Recent Progress of Preparation Strategies in Organic Nanoparticles for Cancer Phototherapeutics
Abstract
:1. Introduction
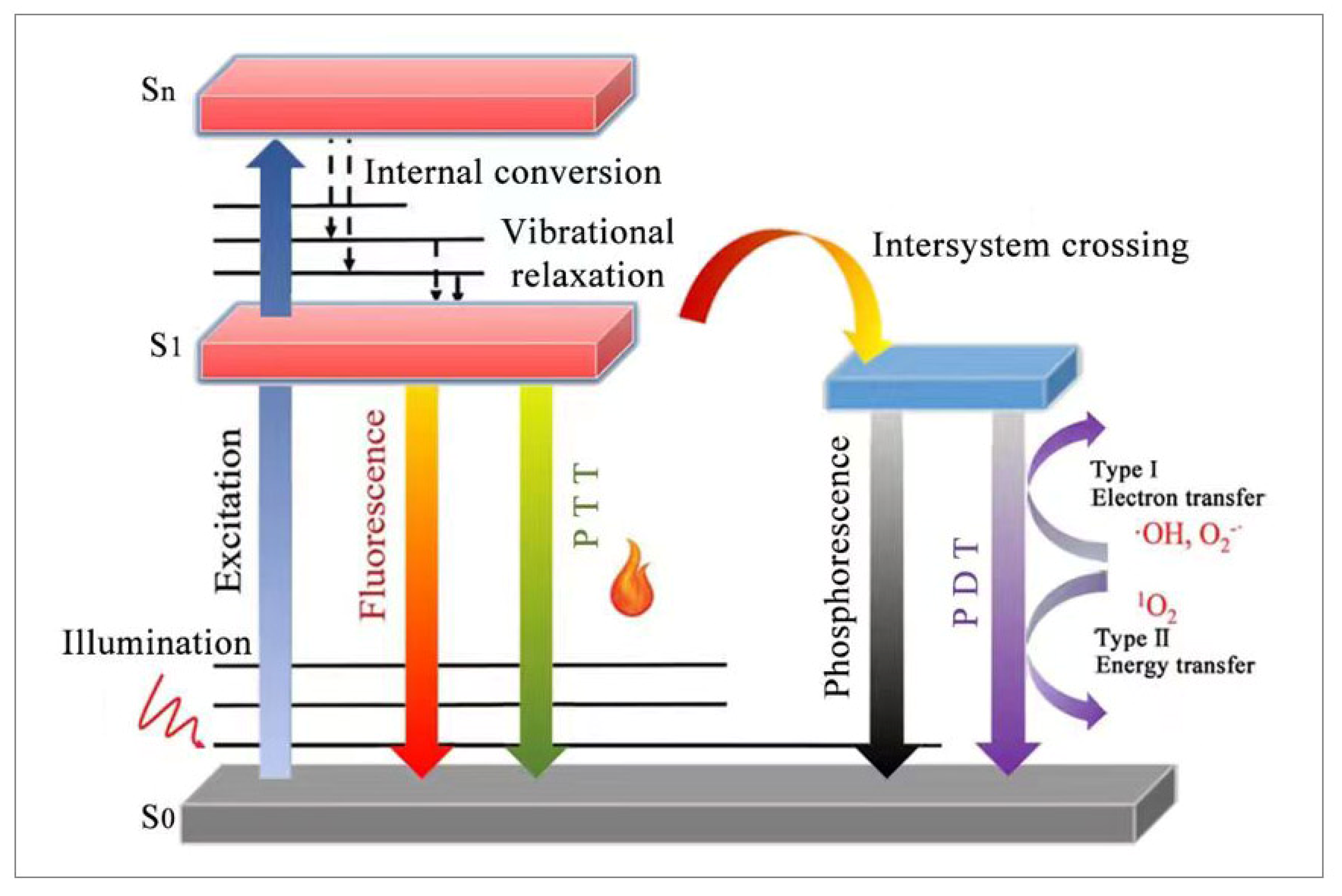
2. Basic Principle of Phototherapy
3. Preparation Strategies of Organic Nanoparticles
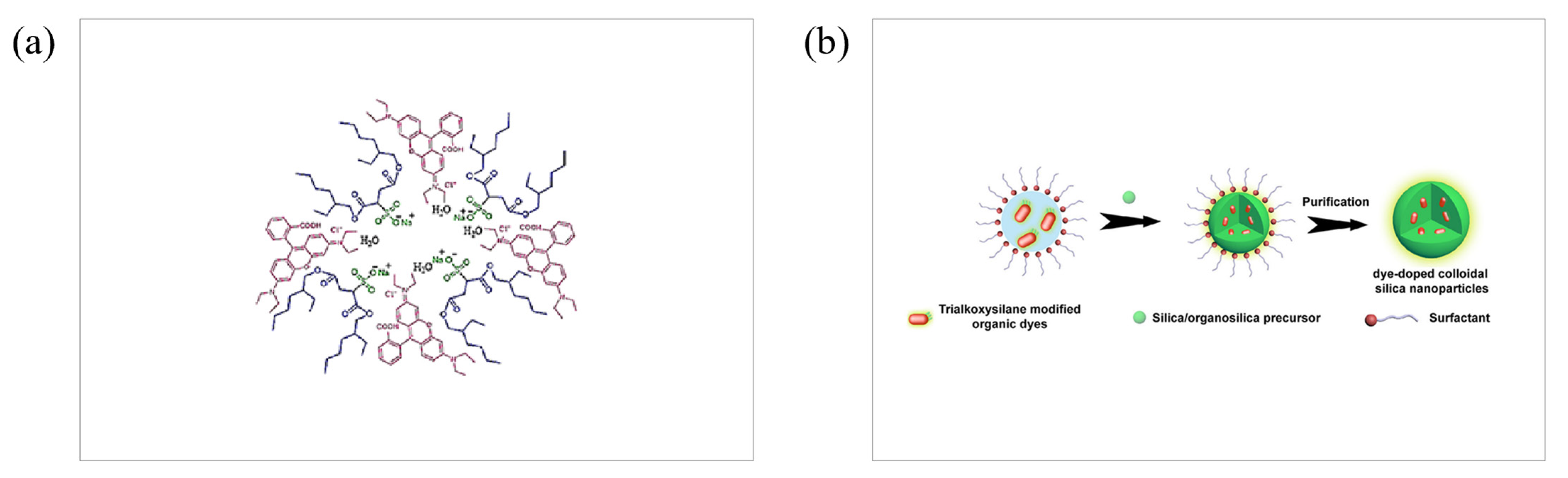
3.1. Co-Precipitation
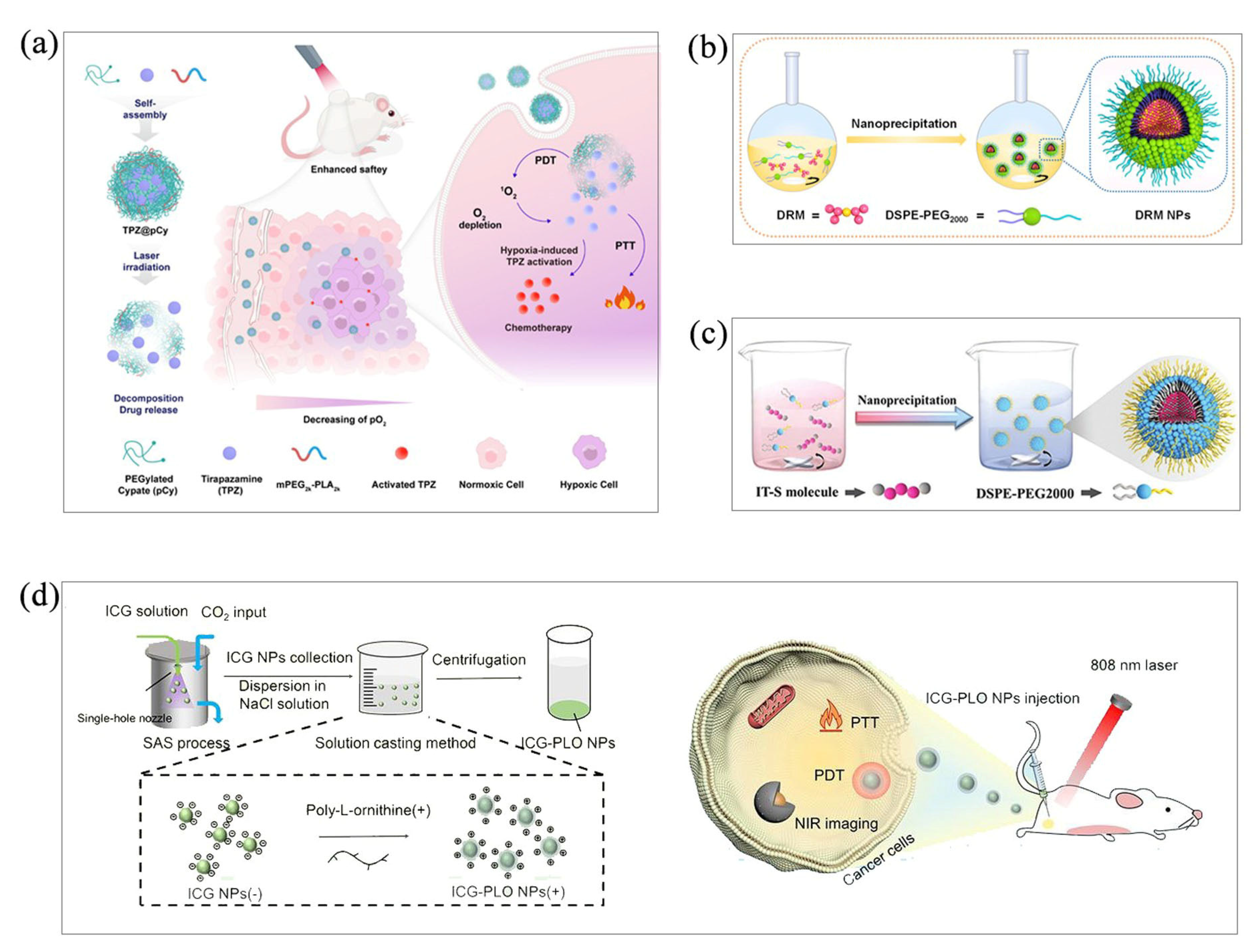
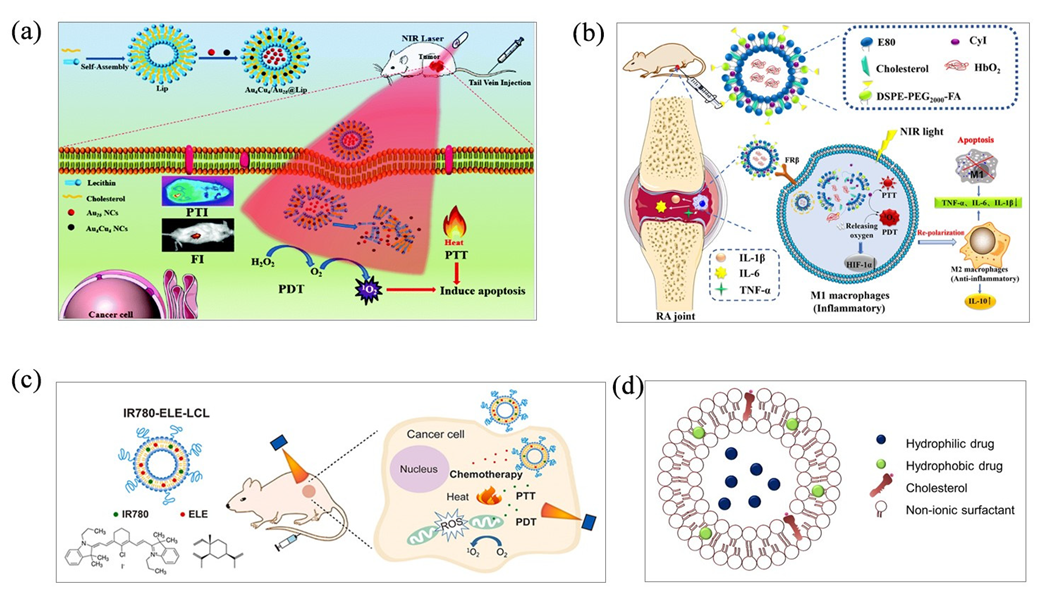
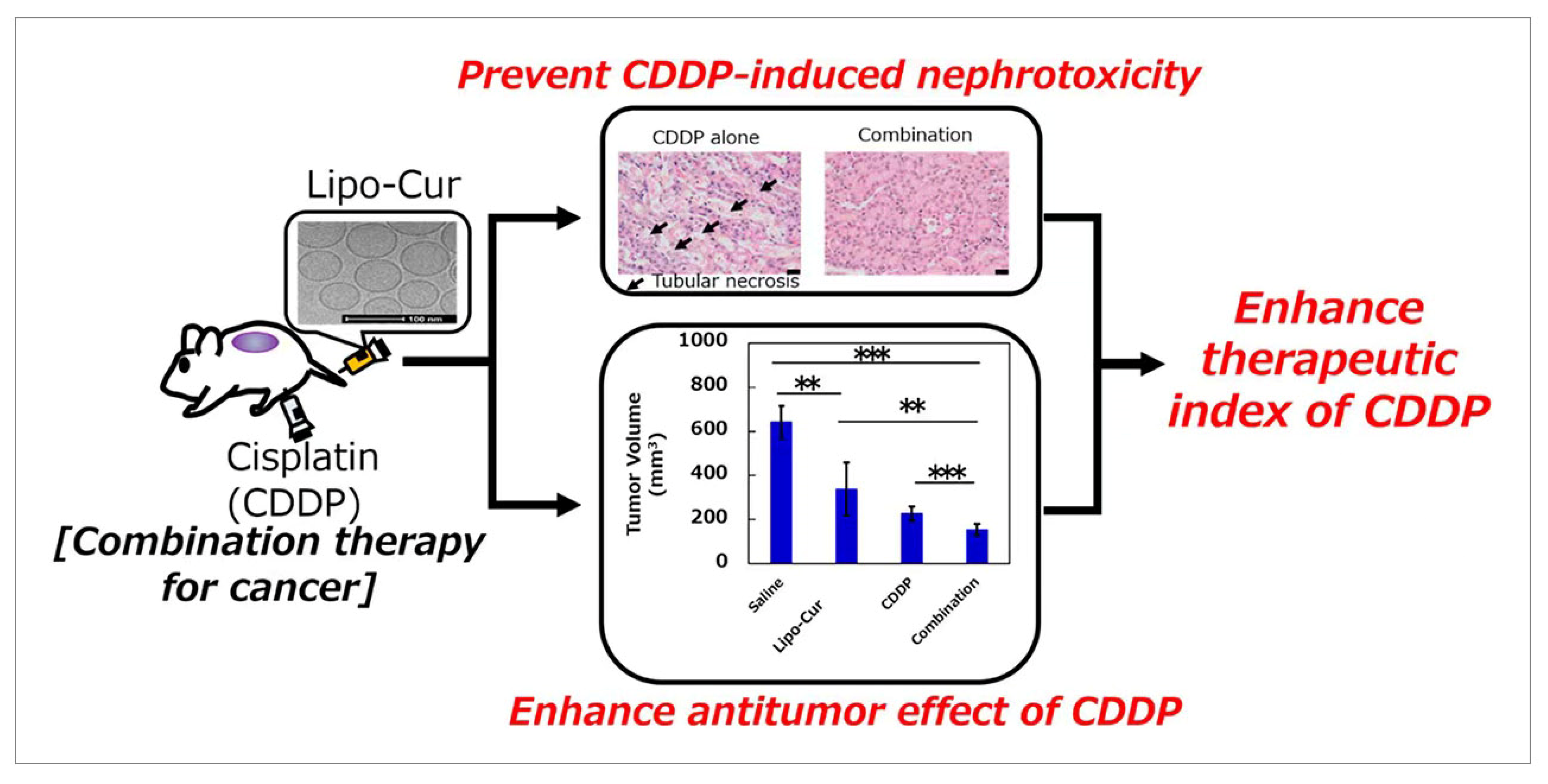
3.2. Thin-Film Hydration
3.3. Microemulsion Method
3.4. Microfluidic Technology
3.5. Biomimetic Technique
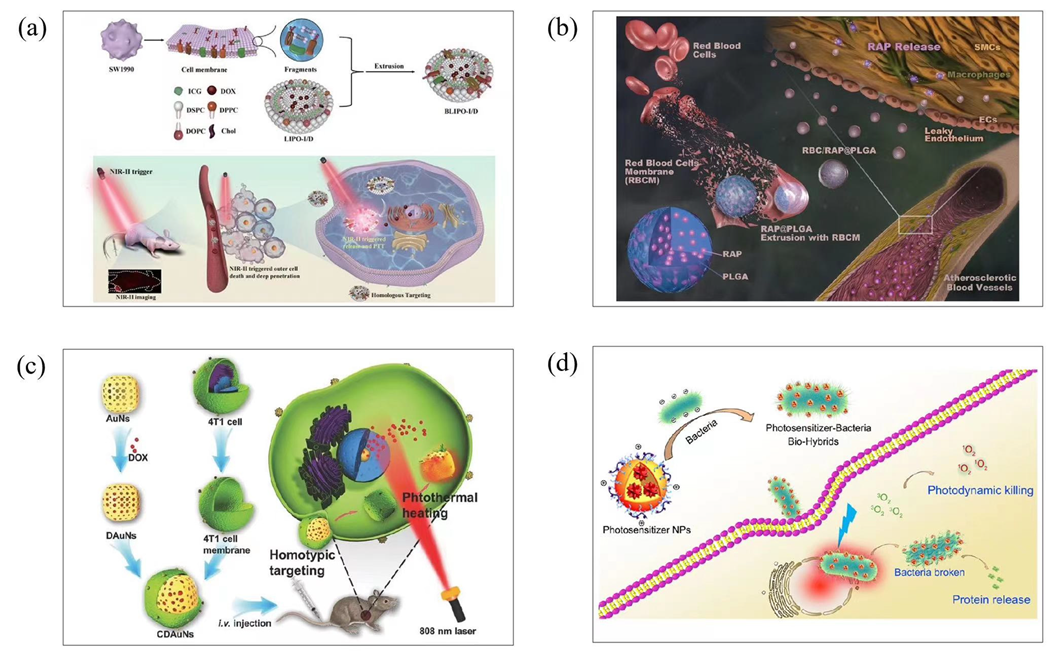
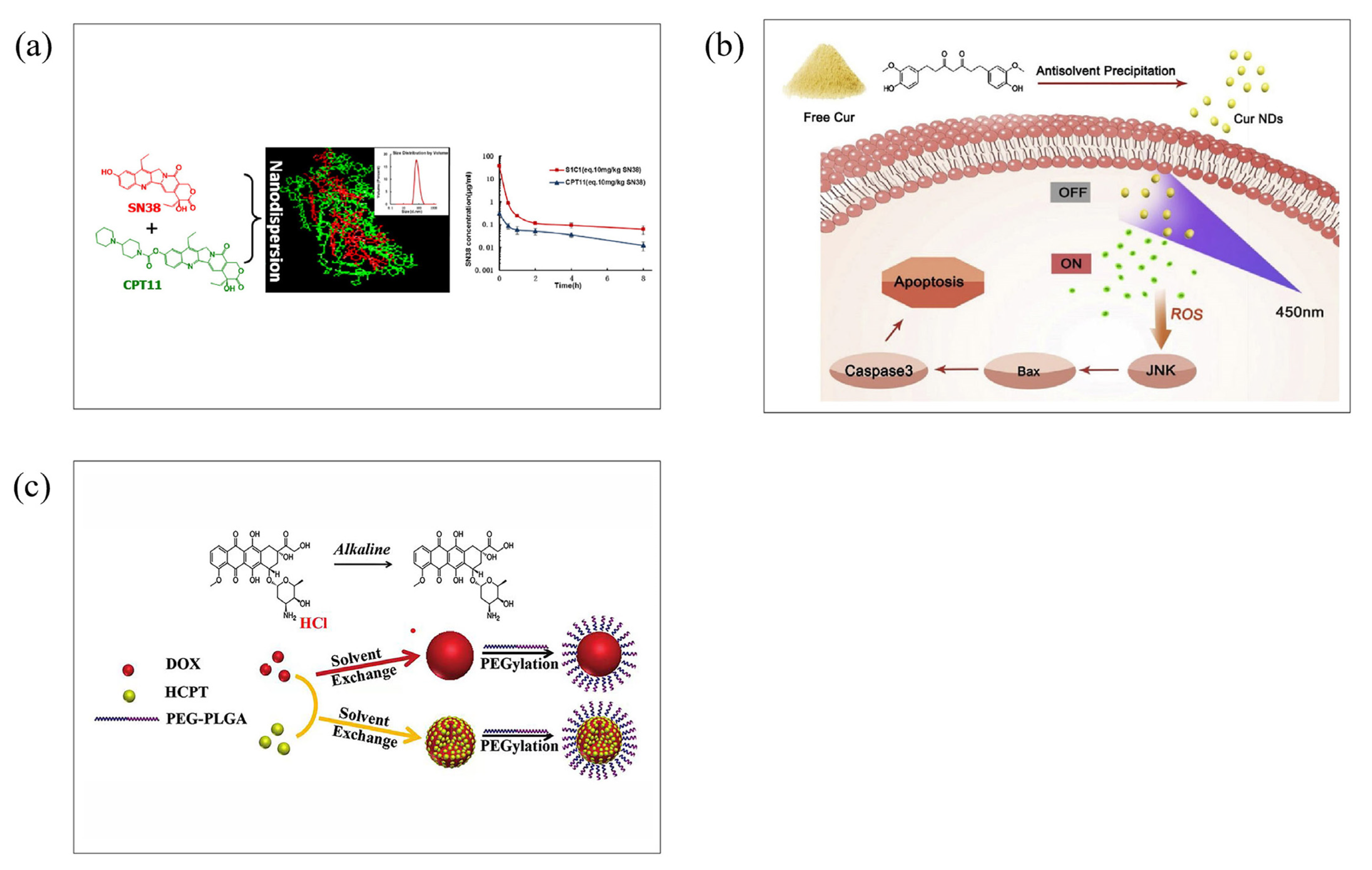
3.6. Carrier-Free Nanoparticle
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.C.; Zhu, L.; Gu, Z.; Dai, L.M. Carbon nanomaterials for phototherapy. Nanophotonics 2022, 11, 4955–4976. [Google Scholar] [CrossRef]
- Liu, J.J.; Shi, J.J.; Nie, W.M.; Wang, S.J.; Liu, G.H.; Cai, K.Y. Recent Progress in the Development of Multifunctional Nanoplatform for Precise Tumor Phototherapy. Adv. Healthc. Mater. 2020, 10, 2001207. [Google Scholar] [CrossRef]
- Zhou, B.; Guo, Z.X.; Lin, Z.X.; Zhang, L.Z.; Jiang, B.P.; Shen, X.C. Recent insights into near-infrared light-responsive carbon dots for bioimaging and cancer phototherapy. Inorg. Chem. Front. 2019, 6, 1116–1128. [Google Scholar] [CrossRef]
- Zhu, W.; Kang, M.M.; Wu, Q.; Zhang, Z.J.; Wu, Y.; Li, C.B.; Li, K.; Wang, L.; Wang, D.; Tang, B.Z. Zwitterionic AIEgens: Rational Molecular Design for NIR-II Fluorescence Imaging-Guided Synergistic Phototherapy. Adv. Funct. Mater. 2021, 31, 2007026. [Google Scholar] [CrossRef]
- Xu, X.; Ho, W.; Zhang, X.; Bertrand, N.; Farokhzad, O. Cancer nanomedicine: From targeted delivery to combination therapy. Trends Mol. Med. 2015, 21, 223–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrastina, A.; Massey, K.A.; Schnitzer, J.E. Overcoming in vivo barriers to targeted nanodelivery. WIREs Nanomed. Nanobiotechno. 2011, 3, 421–437. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Xu, X.; Bertrand, N.; Pridgen, E.; Swami, A.; Farokhzad, O.C. Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine. Adv. Drug Deliv. Rev. 2012, 64, 1363–1384. [Google Scholar] [CrossRef]
- Tang, C.C.; Song, C.H.; Wei, Z.; Liang, C.; Ran, J.C.; Cai, Y.; Dong, X.C.; Han, W. Polycyclic naphthalenediimide-based nanoparticles for NIR-II fluorescence imaging guided phototherapy. Sci. China Chem. 2020, 63, 946–956. [Google Scholar] [CrossRef]
- Wang, S.; Gao, Y.; Liu, Z.; Yang, C.; An, N.; Meng, H.; Yan, M.; Qu, G.; Guo, C. Cell-cargo mediated ZrN nanoparticle for the synergetic phototherapy on both of mice and rabbits. Eur. J. Pharm. Biopharm. 2020, 149, 163–169. [Google Scholar] [CrossRef]
- Long, X.; Zhang, X.; Chen, Q.; Liu, M.; Xiang, Y.; Yang, Y.; Xiao, Z.; Huang, J.; Wang, X.; Liu, C.; et al. Nucleus-Targeting Phototherapy Nanodrugs for High-Effective Anti-Cancer Treatment. Front. Pharmacol. 2022, 13, 905375. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Hou, Y.J.; Yang, X.X.; Liu, R.Q.; Zhao, D.; Guo, C.X.; Zhu, A.C.; Wen, M.N.; Liu, Z.; Qu, G.F.; Meng, H.X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef]
- Zhang, H.H.; Chen, G.H.; Yu, B.; Cong, H.L. Emerging Advanced Nanomaterials for Cancer Photothermal Therapy. Rev. Adv. Mater. Sci. 2018, 53, 131–146. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T.; et al. Emerging combination strategies with phototherapy in cancer nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef]
- Zhou, J.; Cao, C.; Zhang, X.; Zhang, X.; Li, J.; Deng, H.; Wang, S. Gas-assisted phototherapy for cancer treatment. J. Control Release 2023, 360, 564–577. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Zhu, D.Z.; Wang, D.J.; Liu, B.; Du, X.F.; Wei, G.; Zhou, X. Recent advances of smart AIEgens for photoacoustic imaging and phototherapy. Coord. Chem. Rev. 2022, 471, 214725. [Google Scholar] [CrossRef]
- Wang, G.H.; Zhang, F.; Tian, R.; Zhang, L.W.; Fu, G.F.; Yang, L.L.; Zhu, L. Nanotubes-Embedded Indocyanine Green-Hyaluronic Acid Nanoparticles for Photoacoustic-Imaging-Guided Phototherapy. ACS Appl. Mater. Interfaces 2016, 8, 5608–5617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Zhou, W.; Zeng, Z.L.; Fan, Q.L.; Pu, K.Y. Grafted semiconducting polymer amphiphiles for multimodal optical imaging and combination phototherapy. Chem. Sci. 2020, 11, 10553–10570. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Li, H.; Tian, Z.J.; Liu, X.; Guo, Y.; He, J.; Wang, Z.Y.; Zhou, T.; Liu, Y.M. Porphyrin-Based Nanoparticles: A Promising Phototherapy Platform. Chempluschem 2022, 87, e202200156. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Li, D.D.; Lee, C.; Xie, J. Nanoparticle Phototherapy in the Era of Cancer Immunotherapy. Trends Chem. 2020, 2, 1082–1095. [Google Scholar] [CrossRef]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef] [PubMed]
- Ngoune, R.; Peters, A.; von Elverfeldt, D.; Winkler, K.; Putz, G. Accumulating nanoparticles by EPR: A route of no return. J. Control Release 2016, 238, 58–70. [Google Scholar] [CrossRef] [Green Version]
- Caro, C.; Avasthi, A.; Paez-Munoz, J.M.; Leal, M.P.; Garcia-Martin, M.L. Passive targeting of high-grade gliomas via the EPR effect: A closed path for metallic nanoparticles? Biomater. Sci. 2021, 9, 7984–7995. [Google Scholar] [CrossRef]
- Fang, F.; Li, M.; Zhang, J.F.; Lee, C.S. Different Strategies for Organic Nanoparticle Preparation in Biomedicine. ACS Mater. Lett. 2020, 2, 531–549. [Google Scholar] [CrossRef]
- Guo, B.; Middha, E.; Liu, B. Solvent Magic for Organic Particles. ACS Nano 2019, 13, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tong, A.; Niu, P.; Guo, W.; Jin, Y.; Hu, Y.; Tao, P.; Miao, W. Light-Decomposable Polymeric Micelles with Hypoxia-Enhanced Phototherapeutic Efficacy for Combating Metastatic Breast Cancer. Pharmaceutics 2022, 14, 253. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Yin, C.; Lu, G.; Wan, Y.; Huang, Z.; Tan, J.; Li, S.; Luo, J.; Lee, C.S. A Diradicaloid Small Molecular Nanotheranostic with Strong Near-Infrared Absorbance for Effective Cancer Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2021, 13, 15983–15991. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Xiang, C.; Li, S.; Mao, C.; Chen, H.; Chen, J.X.; Tian, S.; Cui, X.; Wan, Y.; Huang, Z.; et al. Single-Photomolecular Nanotheranostics for Synergetic Near-Infrared Fluorescence and Photoacoustic Imaging-Guided Highly Effective Photothermal Ablation. Small 2020, 16, e2002672. [Google Scholar] [CrossRef]
- Prosapio, V.; De Marco, I.; Reverchon, E. Supercritical antisolvent coprecipitation mechanisms. J. Supercrit. Fluids 2018, 138, 247–258. [Google Scholar] [CrossRef]
- Xu, P.Y.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Development of highly stable ICG-polymeric nanoparticles with ultra-high entrapment efficiency using supercritical antisolvent (SAS)-combined solution casting process. Int. J. Pharm. 2022, 629, 122348. [Google Scholar] [CrossRef]
- Thabet, Y.; Elsabahy, M.; Eissa, N.G. Methods for preparation of niosomes: A focus on thin-film hydration method. Methods 2022, 199, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Lee, J.H.; Shin, Y.; Lee, W.; Whang, K.; Kim, D.; Lee, L.P.; Choi, J.W.; Kang, T. General and programmable synthesis of hybrid liposome/metal nanoparticles. Sci. Adv. 2016, 2, e1601838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yang, Y.; Wang, X.; Liu, X.; Cheng, H.; Wang, P.; Shen, Y.; Xie, A.; Zhu, M. Self-assembled Au4Cu4/Au5 NCs@liposome tumor nanotheranostics with PT/fluorescence imaging-guided synergetic PTT/PDT. J. Mater. Chem. B 2021, 9, 6396–6405. [Google Scholar] [CrossRef]
- Han, N.; Shi, Q.; Wang, X.R.; Huang, X.Y.; Ruan, M.Y.; Ren, L.H.; Lang, X.X.; Wu, K.; Du, S.Y. Liposome co-loaded with beta-elemene and IR780 for combined chemo-phototherapy. J. Drug Deliv. Sci. Technol. 2022, 68, 103122. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, R.S.; Dong, Y.X.; Liu, J.J.; Gao, Z.; Zhou, X.Y.; Cao, J. Oxygen supplementation liposomes for rheumatoid arthritis treatment via synergistic phototherapy and repolarization of M1-to-M2 macrophages. Chem. Eng. J. 2023, 459, 141484. [Google Scholar] [CrossRef]
- Hou, M.; Dang, L.; Liu, T.; Guo, Y.; Wang, Z. Novel Fluorescent Microemulsion: Probing Properties, Investigating Mechanism, and Unveiling Potential Application. ACS Appl. Mater. Interfaces 2017, 9, 25747–25754. [Google Scholar] [CrossRef]
- Orte, A.; Ruedas-Rama, M.J.; Paredes, J.M.; Crovetto, L.; Alvarez-Pez, J.M. Dynamics of water-in-oil nanoemulsions revealed by fluorescence lifetime correlation spectroscopy. Langmuir 2011, 27, 12792–12799. [Google Scholar] [CrossRef]
- Rahdar, A.; Bagheri, H. An insight into the effect of nano-confinement on some of photo-physical parameters of dye. Appl. Phys. A 2019, 125, 648. [Google Scholar] [CrossRef]
- Yang, S.B.; Li, Y.S. Fluorescent hybrid silica nanoparticles and their biomedical applications. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1603. [Google Scholar] [CrossRef] [PubMed]
- Hinger, D.; Grafe, S.; Navarro, F.; Spingler, B.; Pandiarajan, D.; Walt, H.; Couffin, A.C.; Maake, C. Lipid nanoemulsions and liposomes improve photodynamic treatment efficacy and tolerance in CAL-33 tumor bearing nude mice. J. Nanobiotechnol. 2016, 14, 71. [Google Scholar] [CrossRef] [Green Version]
- Muehlmann, L.A.; Rodrigues, M.C.; Longo, J.P.; Garcia, M.P.; Py-Daniel, K.R.; Veloso, A.B.; de Souza, P.E.; da Silva, S.W.; Azevedo, R.B. Aluminium-phthalocyanine chloride nanoemulsions for anticancer photodynamic therapy: Development and in vitro activity against monolayers and spheroids of human mammary adenocarcinoma MCF-7 cells. J. Nanobiotechnol. 2015, 13, 36. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Su, P.; Zhang, W.; Raston, C.L. Microfluidic Devices in Fabricating Nano or Micromaterials for Biomedical Applications. Adv. Mater. Technol. 2019, 4, 1900488. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Belliveau, N.; Hafez, I.; Leung, A.K.K.; Huft, J.; Hansen, C.; Cullis, P.R. Bottom-Up Design and Synthesis of Limit Size Lipid Nanoparticle Systems with Aqueous and Triglyceride Cores Using Millisecond Microfluidic Mixing. Langmuir 2012, 28, 3633–3640. [Google Scholar] [CrossRef] [PubMed]
- Batzri, S.; Korn, E.D. Single Bilayer Liposomes Prepared without Sonication. Biochim. Biophys. Acta Biomembr. 1973, 298, 1015–1019. [Google Scholar] [CrossRef]
- Chen, L.; Yang, C.; Xiao, Y.; Yan, X.; Hu, L.; Eggersdorfer, M.; Chen, D.; Weitz, D.A.; Ye, F. Millifluidics, microfluidics, and nanofluidics: Manipulating fluids at varying length scales. Mater. Today Nano 2021, 16, 100136. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Kraft, J.C.; Freeling, J.P.; Wang, Z.; Ho, R.J. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J. Pharm. Sci. 2014, 103, 29–52. [Google Scholar] [CrossRef] [Green Version]
- Hamano, N.; Bottger, R.; Lee, S.E.; Yang, Y.; Kulkarni, J.A.; Ip, S.; Cullis, P.R.; Li, S.D. Robust Microfluidic Technology and New Lipid Composition for Fabrication of Curcumin-Loaded Liposomes: Effect on the Anticancer Activity and Safety of Cisplatin. Mol. Pharm. 2019, 16, 3957–3967. [Google Scholar] [CrossRef]
- Li, S.H.; Zhang, L. Erythrocyte membrane nano-capsules: Biomimetic delivery and controlled release of photothermal-photochemical coupling agents for cancer cell therapy. Dalton. Trans. 2020, 49, 2645–2651. [Google Scholar] [CrossRef]
- Tang, D.; Wang, Y.; Wijaya, A.; Liu, B.Y.; Maruf, A.; Wang, J.X.; Xu, J.X.; Liao, X.L.; Wu, W.; Wang, G.X. ROS-responsive biomimetic nanoparticles for potential application in targeted anti-atherosclerosis. Regen. Biomater. 2021, 8, rbab033. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, K.; Qiu, J.H.; Wang, N.; Qu, K.; Cui, Y.L.; Huang, J.L.; Luo, L.; Zhong, Y.; Tian, T.; et al. Uptake of oxidative stress-mediated extracellular vesicles by vascular endothelial cells under low magnitude shear stress. Bioact. Mater. 2022, 9, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Takeishi, N.; Yamashita, H.; Omori, T.; Yokoyama, N.; Wada, S.; Sugihara-Seki, M. Inertial migration of red blood cells under a Newtonian fluid in a circular channel. J. Fluid. Mech. 2022, 952, A35. [Google Scholar] [CrossRef]
- Su, L.; Liu, Y.; Zhu, Y.; Guo, F.; Arkin, G.; Lin, X.; Xu, J.; Xie, Z.; Zhang, H. Photo-responsive NIR-II biomimetic nanomedicine for efficient cancer-targeted theranostics. Mater. Today Chem. 2022, 24, 100879. [Google Scholar] [CrossRef]
- Sun, H.P.; Su, J.H.; Meng, Q.S.; Yin, Q.; Chen, L.L.; Gu, W.W.; Zhang, Z.W.; Yu, H.J.; Zhang, P.C.; Wang, S.L.; et al. Cancer Cell Membrane-Coated Gold Nanocages with Hyperthermia-Triggered Drug Release and Homotypic Target Inhibit Growth and Metastasis of Breast Cancer (vol 27, 1604300, 2017). Adv. Funct. Mater. 2020, 30, 1604300. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, K.; Qin, X.; Li, T.H.; Qiu, J.H.; Yin, T.Y.; Huang, J.L.; McGinty, S.; Pontrelli, G.; Ren, J.; et al. Biomimetic Nanotherapies: Red Blood Cell Based Core-Shell Structured Nanocomplexes for Atherosclerosis Management. Adv. Sci. 2019, 6, 1900172. [Google Scholar] [CrossRef]
- Hayashi, K.; Zhao, M.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Hoffman, R.M. Cancer Metastasis Directly Eradicated by Targeted Therapy With a Modified Salmonella typhimurium. J. Cell Biochem. 2009, 106, 992–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petros, R.A.; DeSimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, W.B.; Duan, Y.K.; Li, X.Q.; Qi, G.B.; Liu, B. Photosensitizer-Bacteria Biohybrids Promote Photodynamic Cancer Cell Ablation and Intracellular Protein Delivery. Chem. Mater. 2019, 31, 7212–7220. [Google Scholar] [CrossRef]
- Zhong, Y.T.; Cen, Y.; Xu, L.; Li, S.Y.; Cheng, H. Recent Progress in Carrier-Free Nanomedicine for Tumor Phototherapy. Adv. Healthc. Mater. 2023, 12, 2202307. [Google Scholar] [CrossRef]
- Niu, D.; Jiang, Y.Q.; Ji, L.K.; Ouyang, G.H.; Liu, M.H. Self-Assembly through Coordination and pi-Stacking: Controlled Switching of Circularly Polarized Luminescence. Angew. Chem. Int. Ed. 2019, 58, 5946–5950. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Sastry, M.; Rao, M.; Ganesh, K.N. Electrostatic assembly of nanoparticles and biomacromolecules. Acc. Chem. Res. 2002, 35, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, D.C.; Taskinen, K.A. Self-assembly in synthetic macromolecular systems via multiple hydrogen bonding interactions. Chem. Soc. Rev. 2001, 30, 83–93. [Google Scholar] [CrossRef]
- Hu, S.Q.; Lee, E.; Wang, C.; Wang, J.Q.; Zhou, Z.X.; Li, Y.X.; Li, X.Y.; Tang, J.B.; Lee, D.H.; Liu, X.R.; et al. Amphiphilic drugs as surfactants to fabricate excipient-free stable nanodispersions of hydrophobic drugs for cancer chemotherapy. J. Control Release 2015, 220, 175–179. [Google Scholar] [CrossRef]
- Sun, M.D.; Zhang, Y.; He, Y.; Xiong, M.H.; Huang, H.Y.; Pei, S.C.; Liao, J.F.; Wang, Y.S.; Shao, D. Green synthesis of carrier-free curcumin nanodrugs for light-activated breast cancer photodynamic therapy. Colloids. Surf. B 2019, 180, 313–318. [Google Scholar] [CrossRef]
- Zheng, R.J.; Zhao, Q.; Qing, W.X.; Li, S.L.; Liu, Z.H.; Li, Q.Q.; Huang, Y.W. Carrier-Free Delivery of Ultrasmall pi-Conjugated Oligomer Nanoparticles with Photothermal Conversion over 80% for Cancer Theranostics. Small 2022, 18, 2104521. [Google Scholar] [CrossRef]
- Yu, C.T.; Zhou, M.J.; Zhang, X.J.; Wei, W.J.; Chen, X.F.; Zhang, X.H. Smart doxorubicin nanoparticles with high drug payload for enhanced chemotherapy against drug resistance and cancer diagnosis. Nanoscale 2015, 7, 5683–5690. [Google Scholar] [CrossRef]
- Liu, J.; Jin, C.; Yuan, B.; Liu, X.; Chen, Y.; Ji, L.; Chao, H. Selectively lighting up two-photon photodynamic activity in mitochondria with AIE-active iridium(iii) complexes. Commun. Chem. 2017, 53, 2052–2055. [Google Scholar] [CrossRef]
- Cheng, X.X.; Pan, F.S.; Wang, M.R.; Li, W.D.; Song, Y.M.; Liu, G.H.; Yang, H.; Gao, B.X.; Wu, H.; Jiang, Z.Y. Hybrid membranes for pervaporation separations. J. Membr. Sci. 2017, 541, 329–346. [Google Scholar] [CrossRef]
- Galizia, M.; Manning, G.S.; Paul, D.R.; Freeman, B.D. Ion partitioning between brines and ion exchange polymers. Polymer 2020, 165, 91–100. [Google Scholar] [CrossRef]
- Shu, C.; Li, X.G.; Li, H.; Gao, X. Design and optimization of reactive distillation: A review. Front. Chem. Sci. Eng. 2022, 16, 799–818. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Tang, J.; Guo, S.; Zhao, Q.; Li, S. Recent Progress of Preparation Strategies in Organic Nanoparticles for Cancer Phototherapeutics. Molecules 2023, 28, 6038. https://doi.org/10.3390/molecules28166038
Xie Q, Tang J, Guo S, Zhao Q, Li S. Recent Progress of Preparation Strategies in Organic Nanoparticles for Cancer Phototherapeutics. Molecules. 2023; 28(16):6038. https://doi.org/10.3390/molecules28166038
Chicago/Turabian StyleXie, Quanquan, Jiayi Tang, Shengze Guo, Qi Zhao, and Shengliang Li. 2023. "Recent Progress of Preparation Strategies in Organic Nanoparticles for Cancer Phototherapeutics" Molecules 28, no. 16: 6038. https://doi.org/10.3390/molecules28166038





