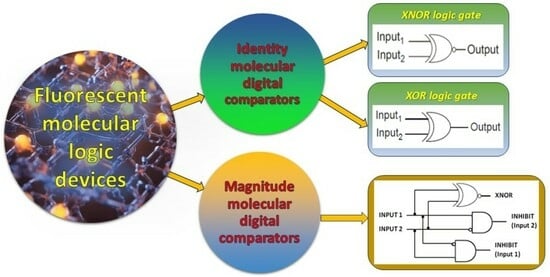
A Tutorial Review on the Fluorescent Probes as a Molecular Logic Circuit—Digital Comparator
Abstract
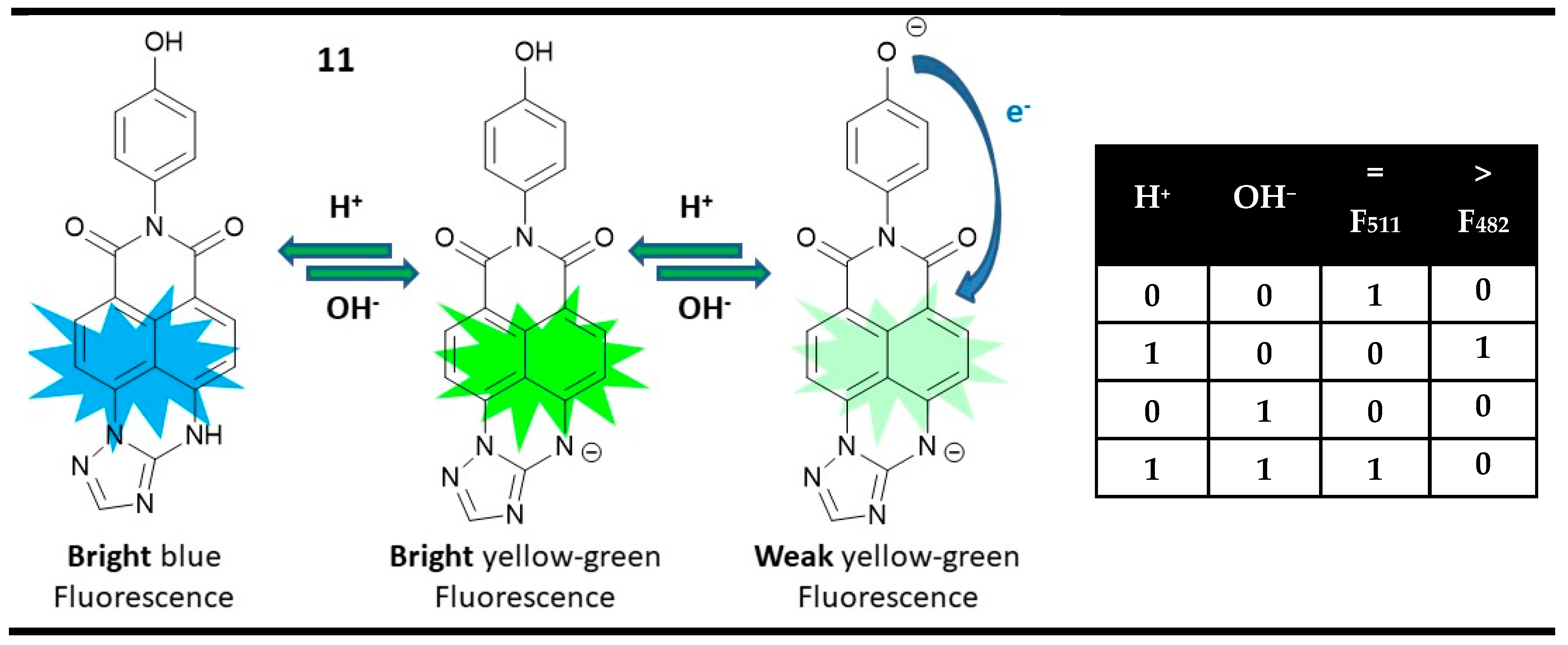
:1. Introduction
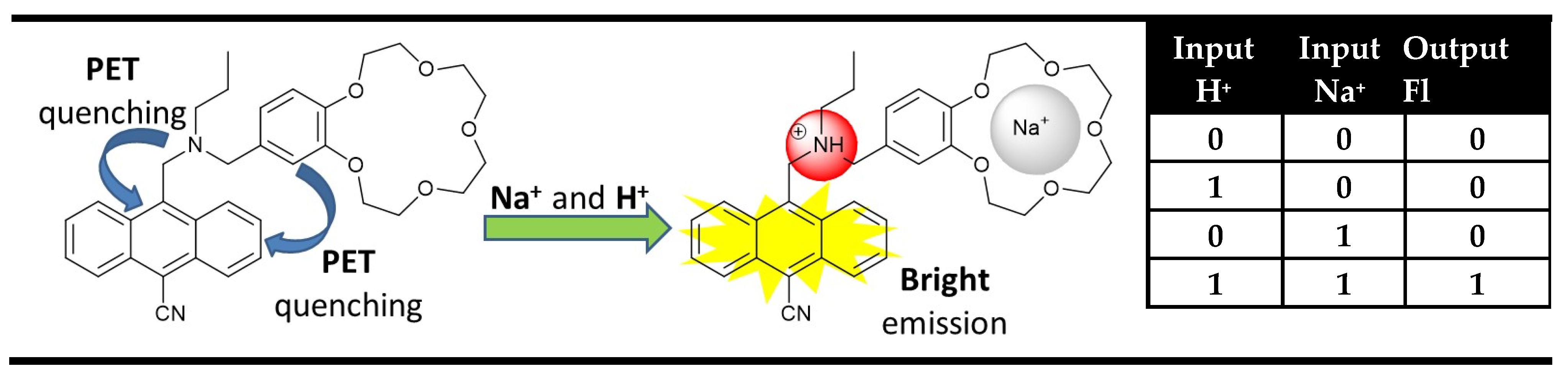
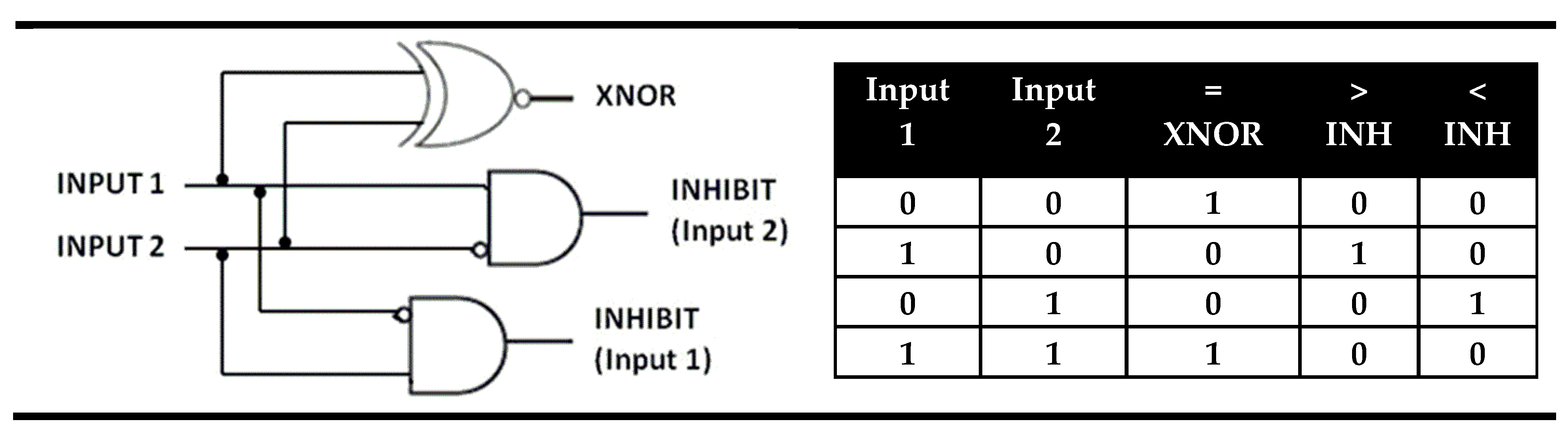
2. Identity Molecular Digital Comparators (XNOR and XOR Molecular Logic Gates)
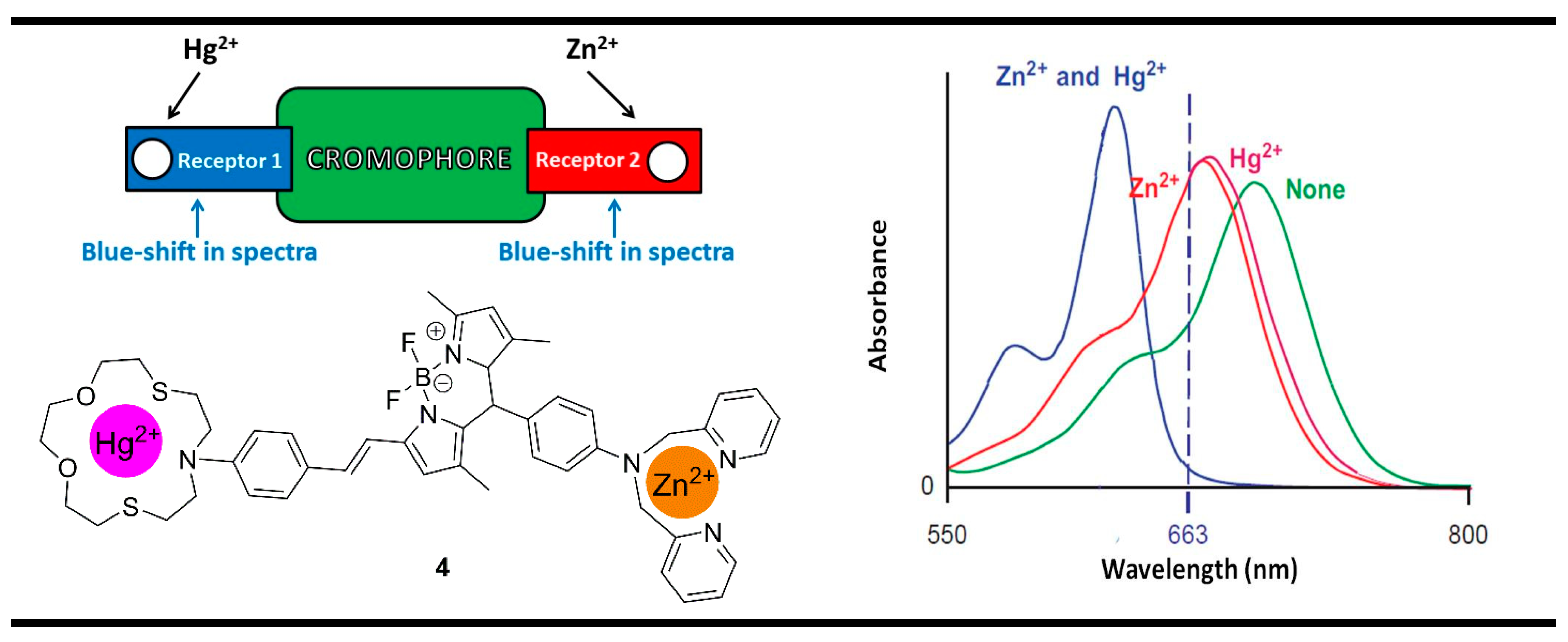
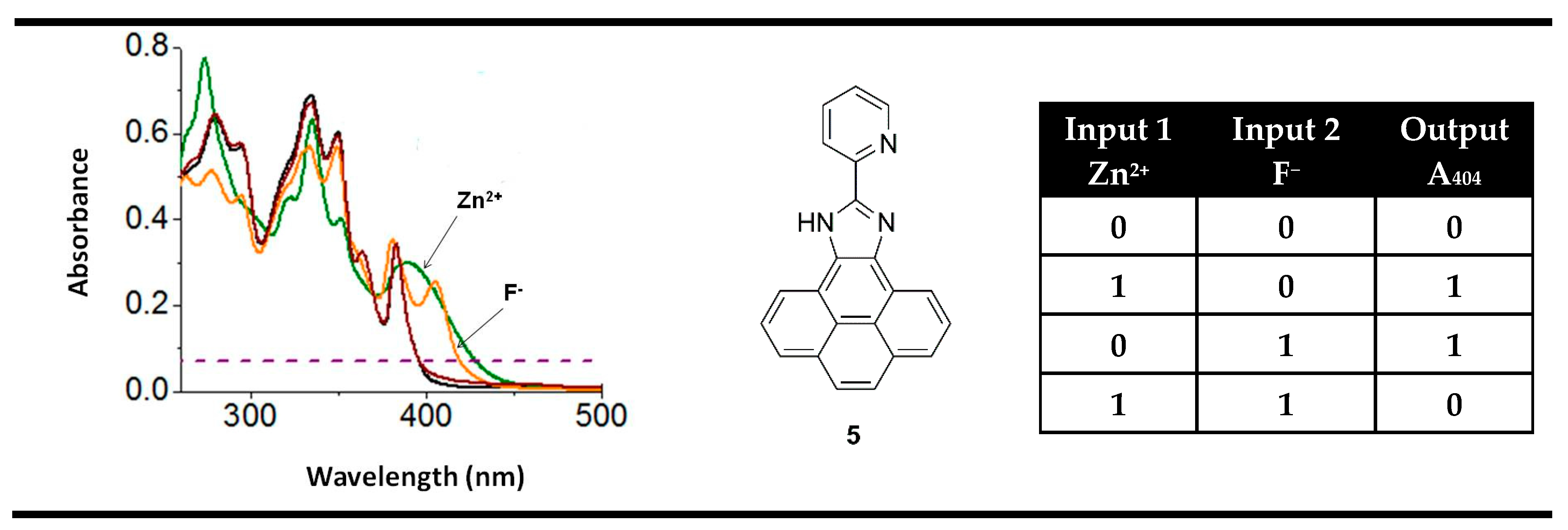
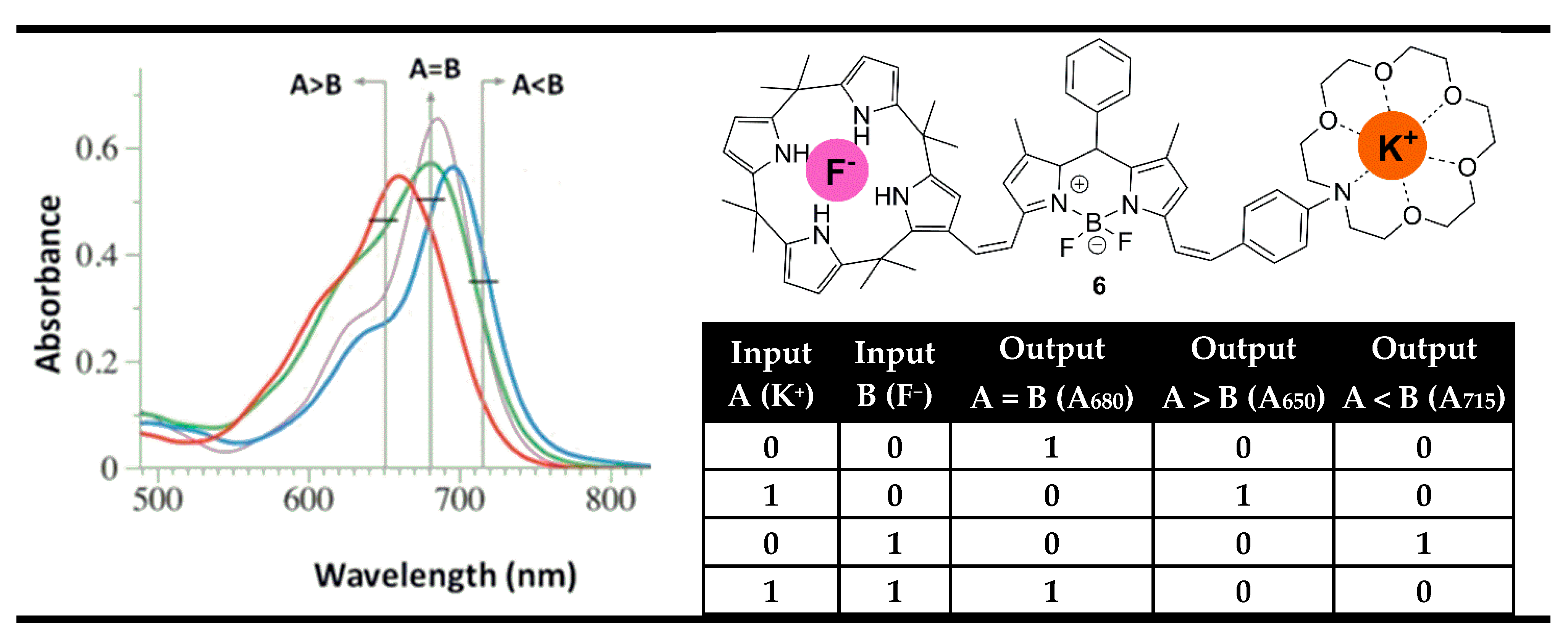
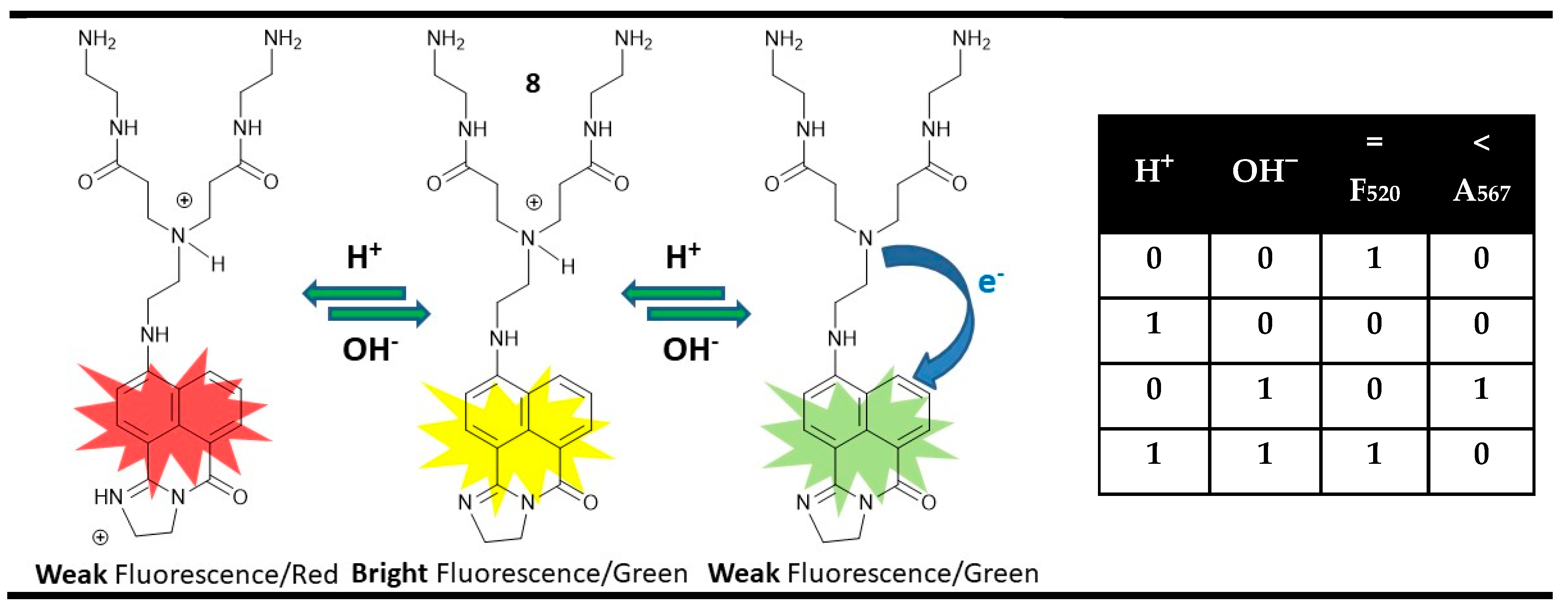
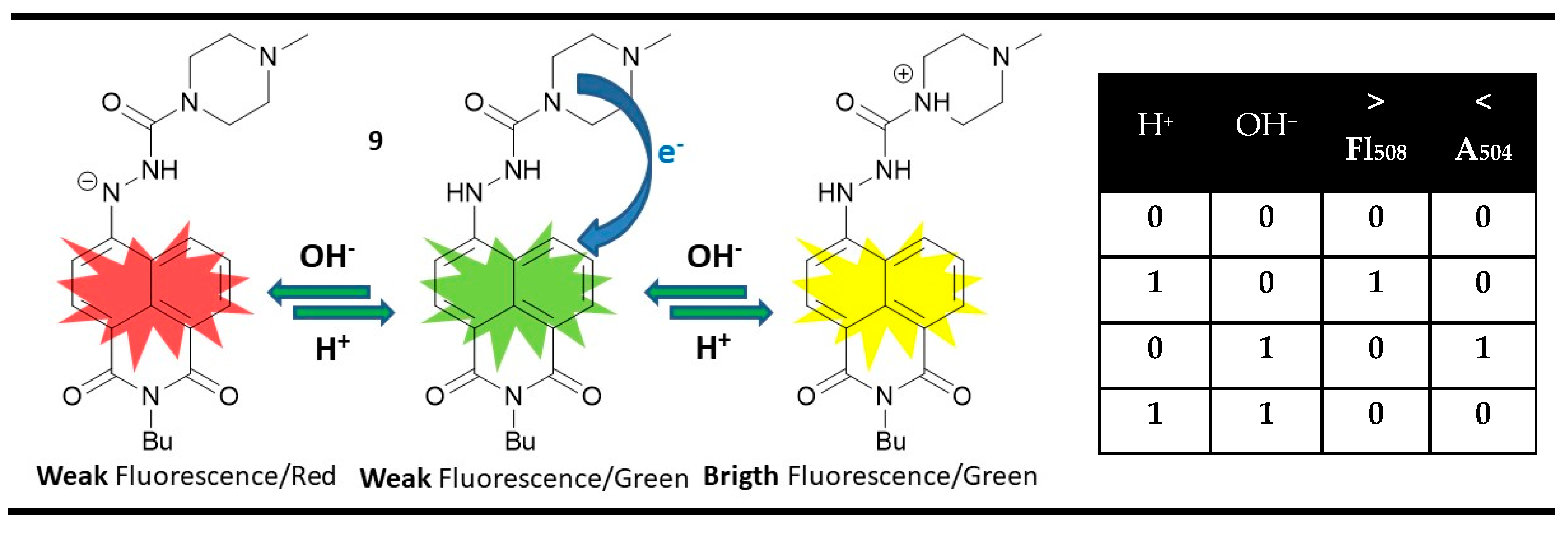
3. Magnitude Molecular Digital Comparators
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Callan, J.F.; de Silva, A.P.; Magri, D.C. Luminescent sensors and switches in the early 21st century. Tetrahedron 2005, 61, 8551–8588. [Google Scholar] [CrossRef]
- Valeur, B.; Leray, I. Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev. 2000, 205, 3–40. [Google Scholar] [CrossRef]
- Kottas, G.S.; Clarke, L.I.; Horinek, D.; Michl, J. Artificial molecular rotors. Chem. Rev. 2005, 105, 1281–1376. [Google Scholar] [CrossRef] [PubMed]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic molecular motors and mechanical machines. Angew. Chem. Int. Ed. 2007, 46, 72–191. [Google Scholar] [CrossRef]
- Balzani, V.; Credi, A.; Raymo, F.M.; Stoddart, J.F. Artificial molecular machines. Angew. Chem. Int. Ed. 2000, 39, 3348–3391. [Google Scholar] [CrossRef]
- Tian, H.; Yang, S. Recent progresses on diarylethene based photochromic switches. Chem. Soc. Rev. 2004, 33, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Rao, S.-J.; Xie, T.; Li, X.; Xu, T.-Y.; Li, D.-W.; Qu, D.-H.; Long, Y.-T.; Tian, H. Muscle-like artificial molecular actuators for nanoparticles. Chem 2018, 4, 1–15. [Google Scholar] [CrossRef]
- Bruns, C.J.; Stoddart, J.F. Rotaxane-based molecular muscles. Acc. Chem. Res. 2014, 47, 2186–2199. [Google Scholar] [CrossRef]
- Juluri, B.K.; Kumar, A.S.; Liu, Y.; Ye, T.; Yang, Y.-W.; Flood, A.H.; Fang, L.; Stoddart, J.; Weiss, P.; Huang, T.J. A mechanical actuator driven electrochemically by artificial molecular muscles. ACS Nano 2009, 3, 291–300. [Google Scholar] [CrossRef]
- Chen, S.; Yang, L.; Leung, F.K.-C.; Kajitani, T.; Stuart, M.C.A.; Fukushima, T.; van Rijn, P.; Feringa, B.L. Photoactuating artificial muscles of motor amphiphiles as an extracellular matrix mimetic scaffold for mesenchymal stem cells. J. Am. Chem. Soc. 2022, 144, 3543–3553. [Google Scholar] [CrossRef]
- Shirai, Y.; Morin, J.-F.; Sasaki, T.; Guerrero, J.M.; Tour, J.M. Recent progress on nanovehicles. Chem. Soc. Rev. 2006, 35, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.; Pishkenari, H.N.; Nem, A. Mechanism of the motion of nanovehicles on hexagonal boron-nitride: A molecular dynamics study. Comput. Mater. Sci. 2022, 207, 111317. [Google Scholar] [CrossRef]
- Kammerer, C.; Erbland, G.; Gisbert, Y.; Nishino, T.; Yasuhara, K.; Rapenne, G. Biomimetic and technomimetic single molecular machines. Chem. Lett. 2019, 48, 299–308. [Google Scholar] [CrossRef]
- Nakashima, K.; Petek, A.; Hori, Y.; Georgiev, A.; Hirashima, S.; Matsushima, Y.; Miura, T.; Antonov, L. Acylhydrazone subunits as a proton cargo delivery system in 7-hydroxyquinoline. Chem. Eur. J. 2021, 27, 11559–11566. [Google Scholar] [CrossRef]
- Chen, W.; Guo, C.; He, Q.; Chi, X.; Lynch, V.M.; Zhang, Z.; Su, J.; Tian, H.; Sessler, J.L. Molecular cursor caliper: A fluorescent sensor for dicarboxylate dianions. J. Am. Chem. Soc. 2019, 141, 14798–14806. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Kolemen, S.; Sedgwick, A.C.; Gunnlaugsson, T.; James, T.D.; Yoon, J.; Akkaya, E.U. Molecular logic gates: The past, present and future. Chem. Soc. Rev. 2018, 47, 2228–2248. [Google Scholar] [CrossRef] [PubMed]
- Slavcheva, S.; Mendoza, J.; Stanimirov, S.; Petkov, I.; Basílio, N.; Pina, F.; Petrov, V. On the multistate of 2′-hydroxyflavylium-flavanone system. Illustrating the concept of a timer with reset at the molecular level. Dyes Pigm. 2018, 158, 465–473. [Google Scholar] [CrossRef]
- Buryak, A.; Zaubitzer, F.; Pozdnoukhov, A.; Severin, K. Indicator displacement assays as molecular timers. J. Am. Chem. Soc. 2008, 130, 11260–11261. [Google Scholar] [CrossRef]
- Yao, C.; Lin, H.; Crory, H.; de Silva, A.P. Supra-molecular agents running tasks intelligently (SMARTI): Recent developments in molecular logic-based computation. Mol. Syst. Des. Eng. 2020, 5, 1325–1353. [Google Scholar] [CrossRef]
- Andréasson, J.; Pischel, U. Light-stimulated molecular and supramolecular systems for information processing and beyond. Coord. Chem. Rev. 2021, 429, 213695. [Google Scholar] [CrossRef]
- Bojinov, V.; Georgiev, N. Molecular sensors and molecular logic gates. J. Univ. Chem. Technol. Metall. 2011, 46, 3–26. [Google Scholar]
- Sarkar, T.; Selvakumar, K.; Motiei, L.; Margulies, D. Message in a molecule. Nat. Commun. 2016, 7, 11374. [Google Scholar] [CrossRef]
- Tong, D.; Duan, H.; Zhuang, H.; Cao, J.; Wei, Z.; Lin, Y. Using T-Hg-T and C-Ag-T: A four-input dual-core molecular logic gate and its new application in cryptography. RSC Adv. 2014, 4, 5363–5366. [Google Scholar] [CrossRef]
- Liu, L.; Liu, P.; Ga, L.; Ai, J. Advances in applications of molecular logic gates. ACS Omega 2021, 6, 30189–30204. [Google Scholar] [CrossRef]
- Magri, D.C. Logical sensing with fluorescent molecular logic gates based on photoinduced electron transfer. Coord. Chem. Rev. 2021, 426, 213598. [Google Scholar] [CrossRef]
- Aviram, A. Molecules for memory, logic, and amplification. J. Am. Chem. Soc. 1988, 110, 5687–5692. [Google Scholar] [CrossRef]
- de Silva, P.; Gunaratne, N.; McCoy, C. A molecular photoionic AND gate based on fluorescent signalling. Nature 1993, 364, 42–44. [Google Scholar] [CrossRef]
- de Silva, A.P. Molecular Logic-Based Computation; Royal Society of Chemistry: Cambridge, UK, 2013. [Google Scholar]
- Li, L.; Zhao, D.; Wang, F.; Zhang, X.; Li, W.; Fan, L. Recent advances in molecular logic gate chemosensors based on luminescent metal organic frameworks. Dalton Trans. 2021, 50, 14967–14977. [Google Scholar] [CrossRef]
- Budyka, M.F. Design principles and action of molecular logic gates. Russ. Chem. Bull. 2014, 63, 1656–1665. [Google Scholar] [CrossRef]
- Andréasson, J.; Pischel, U. Molecules with a sense of logic: A progress report. Chem. Soc. Rev. 2015, 44, 1053–1069. [Google Scholar] [CrossRef]
- Yin, F.; Wang, F.; Fan, C.; Zuo, X.; Li, Q. Biosensors based on DNA logic gates. View 2020, 2, 20200038. [Google Scholar] [CrossRef]
- Huang, Y.; Pu, F.; Ren, J.; Qu, X. Artificial enzyme-based logic operations to mimic an intracellular enzyme-participated redox balance system. Chem. Eur. J. 2017, 23, 9156–9161. [Google Scholar] [CrossRef] [PubMed]
- Katz, E. Boolean logic gates realized with enzyme-catalyzed reactions—Unusual look at usual chemical reactions. ChemPhysChem 2019, 20, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Morihiro, K.; Ankenbruck, N.; Lukasak, B.; Deiters, A. Small molecule release and activation through DNA computing. J. Am. Chem. Soc. 2017, 139, 13909–13915. [Google Scholar] [CrossRef]
- Fan, D.; Wang, J.; Wang, E.; Dong, S. Propelling DNA computing with materials’ power: Recent advancements in innovative DNA logic computing systems and smart bio-applications. Adv. Sci. 2020, 7, 2001766. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, H.; Liu, Y. Unimolecular half-adders and half-subtractors based on acid-base reaction. Front. Chem. China 2009, 4, 292–298. [Google Scholar] [CrossRef]
- Zhao, L.; Xia, W.; Yang, C. Fluorescent 1:2 demultiplexer and half-subtractor based on the hydrolysis of N-salicylidene-3-aminopyridine. Spectrochim. Acta Part A 2014, 117, 397–401. [Google Scholar] [CrossRef]
- Macdonald, J.; Li, Y.; Sutovic, M.; Lederman, H.; Pendri, K.; Lu, W.; Andrews, B.; Stefanovic, D.; Stojanovic, M. Medium scale integration of molecular logic gates in an automaton. Nano Lett. 2006, 6, 2598–2603. [Google Scholar] [CrossRef]
- Chen, X.; Liu, X.; Wang, F.; Li, S.; Chen, C.; Qiang, X.; Shi, X. Massively parallel DNA computing based on domino DNA strand displacement logic gates. ACS Synth. Biol. 2022, 11, 2504–2512. [Google Scholar] [CrossRef]
- Ozlem, S.; Akkaya, E. Thinking outside the silicon box: Molecular AND logic as an additional layer of selectivity in singlet oxygen generation forphotodynamic therapy. J. Am. Chem. Soc. 2009, 131, 48–49. [Google Scholar] [CrossRef]
- Turan, I.S.; Gunaydin, G.; Ayan, S.; Akkaya, E.U. Molecular demultiplexer as a terminator automaton. Nat. Commun. 2018, 9, 805. [Google Scholar] [CrossRef]
- Ling, J.; Naren, G.; Kelly, J.; Moody, T.; de Silva, A.P. Building pH sensors into paperbased small-molecular logic systems for very simple detection of edges of objects. J. Am. Chem. Soc. 2015, 137, 3763–3766. [Google Scholar] [CrossRef]
- Ruan, Q.; Mu, L.; Zeng, X.; Zhao, J.-L.; Zeng, L.; Chen, Z.-M.; Yang, C.; Wei, G.; Redshaw, C. A three-dimensional (time, wavelength and intensity) functioning fluorescent probe for the selective recognition/discrimination of Cu2+, Hg2+, Fe3+ and F− ions. Dalton Trans. 2018, 47, 3674–3678. [Google Scholar] [CrossRef]
- Paglin, S.; Hollister, T.; Delohery, T.; Hackett, N.; McMahill, M.; Sphicas, E.; Domingo, D.; Yahalom, J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001, 61, 439–444. [Google Scholar] [PubMed]
- Xu, M.; Kelley, S.; Glass, T. A multi-component sensor system for detection of amphiphilic compounds. Angew. Chem. Int. Ed. 2018, 130, 12741–12744. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, W.; Liu, T.; Huo, F.; Yin, C. Pinpoint diagnostic kit for heat stroke by monitoring lysosomal pH. Anal. Chem. 2017, 89, 11869–11874. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Du, J.; Hou, J.; Shi, P.; Wang, K.; Zhang, S.; Han, T.; Li, Z. Rewritable optical data storage based on mechanochromic fluorescence materials with aggregationinduced emission. Dyes Pigm. 2019, 160, 830–838. [Google Scholar] [CrossRef]
- Cafferty, B.; Ten, A.; Fink, M.; Morey, S.; Preston, D.; Mrksich, M.; Whitesides, G. Storage of information using small organic molecules. ACS Cent. Sci. 2019, 5, 911–916. [Google Scholar] [CrossRef]
- Ruskowitz, E.; Comerford, M.; Badeau, B.; DeFores, C. Logical stimuli-triggered delivery of small molecules from hydrogel biomaterials. Biomater. Sci. 2019, 7, 542–546. [Google Scholar] [CrossRef]
- Romero, M.; Mateus, P.; Matos, B.; Acuña, A.; García-Río, L.; Arteaga, J.; Pischel, U.; Basílio, N. Binding of flavylium ions to sulfonatocalix[4]arene and implication in the photorelease of biologically relevant guests in water. J. Org. Chem. 2019, 84, 10852–10859. [Google Scholar] [CrossRef]
- Todorov, P.T.; Peneva, P.N.; Georgieva, S.I.; Tchekalarova, J.; Vitkova, V.; Antonova, K.; Georgiev, A. Synthesis, characterization and anticonvulsant activity of new azobenzene-containing VV-hemorphin-5 bio photoswitch. Amino Acids 2019, 51, 549–563. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, N.I.; Bakov, V.V.; Anichina, K.K.; Bojinov, V.B. Fluorescent probes as a tool in diagnostic and drug delivery systems. Pharmaceuticals 2023, 16, 381. [Google Scholar] [CrossRef] [PubMed]
- Daly, B.; Ling, J.; de Silva, A.P. What has supramolecular chemistry done for us? Supramol. Chem. 2016, 28, 2010203. [Google Scholar] [CrossRef]
- Guliyev, R.; Ozturk, S.; Kostereli, Z.; Akkaya, E.U. From virtual to physical: Integration of chemical logic gates. Angew. Chem. Int. Ed. 2011, 50, 9826–9831. [Google Scholar] [CrossRef] [PubMed]
- Said, A.I.; Georgiev, N.I.; Bojinov, V.B. A smart chemosensor: Discriminative multidetection and various logic operations in aqueous solution at biological pH. Spectrochim. Acta Part A 2019, 223, 117304. [Google Scholar] [CrossRef]
- Liu, L.; Ga, L.; Ai, J. Ratiometric fluorescence sensing with logical operation: Theory, design and applications. Biosens. Bioelectron. 2022, 213, 114456. [Google Scholar] [CrossRef]
- Andréasson, J.; Pischel, U. Molecules for security measures: From keypad locks to advanced communication protocols. Chem. Soc. Rev. 2018, 47, 2266–2279. [Google Scholar] [CrossRef]
- Balzani, V.; Credi, A.; Venturi, M. Molecular logic circuits. ChemPhysChem 2003, 4, 49–59. [Google Scholar] [CrossRef]
- de Silva, A.P.; Uchiyama, S. Molecular logic gates and luminescent sensors based on photoinduced electron transfer. Top. Curr. Chem. 2011, 300, 1–28. [Google Scholar] [CrossRef]
- Andréasson, J.; Pischel, U. Smart molecules at work—Mimicking advanced logic operations. Chem. Soc. Rev. 2010, 39, 174–188. [Google Scholar] [CrossRef]
- Guo, Z.; Zhao, P.; Zhu, W.; Huang, X.; Xie, Y.; Tian, H. Intramolecular charge-transfer process based on dicyanomethylene-4H-pyran derivative: An integrated operation of half-subtractor and comparator. J. Phys. Chem. C 2008, 112, 7047–7053. [Google Scholar] [CrossRef]
- Kumar, M.; Kumar, R.; Bhalla, V. ‘On–Off’ reversible switch for Fe3+ and F− mimicking XNOR logic function. Tetrahedron Lett. 2010, 51, 5559–5562. [Google Scholar] [CrossRef]
- de Silva, A.P.; McClenaghan, N.D. Proof-of-principle of molecular-scale arithmetic. J. Am. Chem. Soc. 2000, 122, 3965–3966. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Dimov, S.M.; Asiri, A.M.; Alamry, K.A.; Obaid, A.Y.; Bojinov, V.B. Synthesis, selective pH-sensing activity and logic behavior of highly water-soluble1,8-naphthalimide and dihydroimidazonaphthalimide derivatives. J. Lumin. 2014, 149, 325–332. [Google Scholar] [CrossRef]
- Qian, J.; Xu, Y.; Qian, X.; Zhang, S. A pair of regio-isomeric compounds acting as molecular logic gates with different functions. J. Photochem. Photobiol. A Chem. 2009, 207, 181–189. [Google Scholar] [CrossRef]
- Qian, J.; Xu, Y.; Qian, X.; Wang, J.; Zhang, S. Effects of anionic surfactant SDS on the photophysical properties of two fluorescent molecular sensors. J. Photochem. Photobiol. A Chem. 2008, 200, 402–409. [Google Scholar] [CrossRef]
- Credi, A.; Balzani, V.; Langford, S.; Stoddart, J. Logic operations at the molecular level. An XOR gate based on a molecular machine. J. Am. Chem.Soc. 1997, 119, 2679–2681. [Google Scholar] [CrossRef]
- Coskun, A.; Deniz, E.; Akkaya, E.U. Effective PET and ICT switching of boradiazaindacene emission: A unimolecular, emission-mode, molecular half-subtractor with reconfigurable logic gates. Org. Lett. 2005, 7, 5187–5189. [Google Scholar] [CrossRef]
- Margulies, D.; Melman, G.; Shanzer, A. A molecular full-adder and full-subtractor, an additional step toward a moleculator. J. Am. Chem. Soc. 2006, 128, 4865–4871. [Google Scholar] [CrossRef]
- Langford, S.J.; Yann, T. Molecular logic: A half-subtractor based on tetraphenylporphyrin. J. Am. Chem. Soc. 2003, 125, 11198–11199. [Google Scholar] [CrossRef]
- Qian, J.; Xu, Y.; Qian, X.; Zhang, S. Molecular logic operations based on surfactant nanoaggregates. ChemPhysChem 2008, 9, 1891–1898. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Qian, X.; Xu, Y.; Zhang, S. Multiple molecular logic functions and molecular calculations facilitated by surfactant’s versatility. Chem. Commun. 2008, 35, 4141–4143. [Google Scholar] [CrossRef]
- Bozdemir, O.A.; Guliyev, R.; Buyukcakir, O.; Selcuk, S.; Kolemen, G.; Gulseren, S.; Nalbantoglu, T.; Boyaci, H.; Akkaya, E.U. Selective manipulation of ICT and PET processes in styryl-Bodipy derivatives: Applications in molecular logic and fluorescence sensing of metal ions. J. Am. Chem. Soc. 2010, 132, 8029–8036. [Google Scholar] [CrossRef] [PubMed]
- Mardanya, S.; Karmakar, S.; Das, S.; Baitalik, S. Anion and cation triggered modulation of optical properties of a pyridyl-imidazole receptor rigidly linked to pyrene and construction of INHIBIT, OR and XOR molecular logic gates: Acombined experimental and DFT/TD-DFT investigation. Sens. Actuators B Chem. 2015, 206, 701–713. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Sakr, A.R.; Bojinov, V.B. Design and synthesis of a novel PET and ICT based 1,8-naphthalimide FRET bichromophore as a four-input Disabled–Enabled-OR logic gate. Sens. Actuators B Chem. 2015, 221, 625–634. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Lyulev, M.P.; Bojinov, V.B. Sensor activity and logic behaviour of PET based dihydroimidazonaphthalimide diester. Spectrochim. Acta Part A 2012, 97, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, W.; Zhang, H.-Y.; Li, C.-J. A multifunctional arithmetical processor model integrated inside a single molecule. J. Phys. Chem. B 2006, 110, 14231–14235. [Google Scholar] [CrossRef]
- Gotor, R.; Costero, A.M.; Gil, S.; Parra, M.; Gaviña, P.; Rurack, K. Boolean operations mediated by an ion-pair receptor of a multi-readout molecular logic gate. Chem. Commun. 2013, 49, 11056–11058. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Lyulev, M.P.; Alamry, K.A.; El-Daly, S.A.; Taib, L.A.; Bojinov, V.B. Synthesis, sensor activity, and logic behavior of a highly water-soluble 9,10-dihydro-7H-imidazo[1,2-b]benz[d,e]isoqionolin-7-one dicarboxylic acid. J. Photochem. Photobiol. A Chem. 2015, 297, 31–38. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Yaneva, I.S.; Surleva, A.R.; Asiri, A.M.; Bojinov, V.B. Synthesis, sensor activity and logic behavior of a highly water-solublenaphthalimide derivative. Sens. Actuators B Chem. 2013, 184, 54–63. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Dimitrova, M.D.; Krasteva, P.V.; Bojinov, V.B. A novel water-soluble 1,8-naphthalimide as a fluorescent pH-probe and a molecular logic circuit. J. Lumin. 2017, 187, 383–391. [Google Scholar] [CrossRef]
- Said, A.I.; Georgiev, N.I.; Bojinov, V.B. Sensor activity and logic behavior of dihydroxyphenyl hydrazone derivative as a chemosensor for Cu2+ determination in alkaline aqueous solutions. J. Photochem. Photobiol. A Chem. 2015, 311, 16–24. [Google Scholar] [CrossRef]
- Georgiev, N.I.; Dimitrova, M.D.; Todorova, Y.D.; Bojinov, V.B. Synthesis, chemosensing properties and logic behaviour of a novel ratiometric 1,8-naphthalimide probe based on ICT and PET. Dyes Pigm. 2016, 131, 9–17. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev, N.I.; Bakov, V.V.; Bojinov, V.B. A Tutorial Review on the Fluorescent Probes as a Molecular Logic Circuit—Digital Comparator. Molecules 2023, 28, 6327. https://doi.org/10.3390/molecules28176327
Georgiev NI, Bakov VV, Bojinov VB. A Tutorial Review on the Fluorescent Probes as a Molecular Logic Circuit—Digital Comparator. Molecules. 2023; 28(17):6327. https://doi.org/10.3390/molecules28176327
Chicago/Turabian StyleGeorgiev, Nikolai I., Ventsislav V. Bakov, and Vladimir B. Bojinov. 2023. "A Tutorial Review on the Fluorescent Probes as a Molecular Logic Circuit—Digital Comparator" Molecules 28, no. 17: 6327. https://doi.org/10.3390/molecules28176327