional Epoxy/Nanocomposites Based on Natural Moroccan Clays with High Antimicrobial Activity: Morphological, Thermal and Mechanical Properties. J. Nanomater. 2019, 2019, 2810901. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Liu, Z.; Wang, X.; Pan, Y.T.; Kuehnert, I.; Gehde, M.; Wang, D.Y.; Leuteritz, A. Renewable vanillin based flame retardant for poly(lactic acid): A way to enhance flame retardancy and toughness simultaneously. RSC Adv. 2018, 8, 42189–42199. [Google Scholar] [CrossRef] [PubMed]
- Hamciuc, C.; Vlad-Bubulac, T.; Serbezeanu, D.; Macsim, A.-M.; Lisa, G.; Anghel, I.; Şofran, I.-E. Thermal Properties and Flammability Characteristics of a Series of DGEBA-Based Thermosets Loaded with a Novel Bisphenol Containing DOPO and Phenylphosphonate Units. Materials 2022, 15, 7829. [Google Scholar] [CrossRef]
- ASTM D7309-13; Standard Test Method for Determining Flammability Characteristics of Plastics and Other Solid Materials Using Microscale Combustion Calorimetry. ASTM International: West Conshohocken, PA, USA, 2007.
- Christenson, J.C.; Korgenski, E.K.; Relich, R.F. Laboratory Diagnosis of Infection Due to Bacteria, Fungi, Parasites, and Rickettsiae. In Principles and Practice of Pediatric Infectious Diseases, 5th ed.; Long, S.S., Prober, C.G., Fischer, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1422–1434.e1423. [Google Scholar]









| Sample | Tg1 (°C) | T52 (°C) | T103 (°C) | T304 (°C) | THRI (°C) | Tmax5 (°C) | Char Yield at 700 °C (wt%) |
|---|---|---|---|---|---|---|---|
| EPZ-0 | 194 | 399 | 399 | 315 | 171 | 419 | 19.8 |
| EPZ-1 | 169 | 349 | 369 | 389 | 173 | 391 | 32.5 |
| EPZ-2 | 166 | 332 | 347 | 379 | 176 | 375 | 34.1 |
| EPZ-3 | 172 | 348 | 368 | 385 | 181 | 382 | 30.2 |
| EPZ-4 | 180 | 347 | 367 | 387 | 182 | 387 | 37.7 |
| EPZ-5 | 179 | 345 | 365 | 386 | 181 | 381 | 41.8 |
| Samples vs. Data | EPZ-0 | EPZ-1 | EPZ-2 | EPZ-3 | EPZ-4 | EPZ-5 |
|---|---|---|---|---|---|---|
| CY (wt%) | 11.5 | 28.71 | 25.24 | 21.26 | 27.87 | 34.59 |
| THR (kJ/g) | 25.25 | 20.35 | 19.18 | 19.15 | 18.42 | 17.01 |
| PHRR 1 (W/g) | 376.28 | 380.90 | 289.58 | 347.39 | 370.03 | 316.14 |
| TPHRR 1 (oC) | 410.56 | 391.97 | 379.89 | 387.93 | 390.13 | 389.15 |
| Time 1 (s) | 90.5 | 91.5 | 95.5 | 89.0 | 88.5 | 99.0 |
| PHRR 2 (W/g) | 402.32 | - | 241.91 | - | - | - |
| TPHRR 2 (°C) | 421.22 | - | 395.19 | - | - | - |
| Time 2 (s) | 101 | - | 111 | - | - | - |
| PHRR 3 (W/g) | 458.64 | - | 235.52 | - | - | - |
| TPHRR 3 (°C) | 429.25 | - | 403.08 | - | - | - |
| Time 3 (s) | 109 | - | 119 | - | - | - |
| PHRR 4 (W/g) | 462.90 | - | - | - | - | - |
| TPHRR 4 (°C) | 436.24 | - | - | - | - | - |
| Time 4 (s) | 116 | - | - | - | - | - |
| HRC (J/(g·K)) | 1022.59 | 486.74 | 405.62 | 472.08 | 530.42 | 407.93 |
| Species Bacteria | Incubation Time | EPZ-0 ufc/0.5 mL | EPZ-1 ufc/0.5 mL | EPZ-2 ufc/0.5 mL | EPZ-3 ufc/0.5 mL | EPZ-4 ufc/0.5 mL | EPZ-5 ufc/0.5 mL |
|---|---|---|---|---|---|---|---|
| S. aureus | T0 (Control) | 1.5 × 108 | 1.5 × 108 | 1.5 × 108 | 1.5 × 108 | 1.5 × 108 | 1.5 × 108 |
| 3 h | UQ | UQ | UQ | UQ | UQ | UQ | |
| 24 h | 23.4 × 101 | 23.1 × 101 | 16.2 × 101 | 32.9 × 101 | 19.6 × 101 | 72 × 100 | |
| Log reduction | 5.8068 | 5.81247 | 5.9665 | 5.6588 | 5.8838 | 6.318 | |
| % reduction | 99.9998 | 99.9998 | 99.9998 | 99.9997 | 99.9998 | 99.9999 | |
| 48 h | 32 × 100 | 0 | 0 | 10.1 × 101 | 0 | 0 | |
| Log reduction | 6.6709 | 0 | 0 | 6.1717 | 0 | 0 | |
| % reduction | 99.99997 | 100 | 100 | 99.99993 | 100 | 100 | |
| E. coli | T0 (Control) | 1.5 × 108 | 1.5 × 108 | 1.5 × 108 | 1.5 × 108 | 1.5 × 108 | 1.5 × 108 |
| 3 h | 0 | 0 | 0 | 0 | 0 | 0 | |
| 24 h | 0 | 0 | 0 | 0 | 0 | 0 | |
| 48 h | 0 | 0 | 0 | 0 | 0 | 0 | |
| Log reduction | >6 | >6 | >6 | >6 | >6 | >6 | |
| % reduction | 100 | 100 | 100 | 100 | 100 | 100 |
| Sample | Epoxy Resin (g) | DDS (g) | BPH (g/wt%) | Zeolite-Ag (g/%) | P (%) |
|---|---|---|---|---|---|
| EPZ-0 | 5 | 1.64 | - | - | 0 |
| EPZ-1 | 5 | 1.55 | 0.755/10.35 | - | 1 |
| EPZ-2 | 5 | 1.472 | 1.438/18.179 | - | 2 |
| EPZ-3 | 5 | 1.537 | 0.868/10.55 | 0.822/10 | 1 |
| EPZ-4 | 4 | 1.132 | 0.677/10.65 | 0.44/7 | 1 |
| EPZ-5 | 1 | 0.2976 | 0.1759/10.17 | 0.2559/15 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlad-Bubulac, T.; Hamciuc, C.; Serbezeanu, D.; Macsim, A.-M.; Lisa, G.; Anghel, I.; Preda, D.-M.; Kalvachev, Y.; Rîmbu, C.M. Simultaneous Enhancement of Flame Resistance and Antimicrobial Activity in Epoxy Nanocomposites Containing Phosphorus and Silver-Based Additives. Molecules 2023, 28, 5650. https://doi.org/10.3390/molecules28155650
Vlad-Bubulac T, Hamciuc C, Serbezeanu D, Macsim A-M, Lisa G, Anghel I, Preda D-M, Kalvachev Y, Rîmbu CM. Simultaneous Enhancement of Flame Resistance and Antimicrobial Activity in Epoxy Nanocomposites Containing Phosphorus and Silver-Based Additives. Molecules. 2023; 28(15):5650. https://doi.org/10.3390/molecules28155650
Chicago/Turabian StyleVlad-Bubulac, Tăchiță, Corneliu Hamciuc, Diana Serbezeanu, Ana-Maria Macsim, Gabriela Lisa, Ion Anghel, Dana-Maria Preda, Yuri Kalvachev, and Cristina Mihaela Rîmbu. 2023. "Simultaneous Enhancement of Flame Resistance and Antimicrobial Activity in Epoxy Nanocomposites Containing Phosphorus and Silver-Based Additives" Molecules 28, no. 15: 5650. https://doi.org/10.3390/molecules28155650