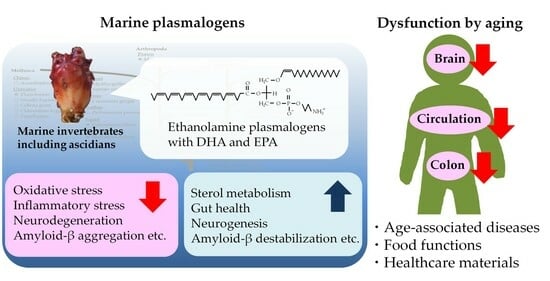
Marine Plasmalogens: A Gift from the Sea with Benefits for Age-Associated Diseases
Abstract
:1. Introduction
2. Structures and Roles of Plasmalogens
2.1. Structures and Distribution
2.2. Biological Role
3. Alterations of Plasmalogens in Aging and Associated Diseases
3.1. Aging
3.2. Alzheimer’s Disease
3.3. Parkinson’s Disease
3.4. Arteriosclerosis
3.5. Cancer
4. Food Functions of Plasmalogens
4.1. Resources
4.2. Alteration during Storage and Cooking
4.3. Absorption and Metabolism
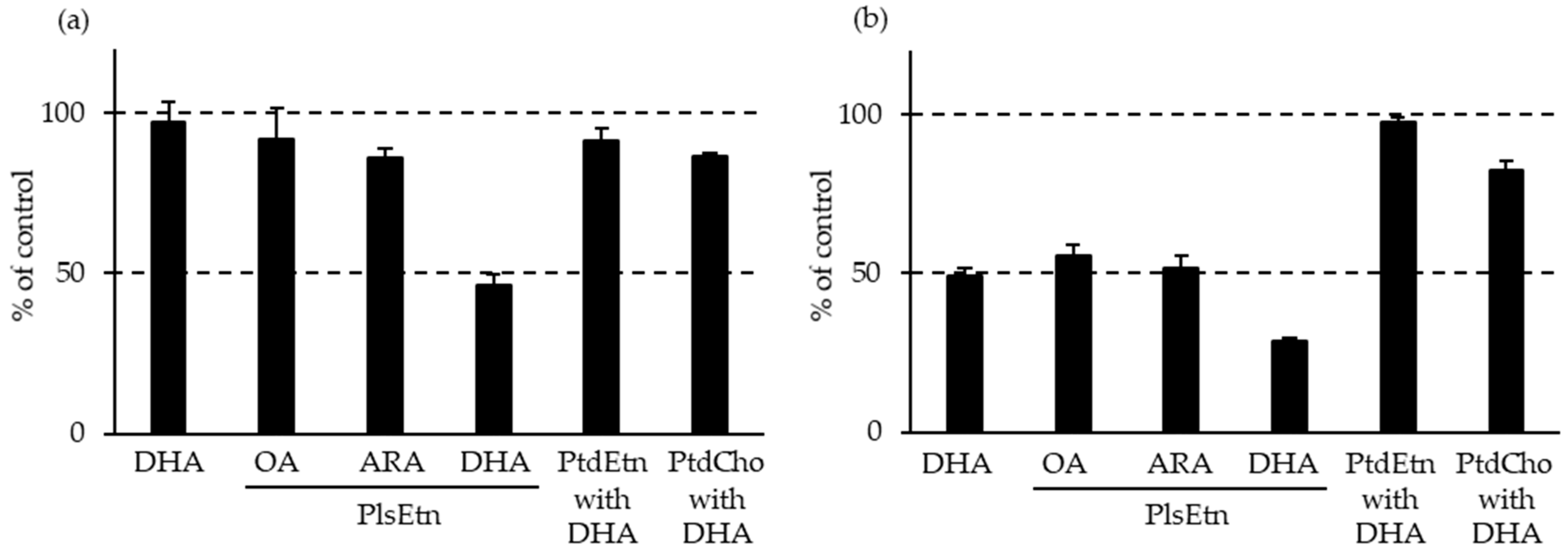
4.4. Impact on Neurodegeneration
| Resource and Composition | Animal | Administration | Effects | Refs. |
|---|---|---|---|---|
| Ascidian viscera EtnGpl composition: 80.4 mol% PlsEtn Fatty composition: 22.0 mol% DHA, 37.1 mol% EPA, 7.6 mol% ARA | Fourth-generation male Wister rats that ate a fish-oil-deficient diet 12 weeks old Aβ40 (4.9–5.5 nmol) and AlCl3 (0.5 μg) infusion into the cerebral ventricle and learning ability check for 12 weeks | Oral gavage of 200 mg (260 μmol) EtnGpl/kg/day for 6 wks from 24 weeks old | Long-term memory ; short-term memory ; short-term memory Plasma levels of 18:0/22:6-, 18:0:20:5-, and 18:0:20:4-PlsEtn  ; RBC and liver levels of 18:0/22:6- and 18:0:20:5-PlsEtn ; RBC and liver levels of 18:0/22:6- and 18:0:20:5-PlsEtn ; cerebral cortex levels of 18:0/22:6-PlsEtn ; cerebral cortex levels of 18:0/22:6-PlsEtn | [156] |
| Egg yolk EtnGpl composition: 4.0 mol% PlsEtn Fatty composition: 2.7 mol% DHA, 0.1 mol% EPA, 13.7 mol% ARA | Long-term memory⬌; short-term memory⬌ Plasma, RBC, liver, and cerebral cortex levels of PlsEtn species⬌ | |||
| Sea cucumber PlsEtn composition: 93.4% PlsEtn Fatty acid composition: 45.6% EPA | Male SD rats 6 weeks old Aβ42 (conc.: unclear) infusion into the cerebral ventricle | Oral gavage of 150 mg EtnGpl/kg/day for 26 days | Memory  In hippocampus: Aβ accumulation  ; tau hyperphosphorylation ; tau hyperphosphorylation  ; inflammation ; inflammation  ; apoptosis ; apoptosis  In cortex: oxidative resistance   | [154] |
| Sea cucumber (PtdEtn enzymatically prepared from PtdCho) PtdEtn composition: 92.6% PtdEtn Fatty acid composition: 49.3% EPA | Memory In hippocampus: Aβ accumulation  ; tau hyperphosphorylation ; tau hyperphosphorylation ; inflammation ; inflammation ; apoptosis ; apoptosis In cortex: oxidative resistance  | |||
| Scallop Pls composition: unclear Fatty acid composition: 28.7% DHA, 26.1% EPA, 10.2% ARA | Male C57/6J mice 6 months old i.p. LPS (250 μg/kg/day) for 7 days at 9 months old | Drinking water containing 0.1 μg/mL for 3 months | In cortex: PKCδ-positive microglial cells | [153] |
| Male triple-transgenic mouse model of AD (PS1, tau, and APP) 3 months old | Drinking water containing 1 μg/mL for 15 months | In cortex: PKCδ-positive microglial cells ; PKCδ protein ; PKCδ protein | ||
| Sea cucumber Pls composition: unclear | Male APP/PS1 mice 20 weeks old | A diet containing 0.1% PlsEtn for 16 weeks | Long-term memory ; short-term memory ; short-term memory In hippocampus: Aβ generation  ; soluble Aβ ; soluble Aβ ; insoluble Aβ ; insoluble Aβ ; tau hyperphosphorylation ; tau hyperphosphorylation ; neurodegeneration ; neurodegeneration ; apoptosis ; apoptosis ; lipid accumulation ; lipid accumulation ; p75NTR ; p75NTR ; TrkA phosphorylation ; TrkA phosphorylation In cortex: Aβ accumulation  ; apoptosis ; apoptosis In brain unclear part: oxidative resistance  In liver: lipid accumulation  ; p75NTR ; p75NTR | [151] |
| Sea cucumber PlsEtn composition: 93.4% PlsEtn Fatty acid composition: 11.4% DHA, 45.6% EPA, 10.1% ARA | Male SAMP8 mice 6 months old | A high-fat diet containing 1% PlsEtn for 2 months | Memory In hippocampus: Aβ generation  ; soluble Aβ ; soluble Aβ ; insoluble Aβ40 ; insoluble Aβ40 ; insoluble Aβ42 ; insoluble Aβ42 In white matter: oxidative resistance  In brain unclear part: tau hyperphosphorylation  ; glial activation ; glial activation ; inflammation ; inflammation ; apoptosis ; apoptosis ; ARA content ; ARA content | [166] |
| Ascidian Pls composition: unclear | Female C57BL/6J mice 16 months old | Oral gavage of 200 mg Pls/kg/day for 2 months | Memory In hippocampus: synaptic conditions (number, form, genesis)  ; neurogenesis ; neurogenesis ; glial activation ; glial activation ; cytokine mRNA levels ; cytokine mRNA levels | [150] |
| Chicken breast muscle Pls composition: 96.5% PlsEtn, 2.5% PlsCho Fatty acid composition: 23.8% DHA, 0.9% EPA, 21.9% ARA | Male C57BL/6 mice 8 weeks old | Diet containing 0.01% Pls for 6 weeks | Memory In hippocampus: Pls level  ; neurogenesis ; neurogenesis | [68] |
| Chicken breast muscle Pls composition: 96.5% PlsEtn, 2.5% PlsCho Fatty acid composition of PlsEtn: 23.8% DHA, 0.9% EPA, 21.9% ARA | Drinking water containing 0.01% Pls (w/v) for 6 weeks | Long-term memory⬌; short-term memory | ||
| Scallop Pls composition: 96.5% PlsEtn, 2.5% PlsCho Fatty acid composition of PlsEtn: 37.1% DHA, 27.8% EPA, 24.9% ARA | Long-term memory ; short-term memory ; short-term memory | |||
| Chicken breast muscle Pls composition: unclear | Male C57/6J mice 7 months old i.p. LPS (250 μg/kg/day) for 7 days at 10 months old | Drinking water containing 0.1 or 10 μg Pls/mL for 3 months | Memory In cortex and hippocampus: Aβ accumulation  ; glial activation ; glial activation ; cytokine mRNA levels ; cytokine mRNA levels | [152] |
| Chicken breast muscle Pls composition: 47.6% PlsEtn (18.6% DHA, 24.9% ARA), 49.3% PlsCho (2.3% DHA, 17.2% ARA) | Male C57/6J mice 10 months old i.p. LPS (250 μg/kg/day) for 7 days | i.p. 20 mg Pls/kg/day for 7 days along with LPS treatment | In PFC and hippocampus: Aβ accumulation ; glial activation ; glial activation ; cytokine mRNA levels ; cytokine mRNA levels ; PlsEtn levels ; PlsEtn levels | [155] |
 , decrease;
, decrease;  increase; ⬌, no change.
increase; ⬌, no change. 4.5. Impact on Arteriosclerosis
4.6. Impact on Colon Impairments
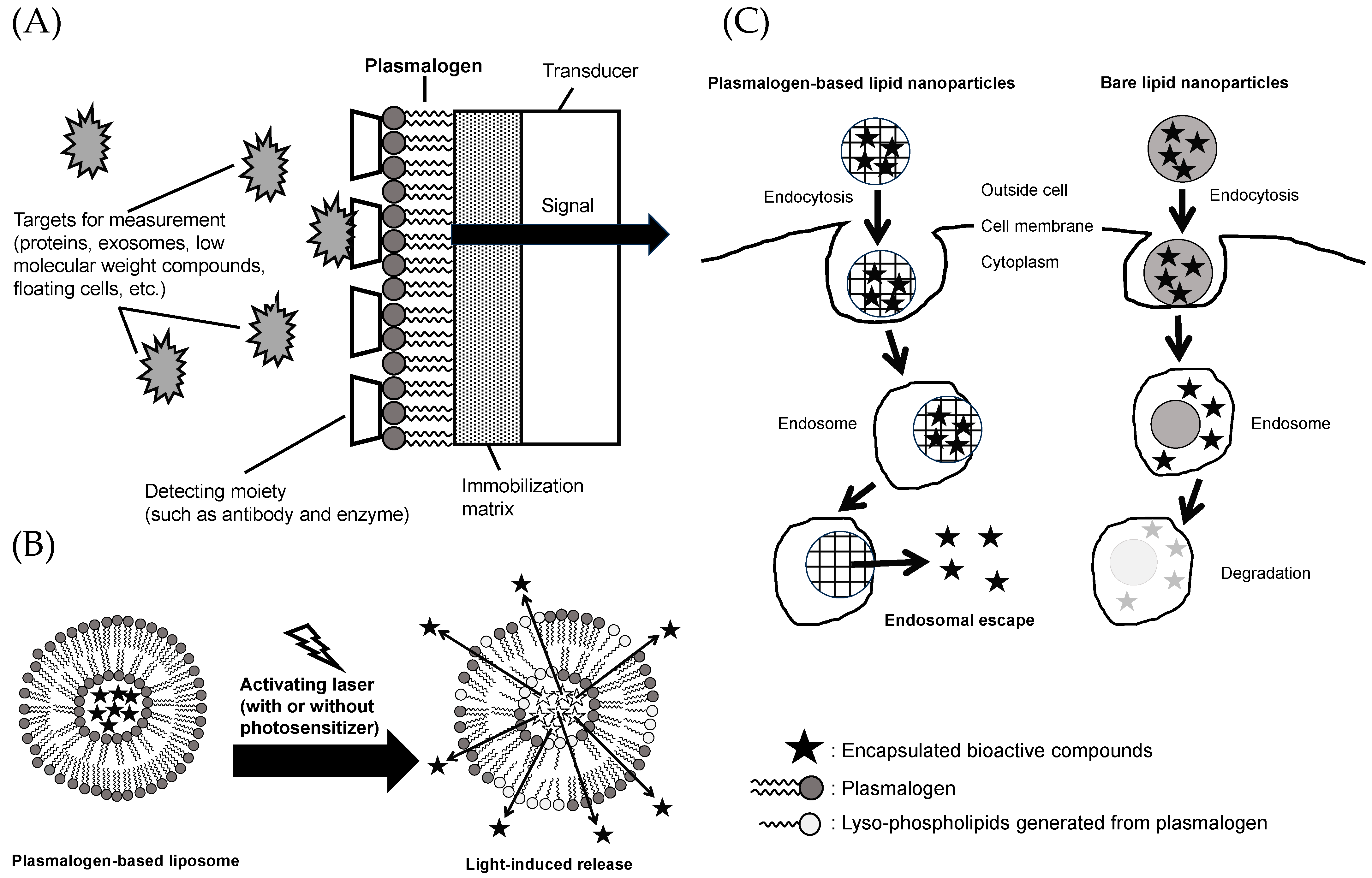
5. Plasmalogens as Healthcare Materials
5.1. Materials for Functional Membrane of Biosensors
5.2. Materials for Light-Activated Liposomes
5.3. Materials for Nanoparticles with Endosomal Escape Capabilities
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Selkoe, D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56, S14–S19. [Google Scholar] [CrossRef]
- Patel, A.K.; Albarico, F.; Perumal, P.K.; Vadrale, A.P.; Nian, C.T.; Chau, H.T.B.; Anwar, C.; Wani, H.; Pal, A.; Saini, R.; et al. Algae as an emerging source of bioactive pigments. Bioresour. Technol. 2022, 351, 126910. [Google Scholar] [CrossRef]
- Yamashita, S.; Honjo, A.; Aruga, M.; Nakagawa, K.; Miyazawa, T. Preparation of marine plasmalogen and selective identification of molecular species by LC-MS/MS. J. Oleo Sci. 2014, 63, 423–430. [Google Scholar] [CrossRef]
- Yamashita, S.; Abe, A.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Separation and detection of plasmalogen in marine invertebrates by high-performance liquid chromatography with evaporative light-scattering detection. Lipids 2014, 49, 1261–1273. [Google Scholar] [CrossRef]
- Yamashita, S.; Kanno, S.; Honjo, A.; Otoki, Y.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Analysis of Plasmalogen Species in Foodstuffs. Lipids 2016, 51, 199–210. [Google Scholar] [CrossRef]
- Brosche, T. Plasmalogen phospholipids—Facts and theses to their antioxidative qualities. Arch. Gerontol. Geriatr. 1997, 25, 73–81. [Google Scholar] [CrossRef]
- Han, X.; Holtzman, D.M.; McKeel, D.W., Jr. Plasmalogen deficiency in early Alzheimer’s disease subjects and in animal models: Molecular characterization using electrospray ionization mass spectrometry. J. Neurochem. 2001, 77, 1168–1180. [Google Scholar] [CrossRef]
- Fujino, T.; Yamada, T.; Asada, T.; Tsuboi, Y.; Wakana, C.; Mawatari, S.; Kono, S. Efficacy and Blood Plasmalogen Changes by Oral Administration of Plasmalogen in Patients with Mild Alzheimer’s Disease and Mild Cognitive Impairment: A Multicenter, Randomized, Double-blind, Placebo-controlled Trial. EBioMedicine 2017, 17, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, A.; Aizawa, T.; Kubomura, D.; Akahori, Y.; Yamashita, S.; Nakagawa, K.; Miyazawa, T. Effects of Ascidian-Derived Ethanolamine Plasmalogen on Cognitive Function and Its Safety -A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study-. Pharmacometrics 2023, 104, 17–24. [Google Scholar]
- Mawatari, S.; Katafuchi, T.; Miake, K.; Fujino, T. Dietary plasmalogen increases erythrocyte membrane plasmalogen in rats. Lipids Health Dis. 2012, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Braverman, N.E.; Moser, A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta 2012, 1822, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Bozelli, J.C., Jr.; Azher, S.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12, 730829. [Google Scholar] [CrossRef]
- Wu, L.C.; Pfeiffer, D.R.; Calhoon, E.A.; Madiai, F.; Marcucci, G.; Liu, S.; Jurkowitz, M.S. Purification, identification, and cloning of lysoplasmalogenase, the enzyme that catalyzes hydrolysis of the vinyl ether bond of lysoplasmalogen. J. Biol. Chem. 2011, 286, 24916–24930. [Google Scholar] [CrossRef]
- Panganamala, R.V.; Horrocks, L.A.; Geer, J.C.; Cornwell, D.G. Positions of double bonds in the monounsaturated alk-1-enyl groups from the plasmalogens of human heart and brain. Chem. Phys. Lipids 1971, 6, 97–102. [Google Scholar] [CrossRef]
- Heymans, H.S.; Schutgens, R.B.; Tan, R.; van den Bosch, H.; Borst, P. Severe plasmalogen deficiency in tissues of infants without peroxisomes (Zellweger syndrome). Nature 1983, 306, 69–70. [Google Scholar] [CrossRef]
- Guan, Z.; Grünler, J.; Piao, S.; Sindelar, P.J. Separation and Quantitation of Phospholipids and Their Ether Analogues by High-Performance Liquid Chromatography. Anal. Biochem. 2001, 297, 137–143. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Sampson, E.L. Lipid composition of the normal human brain: Gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 537–544. [Google Scholar] [CrossRef]
- Seikagaku Data Book Editorial Board. Seikagaku Data Book I; The Japanese Biochemical Society, Ed.; Tokyo Kagaku Dojin: Tokyo, Japan, 1979. [Google Scholar]
- Rapport, M.M.; Lerner, B. The structure of plasmalogens IV. Lipids in normal and neoplastic tissues of man and in normal tissues of rabbit and rat. Biochim. Biophys. Acta 1959, 33, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B.P.; Frais, F.F. Changes in Plasmalogen Content of Human Heart and Skeletal Muscle with Age and Development. Nature 1967, 215, 993–994. [Google Scholar] [CrossRef] [PubMed]
- Takamura, H.; Kasai, H.; Arita, H.; Kito, M. Phospholipid molecular species in human umbilical artery and vein endothelial cells. J. Lipid Res. 1990, 31, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Dueck, D.A.; Chan, M.; Tran, K.; Wong, J.T.; Jay, F.T.; Littman, C.; Stimpson, R.; Choy, P.C. The modulation of choline phosphoglyceride metabolism in human colon cancer. Mol. Cell Biochem. 1996, 162, 97–103. [Google Scholar] [CrossRef]
- Deeley, J.M.; Thomas, M.C.; Truscott, R.J.W.; Mitchell, T.W.; Blanksby, S.J. Identification of Abundant Alkyl Ether Glycerophospholipids in the Human Lens by Tandem Mass Spectrometry Techniques. Anal. Chem. 2009, 81, 1920–1930. [Google Scholar] [CrossRef]
- Chabot, M.C.; Greene, D.G.; Brockschmidt, J.K.; Capizzi, R.L.; Wykle, R.L. Ether-linked phosphoglyceride content of human leukemia cells. Cancer Res. 1990, 50, 7174–7178. [Google Scholar]
- Ojima-Uchiyama, A.; Masuzawa, Y.; Sugiura, T.; Waku, K.; Saito, H.; Yui, Y.; Tomioka, H. Phospholipid analysis of human eosinophils: High levels of alkylacylglycerophosphocholine (PAF precursor). Lipids 1988, 23, 815–817. [Google Scholar] [CrossRef]
- Farquhar, J.W.; Ahrens, E.H., Jr. Effects of dietary fats on human erythrocyte fatty acid patterns. J. Clin. Investig. 1963, 42, 675–685. [Google Scholar] [CrossRef]
- Engelmann, B.; Streich, S.; Schonthier, U.M.; Richter, W.O.; Duhm, J. Changes of membrane phospholipid composition of human erythrocytes in hyperlipidemias. I. Increased phosphatidylcholine and reduced sphingomyelin in patients with elevated levels of triacylglycerol-rich lipoproteins. Biochim. Biophys. Acta 1992, 1165, 32–37. [Google Scholar] [CrossRef]
- Mawatari, S.; Fukata, M.; Arita, T.; Maruyama, T.; Kono, S.; Fujino, T. Decreases of ethanolamine plasmalogen and phosphatidylcholine in erythrocyte are a common phenomenon in Alzheimer’s, Parkinson’s, and coronary artery diseases. Brain Res. Bull. 2022, 189, 5–10. [Google Scholar] [CrossRef]
- Brautigam, C.; Engelmann, B.; Reiss, D.; Reinhardt, U.; Thiery, J.; Richter, W.O.; Brosche, T. Plasmalogen phospholipids in plasma lipoproteins of normolipidemic donors and patients with hypercholesterolemia treated by LDL apheresis. Atherosclerosis 1996, 119, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Maeba, R.; Maeda, T.; Kinoshita, M.; Takao, K.; Takenaka, H.; Kusano, J.; Yoshimura, N.; Takeoka, Y.; Yasuda, D.; Okazaki, T.; et al. Plasmalogens in human serum positively correlate with high- density lipoprotein and decrease with aging. J. Atheroscler. Thromb. 2007, 14, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, A.; Sakurai, T.; Nishimukai, M.; Takahashi, Y.; Nagasaka, A.; Hui, S.P.; Hara, H.; Chiba, H. Composition of plasmalogens in serum lipoproteins from patients with non-alcoholic steatohepatitis and their susceptibility to oxidation. Clin. Chim. Acta 2019, 493, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Takamura, H.; Tanaka, K.; Matsuura, T.; Kito, M. Ether phospholipid molecular species in human platelets. J. Biochem. 1989, 105, 168–172. [Google Scholar] [CrossRef]
- Wallner, S.; Orso, E.; Grandl, M.; Konovalova, T.; Liebisch, G.; Schmitz, G. Phosphatidylcholine and phosphatidylethanolamine plasmalogens in lipid loaded human macrophages. PLoS ONE 2018, 13, e0205706. [Google Scholar] [CrossRef]
- Poulos, A.; White, I.G. The phospholipid composition of human spermatozoa and seminal plasma. J. Reprod. Fertil. 1973, 35, 265–272. [Google Scholar] [CrossRef]
- Hoffman-Kuczynski, B.; Reo, N.V. Administration of myo-inositol plus ethanolamine elevates phosphatidylethanolamine plasmalogen in the rat cerebellum. Neurochem. Res. 2005, 30, 47–60. [Google Scholar] [CrossRef]
- Breckenridge, W.C.; Morgan, I.G.; Zanetta, J.P.; Vincendon, G. Adult rat brain synaptic vesicles. II. Lipid composition. Biochim. Biophys. Acta 1973, 320, 681–686. [Google Scholar] [CrossRef]
- Novák, F.; Tvrzická, E.; Hamplová, B.; Kolář, F.; Nováková, O. Postnatal development of phospholipids and their fatty acid profile in rat heart. Mol. Cell Biochem. 2006, 293, 23–33. [Google Scholar] [CrossRef]
- Post, J.A.; Verkleij, A.J.; Roelofsen, B.; de Kamp, J.A.F.O. Plasmalogen content and distribution in the sarcolemma of cultured neonatal rat myocytes. FEBS Lett. 1988, 240, 78–82. [Google Scholar] [CrossRef]
- Blank, M.L.; Cress, E.A.; Smith, Z.L.; Snyder, F. Dietary supplementation with ether-linked lipids and tissue lipid composition. Lipids 1991, 26, 166–169. [Google Scholar] [CrossRef]
- Vance, J.E. Lipoproteins secreted by cultured rat hepatocytes contain the antioxidant 1-alk-1-enyl-2-acylglycerophosphoethanolamine. Biochim. Biophys. Acta 1990, 1045, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Surinlert, P.; Asuvapongpatana, S.; Srakaew, N.; Daungchinda, T.; Setou, M.; Weerachatyanukul, W. Changes of fatty acids in phosphatidylcholine on sperm membrane during Macrobrachium rosenbergii sperm transit through spermatic duct and lipid analysis in spermatic vesicles. Aquaculture 2016, 456, 62–69. [Google Scholar] [CrossRef]
- Kimura, T.; Kimura, A.K.; Ren, M.; Berno, B.; Xu, Y.; Schlame, M.; Epand, R.M. Substantial Decrease in Plasmalogen in the Heart Associated with Tafazzin Deficiency. Biochemistry 2018, 57, 2162–2175. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.W. Identification of plasmalogen as the major phospholipid constituent of cardiac sarcoplasmic reticulum. Biochemistry 1985, 24, 1662–1668. [Google Scholar] [CrossRef]
- Sugiura, T.; Nakajima, M.; Sekiguchi, N.; Nakagawa, Y.; Waku, K. Different fatty chain compositions of alkenylacyl, alkylacyl and diacyl phospholipids in rabbit alveolar macrophages: High amounts of arachidonic acid in ether phospholipids. Lipids 1983, 18, 125–129. [Google Scholar]
- Amunugama, K.; Jellinek, M.J.; Kilroy, M.P.; Albert, C.J.; Rasi, V.; Hoft, D.F.; Shashaty, M.G.S.; Meyer, N.J.; Ford, D.A. Identification of novel neutrophil very long chain plasmalogen molecular species and their myeloperoxidase mediated oxidation products in human sepsis. Redox Biol. 2021, 48, 102208. [Google Scholar] [CrossRef] [PubMed]
- Wood, P.L.; Khan, M.A.; Smith, T.; Ehrmantraut, G.; Jin, W.; Cui, W.; Braverman, N.E.; Goodenowe, D.B. In vitro and in vivo plasmalogen replacement evaluations in rhizomelic chrondrodysplasia punctata and Pelizaeus-Merzbacher disease using PPI-1011, an ether lipid plasmalogen precursor. Lipids Health Dis. 2011, 10, 182. [Google Scholar] [CrossRef]
- Goodenowe, D.B.; Cook, L.L.; Liu, J.; Lu, Y.; Jayasinghe, D.A.; Ahiahonu, P.W.; Heath, D.; Yamazaki, Y.; Flax, J.; Krenitsky, K.F.; et al. Peripheral ethanolamine plasmalogen deficiency: A logical causative factor in Alzheimer’s disease and dementia. J. Lipid Res. 2007, 48, 2485–2498. [Google Scholar] [CrossRef]
- Vecchini, A.; Del Rosso, F.; Binaglia, L.; Dhalla, N.S.; Panagia, V. Molecular defects in sarcolemmal glycerophospholipid subclasses in diabetic cardiomyopathy. J. Mol. Cell Cardiol. 2000, 32, 1061–1074. [Google Scholar] [CrossRef] [PubMed]
- Bizeau, J.B.; Albouery, M.; Gregoire, S.; Buteau, B.; Martine, L.; Crepin, M.; Bron, A.M.; Berdeaux, O.; Acar, N.; Chassaing, B.; et al. Dietary Inulin Supplementation Affects Specific Plasmalogen Species in the Brain. Nutrients 2022, 14, 3097. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Sheikh, A.M.; Haque, A.; Osago, H.; Sakai, H.; Shibly, A.Z.; Yano, S.; Michikawa, M.; Hossain, S.; Tabassum, S.; et al. Time-Dependent Analysis of Plasmalogens in the Hippocampus of an Alzheimer’s Disease Mouse Model: A Role of Ethanolamine Plasmalogen. Brain Sci. 2021, 11, 1603. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.M.; Astudillo, A.M.; Casas, J.; Balboa, M.A.; Balsinde, J. Regulation of Phagocytosis in Macrophages by Membrane Ethanolamine Plasmalogens. Front. Immunol. 2018, 9, 1723. [Google Scholar] [CrossRef]
- Portilla, D.; Creer, M.H. Plasmalogen phospholipid hydrolysis during hypoxic injury of rabbit proximal tubules. Kidney Int. 1995, 47, 1087–1094. [Google Scholar] [CrossRef]
- Koivuniemi, A. The biophysical properties of plasmalogens originating from their unique molecular architecture. FEBS Lett. 2017, 591, 2700–2713. [Google Scholar]
- West, A.; Zoni, V.; Teague, W.E., Jr.; Leonard, A.N.; Vanni, S.; Gawrisch, K.; Tristram-Nagle, S.; Sachs, J.N.; Klauda, J.B. How Do Ethanolamine Plasmalogens Contribute to Order and Structure of Neurological Membranes? J. Phys. Chem. B 2020, 124, 828–839. [Google Scholar] [CrossRef]
- Maeba, R.; Yusufu, Y.; Shimasaki, H.; Ueta, N. Comparison of the oxidizability of various glycerophospholipids in bilayers by the oxygen uptake method. Lipids 2002, 37, 893–900. [Google Scholar] [CrossRef]
- Broniec, A.; Klosinski, R.; Pawlak, A.; Wrona-Krol, M.; Thompson, D.; Sarna, T. Interactions of plasmalogens and their diacyl analogs with singlet oxygen in selected model systems. Free Radic. Biol. Med. 2011, 50, 892–898. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A. Plasmalogens, phospholipase A2, and docosahexaenoic acid turnover in brain tissue. J. Mol. Neurosci. 2001, 16, 263–272; discussion 279–284. [Google Scholar] [CrossRef]
- Christie, W.W.; Harwood, J.L. Oxidation of polyunsaturated fatty acids to produce lipid mediators. Essays Biochem. 2020, 64, 401–421. [Google Scholar]
- Hayashi, D.; Mouchlis, V.D.; Dennis, E.A. Each phospholipase A(2) type exhibits distinct selectivity toward sn-1 ester, alkyl ether, and vinyl ether phospholipids. Biochim. Biophys. Acta 2022, 1867, 159067. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.A.; Morand, O.H.; Raetz, C.R. A possible role for plasmalogens in protecting animal cells against photosensitized killing. J. Biol. Chem. 1988, 263, 11590–11596. [Google Scholar]
- Honsho, M.; Fujiki, Y. Regulation of plasmalogen biosynthesis in mammalian cells and tissues. Brain Res. Bull. 2023, 194, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Dorninger, F.; Brodde, A.; Braverman, N.E.; Moser, A.B.; Just, W.W.; Forss-Petter, S.; Brügger, B.; Berger, J. Homeostasis of phospholipids—The level of phosphatidylethanolamine tightly adapts to changes in ethanolamine plasmalogens. Biochim. Biophys. Acta 2015, 1851, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Glaser, P.E.; Gross, R.W. Plasmenylethanolamine facilitates rapid membrane fusion: A stopped-flow kinetic investigation correlating the propensity of a major plasma membrane constituent to adopt an HII phase with its ability to promote membrane fusion. Biochemistry 1994, 33, 5805–5812. [Google Scholar] [CrossRef]
- Malheiro, A.R.; Correia, B.; Ferreira da Silva, T.; Bessa-Neto, D.; Van Veldhoven, P.P.; Brites, P. Leukodystrophy caused by plasmalogen deficiency rescued by glyceryl 1-myristyl ether treatment. Brain Pathol. 2019, 29, 622–639. [Google Scholar] [CrossRef]
- Hossain, M.S.; Mawatari, S.; Fujino, T. Plasmalogens, the Vinyl Ether-Linked Glycerophospholipids, Enhance Learning and Memory by Regulating Brain-Derived Neurotrophic Factor. Front. Cell Dev. Biol. 2022, 10, 828282. [Google Scholar]
- Cui, W.; Liu, D.; Gu, W.; Chu, B. Peroxisome-driven ether-linked phospholipids biosynthesis is essential for ferroptosis. Cell Death Differ. 2021, 28, 2536–2551. [Google Scholar] [CrossRef]
- Zoeller, R.A.; Grazia, T.J.; LaCamera, P.; Park, J.; Gaposchkin, D.P.; Farber, H.W. Increasing plasmalogen levels protects human endothelial cells during hypoxia. Am. J. Physiol.-Heart Circ. Physiol. 2002, 283, H671–H679. [Google Scholar] [CrossRef]
- Hahnel, D.; Thiery, J.; Brosche, T.; Engelmann, B. Role of plasmalogens in the enhanced resistance of LDL to copper-induced oxidation after LDL apheresis. Arter. Thromb. Vasc. Biol. 1999, 19, 2431–2438. [Google Scholar] [CrossRef]
- Rasmiena, A.A.; Barlow, C.K.; Stefanovic, N.; Huynh, K.; Tan, R.; Sharma, A.; Tull, D.; de Haan, J.B.; Meikle, P.J. Plasmalogen modulation attenuates atherosclerosis in ApoE- and ApoE/GPx1-deficient mice. Atherosclerosis 2015, 243, 598–608. [Google Scholar] [CrossRef] [PubMed]
- Gil-de-Gómez, L.; Astudillo, A.M.; Lebrero, P.; Balboa, M.A.; Balsinde, J. Essential Role for Ethanolamine Plasmalogen Hydrolysis in Bacterial Lipopolysaccharide Priming of Macrophages for Enhanced Arachidonic Acid Release. Front. Immunol. 2017, 8, 1251. [Google Scholar] [CrossRef] [PubMed]
- Maeba, R. Strange Phospholipids—Plasmalogen. Oleoscience 2005, 5, 405–415. [Google Scholar] [CrossRef]
- Kimura, T.; Kimura, A.K.; Ren, M.; Monteiro, V.; Xu, Y.; Berno, B.; Schlame, M.; Epand, R.M. Plasmalogen loss caused by remodeling deficiency in mitochondria. Life Sci. Alliance 2019, 2, e201900348. [Google Scholar] [CrossRef]
- Kinoshita, M.; Oikawa, S.; Hayasaka, K.; Sekikawa, A.; Nagashima, T.; Toyota, T.; Miyazawa, T. Age-related increases in plasma phosphatidylcholine hydroperoxide concentrations in control subjects and patients with hyperlipidemia. Clin. Chem. 2000, 46, 822–828. [Google Scholar] [CrossRef]
- Miyazawa, T.; Suzuki, T.; Fujimoto, K.; Kinoshita, M. Age-related change of phosphatidylcholine hydroperoxide and phosphatidylethanolamine hydroperoxide levels in normal human red blood cells. Mech. Ageing Dev. 1996, 86, 145–150. [Google Scholar] [CrossRef]
- Miyazawa, T. Lipid hydroperoxides in nutrition, health, and diseases. Proc. Jpn. Acad. Ser. B 2021, 97, 161–196. [Google Scholar] [CrossRef]
- Asai, A.; Okajima, F.; Nakagawa, K.; Ibusuki, D.; Tanimura, K.; Nakajima, Y.; Nagao, M.; Sudo, M.; Harada, T.; Miyazawa, T.; et al. Phosphatidylcholine hydroperoxide-induced THP-1 cell adhesion to intracellular adhesion molecule-1. J. Lipid Res. 2009, 50, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Rouser, G.; Yamamoto, A. Curvilinear regression course of human brain lipid composition changes with age. Lipids 1968, 3, 284–287. [Google Scholar] [CrossRef]
- Weisser, M.; Vieth, M.; Stolte, M.; Riederer, P.; Pfeuffer, R.; Leblhuber, F.; Spiteller, G. Dramatic increase of alpha-hydroxyaldehydes derived from plasmalogens in the aged human brain. Chem. Phys. Lipids 1997, 90, 135–142. [Google Scholar] [CrossRef]
- Bartzokis, G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol. Aging 2011, 32, 1341–1371. [Google Scholar] [CrossRef] [PubMed]
- Terlecky, S.R.; Koepke, J.I.; Walton, P.A. Peroxisomes and aging. Biochim. Biophys. Acta 2006, 1763, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.M.; Yang, K.; Liu, G.; Moon, S.H.; Dilthey, B.G.; Gross, R.W. Cytochrome c is an oxidative stress-activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage. J. Biol. Chem. 2018, 293, 8693–8709. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, D.; Barker, T. 2020 Alzheimer’s disease facts and figures. In Alzheimers Dement; Cambridge University Press: Cambridge, UK, 2020. [Google Scholar]
- Mattson, M.P.; Begley, J.G.; Mark, R.J.; Furukawa, K. Abeta25-35 induces rapid lysis of red blood cells: Contrast with Abeta1-42 and examination of underlying mechanisms. Brain Res. 1997, 771, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Eckley, D.M.; Williamson, J.D.; Launer, L.J.; Rifkind, J.M. Do red blood cell-beta-amyloid interactions alter oxygen delivery in Alzheimer’s disease? Adv. Exp. Med. Biol. 2008, 614, 29–35. [Google Scholar]
- Jayakumar, R.; Kusiak, J.W.; Chrest, F.J.; Demehin, A.A.; Murali, J.; Wersto, R.P.; Nagababu, E.; Ravi, L.; Rifkind, J.M. Red cell perturbations by amyloid beta-protein. Biochim. Biophys. Acta 2003, 1622, 20–28. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Sookwong, P.; Tsuduki, T.; Satoh, A.; Miyazawa, T. Amyloid beta-induced erythrocytic damage and its attenuation by carotenoids. FEBS Lett. 2011, 585, 1249–1254. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kiko, T.; Kuriwada, S.; Miyazawa, T.; Kimura, F.; Miyazawa, T. Amyloid beta induces adhesion of erythrocytes to endothelial cells and affects endothelial viability and functionality. Biosci. Biotechnol. Biochem. 2011, 75, 2030–2033. [Google Scholar] [CrossRef]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Suzuki, T.; Arai, H.; Miyazawa, T. Significance of lutein in red blood cells of Alzheimer’s disease patients. J. Alzheimers Dis. 2012, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Kiko, T.; Nakagawa, K.; Satoh, A.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. Amyloid beta levels in human red blood cells. PLoS ONE 2012, 7, e49620. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, L.; Rafique, S.; Xuereb, J.H.; Rapoport, S.I.; Gershfeld, N.L. Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer’s disease brain. Brain Res. 1995, 698, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Ma, K.; Gao, F.; Kim, H.-W.; Rapoport, S.I.; Rao, J.S. Disturbed Choline Plasmalogen and Phospholipid Fatty Acid Concentrations in Alzheimer’s Disease Prefrontal Cortex. J. Alzheimers Dis. 2011, 24, 507–517. [Google Scholar] [CrossRef]
- Yamashita, S.; Kiko, T.; Fujiwara, H.; Hashimoto, M.; Nakagawa, K.; Kinoshita, M.; Furukawa, K.; Arai, H.; Miyazawa, T. Alterations in the Levels of Amyloid-beta, Phospholipid Hydroperoxide, and Plasmalogen in the Blood of Patients with Alzheimer’s Disease: Possible Interactions between Amyloid-beta and These Lipids. J. Alzheimers Dis. 2016, 50, 527–537. [Google Scholar] [CrossRef]
- Trist, B.G.; Hare, D.J.; Double, K.L. Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 2019, 18, e13031. [Google Scholar] [CrossRef]
- Lim, E.W.; Aarsland, D.; Ffytche, D.; Taddei, R.N.; van Wamelen, D.J.; Wan, Y.M.; Tan, E.K.; Ray Chaudhuri, K.; Kings Parcog groupMDS Nonmotor Study Group. Amyloid-beta and Parkinson’s disease. J. Neurol. 2019, 266, 2605–2619. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Kinoshita, M.; Shimokado, K. Autocrine FGF-2 is responsible for the cell density-dependent susceptibility to apoptosis of HUVEC: A role of a calpain inhibitor-sensitive mechanism. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2323–2329. [Google Scholar] [CrossRef]
- Harada-Shiba, M.; Kinoshita, M.; Kamido, H.; Shimokado, K. Oxidized low density lipoprotein induces apoptosis in cultured human umbilical vein endothelial cells by common and unique mechanisms. J. Biol. Chem. 1998, 273, 9681–9687. [Google Scholar] [CrossRef]
- Brosche, T. Plasmalogen levels in serum from patients with impaired carbohydrate or lipid metabolism and in elderly subjects with normal metabolic values. Arch. Gerontol. Geriatr. 2001, 32, 283–294. [Google Scholar] [CrossRef]
- Nishimukai, M.; Maeba, R.; Yamazaki, Y.; Nezu, T.; Sakurai, T.; Takahashi, Y.; Hui, S.P.; Chiba, H.; Okazaki, T.; Hara, H. Serum choline plasmalogens, particularly those with oleic acid in sn-2, are associated with proatherogenic state. J. Lipid Res. 2014, 55, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Nishimukai, M.; Maeba, R.; Ikuta, A.; Asakawa, N.; Kamiya, K.; Yamada, S.; Yokota, T.; Sakakibara, M.; Tsutsui, H.; Sakurai, T.; et al. Serum choline plasmalogens-those with oleic acid in sn-2-are biomarkers for coronary artery disease. Clin. Chim. Acta 2014, 437, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Meikle, P.J.; Wong, G.; Tsorotes, D.; Barlow, C.K.; Weir, J.M.; Christopher, M.J.; MacIntosh, G.L.; Goudey, B.; Stern, L.; Kowalczyk, A.; et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2723–2732. [Google Scholar] [CrossRef] [PubMed]
- Sutter, I.; Klingenberg, R.; Othman, A.; Rohrer, L.; Landmesser, U.; Heg, D.; Rodondi, N.; Mach, F.; Windecker, S.; Matter, C.M.; et al. Decreased phosphatidylcholine plasmalogens—A putative novel lipid signature in patients with stable coronary artery disease and acute myocardial infarction. Atherosclerosis 2016, 246, 130–140. [Google Scholar] [CrossRef]
- Dudda, A.; Spiteller, G.; Kobelt, F. Lipid oxidation products in ischemic porcine heart tissue. Chem. Phys. Lipids 1996, 82, 39–51. [Google Scholar] [CrossRef]
- Lukacova, N.; Halat, G.; Chavko, M.; Marsala, J. Ischemia-reperfusion injury in the spinal cord of rabbits strongly enhances lipid peroxidation and modifies phospholipid profiles. Neurochem. Res. 1996, 21, 869–873. [Google Scholar] [CrossRef]
- Lukacova, N.; Marsala, M.; Halat, G.; Marsala, J. Neuroprotective effect of graded postischemic reoxygenation in spinal cord ischemia in the rabbit. Brain Res. Bull. 1997, 43, 457–465. [Google Scholar] [CrossRef]
- Bartsch, H.; Nair, J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage, and repair. Langenbeck’s Arch. Surg. 2006, 391, 499–510. [Google Scholar] [CrossRef]
- Matsuno, Y.; Atsumi, Y.; Shimizu, A.; Katayama, K.; Fujimori, H.; Hyodo, M.; Minakawa, Y.; Nakatsu, Y.; Kaneko, S.; Hamamoto, R.; et al. Replication stress triggers microsatellite destabilization and hypermutation leading to clonal expansion in vitro. Nat. Commun. 2019, 10, 3925. [Google Scholar] [CrossRef]
- Messias, M.C.F.; Mecatti, G.C.; Priolli, D.G.; de Oliveira Carvalho, P. Plasmalogen lipids: Functional mechanism and their involvement in gastrointestinal cancer. Lipids Health Dis. 2018, 17, 41. [Google Scholar] [CrossRef] [PubMed]
- Dahabieh, M.S.; Di Pietro, E.; Jangal, M.; Goncalves, C.; Witcher, M.; Braverman, N.E.; del Rincón, S.V. Peroxisomes and cancer: The role of a metabolic specialist in a disease of aberrant metabolism. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2018, 1870, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Tamiya-Koizumi, K.; Otoki, Y.; Nakagawa, K.; Kannagi, R.; Mizutani, N.; Suzuki, M.; Kyogashima, M.; Iwaki, S.; Aoyama, M.; Murate, T.; et al. Cellular concentrations of plasmalogen species containing a polyunsaturated fatty acid significantly increase under hypoxia in human colorectal cancer, Caco2 cells. Biochem. Biophys. Res. Commun. 2022, 611, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Lv, C.-Q.; Xu, L.; Yang, H. Plasma Content Variation and Correlation of Plasmalogen and GIS, TC, and TPL in Gastric Carcinoma Patients: A Comparative Study. Med. Sci. Monit. Basic. Res. 2015, 21, 157–160. [Google Scholar]
- Merchant, T.E.; de Graaf, P.W.; Minsky, B.D.; Obertop, H.; Glonek, T. Esophageal cancer phospholipid characterization by31P NMR. NMR Biomed. 1993, 6, 187–193. [Google Scholar] [CrossRef]
- Ritchie, S.A.; Akita, H.; Takemasa, I.; Eguchi, H.; Pastural, E.; Nagano, H.; Monden, M.; Doki, Y.; Mori, M.; Jin, W.; et al. Metabolic system alterations in pancreatic cancer patient serum: Potential for early detection. BMC Cancer 2013, 13, 416. [Google Scholar] [CrossRef]
- Murakami, M.; Miki, Y.; Sato, H.; Murase, R.; Taketomi, Y.; Yamamoto, K. Group IID, IIE, IIF and III secreted phospholipase A2s. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2019, 1864, 803–818. [Google Scholar] [CrossRef]
- Abe, C.; Miyazawa, T.; Miyazawa, T. Current Use of Fenton Reaction in Drugs and Food. Molecules 2022, 27, 5451. [Google Scholar] [CrossRef]
- Zou, Y.; Henry, W.S.; Ricq, E.L.; Graham, E.T.; Phadnis, V.V.; Maretich, P.; Paradkar, S.; Boehnke, N.; Deik, A.A.; Reinhardt, F.; et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 2020, 585, 603–608. [Google Scholar] [CrossRef]
- Pedrono, F.; Martin, B.; Leduc, C.; Le Lan, J.; Saiag, B.; Legrand, P.; Moulinoux, J.-P.; Legrand, A.B. Natural Alkylglycerols Restrain Growth and Metastasis of Grafted Tumors in Mice. Nutr. Cancer 2004, 48, 64–69. [Google Scholar] [CrossRef]
- Miake, K.; Yunoki, K.; Kawamura, J.; Fuchu, H.; Sugiyama, M.; Ohnishi, M. The Plasmalogen composition of post laying-eggs hen and the production method for edible use. Jpn. J. Zootech. Sci. 2014, 85, 153–161. [Google Scholar]
- Pham, T.H.; Manful, C.F.; Pumphrey, R.P.; Hamilton, M.C.; Adigun, O.A.; Vidal, N.P.; Thomas, R.H. Big game cervid meat as a potential good source of plasmalogens for functional foods. J. Food Compos. Anal. 2021, 96, 103724. [Google Scholar] [CrossRef]
- Nishimukai, M.; Yamashita, M.; Watanabe, Y.; Yamazaki, Y.; Nezu, T.; Maeba, R.; Hara, H. Lymphatic absorption of choline plasmalogen is much higher than that of ethanolamine plasmalogen in rats. Eur. J. Nutr. 2011, 50, 427–436. [Google Scholar] [CrossRef]
- Otoki, Y.; Kato, S.; Nakagawa, K.; Harvey, D.J.; Jin, L.-W.; Dugger, B.N.; Taha, A.Y. Lipidomic Analysis of Postmortem Prefrontal Cortex Phospholipids Reveals Changes in Choline Plasmalogen Containing Docosahexaenoic Acid and Stearic Acid Between Cases With and Without Alzheimer’s Disease. Neuromolecular Med. 2021, 23, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, Z.; Jia, J.; Chiba, H.; Hui, S.-P. Quantitative and Comparative Investigation of Plasmalogen Species in Daily Foodstuffs. Foods 2021, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liao, J.; Wang, H.; Zhu, X.; Li, L.; Lu, W.; Song, G.; Shen, Q. Quantitative and comparative study of plasmalogen molecular species in six edible shellfishes by hydrophilic interaction chromatography mass spectrometry. Food Chem. 2021, 334, 127558. [Google Scholar] [CrossRef] [PubMed]
- Řezanka, T.; Křesinová, Z.; Kolouchová, I.; Sigler, K. Lipidomic analysis of bacterial plasmalogens. Folia Microbiol. 2012, 57, 463–472. [Google Scholar] [CrossRef]
- Sato, N.; Kanehama, A.; Kashiwagi, A.; Yamada, M.; Nishimukai, M. Lymphatic Absorption of Microbial Plasmalogens in Rats. Front. Cell Dev. Biol. 2022, 10, 836186. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, X.; Cong, P.; Liu, Y.; Wang, Y.; Xu, J.; Xue, C. Mechanism of Phospholipid Hydrolysis for Oyster Crassostrea plicatula Phospholipids During Storage Using Shotgun Lipidomics. Lipids 2017, 52, 1045–1058. [Google Scholar] [CrossRef]
- Chen, Z.; Jia, J.; Wu, Y.; Chiba, H.; Hui, S.-P. LC/MS analysis of storage-induced plasmalogen loss in ready-to-eat fish. Food Chem. 2022, 383, 132320. [Google Scholar] [CrossRef]
- Yamashita, S.; Shimada, K.; Sakurai, R.; Yasuda, N.; Oikawa, N.; Kamiyoshihara, R.; Otoki, Y.; Nakagawa, K.; Miyazawa, T.; Kinoshita, M. Decrease in Intramuscular Levels of Phosphatidylethanolamine Bearing Arachidonic Acid During Postmortem Aging Depends on Meat Cuts and Breed. Eur. J. Lipid Sci. Technol. 2019, 121, 1800370. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Z.; Chiba, H.; Hui, S.-P. Plasmalogen fingerprint alteration and content reduction in beef during boiling, roasting, and frying. Food Chem. 2020, 322, 126764. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Ibusuki, D.; Yamashita, S.; Nakagawa, K. Analysis of Amadori-glycated Phosphatidylethanolamine in the Plasma of Healthy Subjects and Diabetic Patients by Liquid Chromatography-Tandem Mass Spectrometry. Ann. N. Y. Acad. Sci. 2008, 1126, 291–294. [Google Scholar] [CrossRef]
- Hara, H.; Wakisaka, T.; Aoyama, Y. Lymphatic absorption of plasmalogen in rats. Br. J. Nutr. 2003, 90, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Nishimukai, M.; Wakisaka, T.; Hara, H. Ingestion of plasmalogen markedly increased plasmalogen levels of blood plasma in rats. Lipids 2003, 38, 1227–1235. [Google Scholar] [CrossRef]
- Takahashi, T.; Kamiyoshihara, R.; Otoki, Y.; Ito, J.; Kato, S.; Suzuki, T.; Yamashita, S.; Eitsuka, T.; Ikeda, I.; Nakagawa, K. Structural changes of ethanolamine plasmalogen during intestinal absorption. Food Funct. 2020, 11, 8068–8076. [Google Scholar] [CrossRef]
- Yamashita, S.; Fujiwara, K.; Tominaga, Y.; Nguma, E.; Takahashi, T.; Otoki, Y.; Yamamoto, A.; Higuchi, O.; Nakagawa, K.; Kinoshita, M.; et al. Absorption Kinetics of Ethanolamine Plasmalogen and Its Hydrolysate in Mice. J. Oleo Sci. 2021, 70, 263–273. [Google Scholar] [CrossRef]
- Hashidate-Yoshida, T.; Harayama, T.; Hishikawa, D.; Morimoto, R.; Hamano, F.; Tokuoka, S.M.; Eto, M.; Tamura-Nakano, M.; Yanobu-Takanashi, R.; Mukumoto, Y.; et al. Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. eLife 2015, 4, e06328. [Google Scholar] [CrossRef]
- Engelmann, B.; Bräutigam, C.; Kulschar, R.; Duhm, J.; Prenner, E.; Hermetter, A.; Richter, W.O.; Thiery, J.; Seidel, D. Reversible reduction of phospholipid bound arachidonic acid after low density lipoprotein apheresis. Evidence for rapid incorporation of plasmalogen phosphatidylethanolamine into the red blood cell membrane. Biochim. Biophys. Acta 1994, 1196, 154–164. [Google Scholar] [CrossRef]
- Mawatari, S.; Ohara, S.; Taniwaki, Y.; Tsuboi, Y.; Maruyama, T.; Fujino, T. Improvement of Blood Plasmalogens and Clinical Symptoms in Parkinson’s Disease by Oral Administration of Ether Phospholipids: A Preliminary Report. Park. Dis. 2020, 2020, 2671070. [Google Scholar] [CrossRef]
- Fujino, T.; Yamada, T.; Asada, T.; Ichimaru, M.; Tsuboi, Y.; Wakana, C.; Mawatari, S. Effects of Plasmalogen on Patients with Mild Cognitive Impairment: A Randomized, Placebo-Controlled Trial in Japan. J. Alzheimers Dis. Park. 2018, 8, 419. [Google Scholar] [CrossRef]
- Fujino, T.; Yamada, T.; Mawatari, S.; Shinfuku, N.; Tsuboi, Y.; Wakana, C.; Kono, S. Effects of Plasmalogen on Patients with Moderate-to-Severe Alzheimer’s Disease and Blood Plasmalogen Changes: A Multi-Center, Open-Label Study. J. Alzheimers Dis. Park. 2019, 9, 474. [Google Scholar]
- Watanabe, H.; Okawara, M.; Matahira, Y.; Mano, T.; Wada, T.; Suzuki, N.; Takara, T. The Impact of Ascidian (Halocynthia roretzi)-derived Plasmalogen on Cognitive Function in Healthy Humans: A Randomized, Double-blind, Placebo-controlled Trial. J. Oleo Sci. 2020, 69, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Kotoura, S.; Yuasa, K.; Koikeda, T. Effects of Dietary Plasmalogens on Brain Function in the Healthy Subjects—Randomized Double-blind Placebo-controlled Parallel Group Comparison. Jpn. Pharmacol. Ther. 2017, 45, 1511–1521. [Google Scholar]
- Kawamura, J.; Kotoura, S.; Ando, T.; Kawasaki, Y.; Ebihara, S. The Evaluation Test of Brain Function by Oral Consumption of the Food Which Contain Plasmalogen—Randomized, Placebo-controlled, Double-blind Parallel-group Study. Jpn. Pharmacol. Ther. 2019, 47, 739–749. [Google Scholar]
- Janssen, C.I.F.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Olivera-Pueyo, J.; Pelegrin-Valero, C. Dietary supplements for cognitive impairment. Actas Esp. Psiquiatr. 2017, 45, 37–47. [Google Scholar]
- Gu, J.; Chen, L.; Sun, R.; Wang, J.-L.; Wang, J.; Lin, Y.; Lei, S.; Zhang, Y.; Lv, D.; Jiang, F.; et al. Plasmalogens Eliminate Aging-Associated Synaptic Defects and Microglia-Mediated Neuroinflammation in Mice. Front. Mol. Biosci. 2022, 9, 815320. [Google Scholar] [CrossRef]
- Liu, Y.; Cong, P.; Zhang, T.; Wang, R.; Wang, X.; Liu, J.; Wang, X.; Xu, J.; Wang, Y.; Wang, J.; et al. Plasmalogen attenuates the development of hepatic steatosis and cognitive deficit through mechanism involving p75NTR inhibition. Redox Biol. 2021, 43, 102002. [Google Scholar] [CrossRef]
- Hossain, M.S.; Tajima, A.; Kotoura, S.; Katafuchi, T. Oral ingestion of plasmalogens can attenuate the LPS-induced memory loss and microglial activation. Biochem. Biophys. Res. Commun. 2018, 496, 1033–1039. [Google Scholar] [CrossRef]
- Sejimo, S.; Hossain, M.S.; Akashi, K. Scallop-derived plasmalogens attenuate the activation of PKCδ associated with the brain inflammation. Biochem. Biophys. Res. Commun. 2018, 503, 837–842. [Google Scholar] [CrossRef]
- Che, H.; Li, Q.; Zhang, T.; Ding, L.; Zhang, L.; Shi, H.; Yanagita, T.; Xue, C.; Chang, Y.; Wang, Y. A comparative study of EPA-enriched ethanolamine plasmalogen and EPA-enriched phosphatidylethanolamine on Aβ42 induced cognitive deficiency in a rat model of Alzheimer’s disease. Food Funct. 2018, 9, 3008–3017. [Google Scholar] [CrossRef]
- Ifuku, M.; Katafuchi, T.; Mawatari, S.; Noda, M.; Miake, K.; Sugiyama, M.; Fujino, T. Anti-inflammatory/anti-amyloidogenic effects of plasmalogens in lipopolysaccharide-induced neuroinflammation in adult mice. J. Neuroinflamm. 2012, 9, 197. [Google Scholar] [CrossRef]
- Yamashita, S.; Hashimoto, M.; Haque, A.M.; Nakagawa, K.; Kinoshita, M.; Shido, O.; Miyazawa, T. Oral Administration of Ethanolamine Glycerophospholipid Containing a High Level of Plasmalogen Improves Memory Impairment in Amyloid beta-Infused Rats. Lipids 2017, 52, 575–585. [Google Scholar] [CrossRef]
- Yamashita, S.; Kanno, S.; Nakagawa, K.; Kinoshita, M.; Miyazawa, T. Extrinsic plasmalogens suppress neuronal apoptosis in mouse neuroblastoma Neuro-2A cells: Importance of plasmalogen molecular species. RSC Adv. 2015, 5, 61012–61020. [Google Scholar] [CrossRef]
- Youssef, M.; Ibrahim, A.; Akashi, K.; Hossain, M.S. PUFA-Plasmalogens Attenuate the LPS-Induced Nitric Oxide Production by Inhibiting the NF-kB, p38 MAPK and JNK Pathways in Microglial Cells. Neuroscience 2019, 397, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Futai, E.; Kan, E.; Abe, N.; Uchida, T.; Kamio, Y.; Kaneko, J. Phosphatidylethanolamine plasmalogen enhances the inhibiting effect of phosphatidylethanolamine on γ-secretase activity. J. Biochem. 2014, 157, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, T.; Itaya, M.; Burdeos, G.C.; Nakagawa, K.; Miyazawa, T. A Critical Review of the Use of Surfactant-Coated Nanoparticles in Nanomedicine and Food Nanotechnology. Int. J. Nanomed. 2021, 16, 3937–3999. [Google Scholar] [CrossRef]
- Hino, K.; Kaneko, S.; Harasawa, T.; Kimura, T.; Takei, S.; Shinohara, M.; Yamazaki, F.; Morita, S.-y.; Sato, S.; Kubo, Y.; et al. Change in Brain Plasmalogen Composition by Exposure to Prenatal Undernutrition Leads to Behavioral Impairment of Rats. J. Neurosci. 2019, 39, 7689–7702. [Google Scholar] [CrossRef]
- Miville-Godbout, E.; Bourque, M.; Morissette, M.; Al-Sweidi, S.; Smith, T.; Mochizuki, A.; Senanayake, V.; Jayasinghe, D.; Wang, L.; Goodenowe, D.; et al. Plasmalogen Augmentation Reverses Striatal Dopamine Loss in MPTP Mice. PLoS ONE 2016, 11, e0151020. [Google Scholar] [CrossRef]
- Fallatah, W.; Smith, T.; Cui, W.; Jayasinghe, D.; Di Pietro, E.; Ritchie, S.A.; Braverman, N. Oral administration of a synthetic vinyl-ether plasmalogen normalizes open field activity in a mouse model of Rhizomelic chondrodysplasia punctata. Dis. Model. Mech. 2020, 13, dmm042499. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, J.; Smith, T.; Lamontagne-Proulx, J.; Bourque, M.; Al Sweidi, S.; Jayasinghe, D.; Ritchie, S.; Di Paolo, T.; Soulet, D. Neuroprotection and immunomodulation in the gut of parkinsonian mice with a plasmalogen precursor. Brain Res. 2019, 1725, 146460. [Google Scholar] [CrossRef] [PubMed]
- Dorninger, F.; Vaz, F.M.; Waterham, H.R.; van Klinken, J.B.; Zeitler, G.; Forss-Petter, S.; Berger, J.; Wiesinger, C. Ether lipid transfer across the blood-brain and placental barriers does not improve by inactivation of the most abundant ABC transporters. Brain Res. Bull. 2022, 189, 69–79. [Google Scholar] [CrossRef]
- Che, H.; Zhou, M.; Zhang, T.; Zhang, L.; Ding, L.; Yanagita, T.; Xu, J.; Xue, C.; Wang, Y. EPA enriched ethanolamine plasmalogens significantly improve cognition of Alzheimer’s disease mouse model by suppressing β-amyloid generation. J. Funct. Foods 2018, 41, 9–18. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, L.; Shi, H.; Xue, C.; Yanagita, T.; Zhang, T.; Wang, Y. EPA-enriched ethanolamine plasmalogen alleviates atherosclerosis via mediating bile acids metabolism. J. Funct. Foods 2020, 66, 103824. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, L.-Y.; Shi, H.-H.; Wang, C.-C.; Jiang, X.-M.; Xue, C.-H.; Yanagita, T.; Zhang, T.-T.; Wang, Y.-M. Eicosapentaenoic Acid-Enriched Phosphoethanolamine Plasmalogens Alleviated Atherosclerosis by Remodeling Gut Microbiota to Regulate Bile Acid Metabolism in LDLR–/– Mice. J. Agric. Food Chem. 2020, 68, 5339–5348. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Q.; Wang, X.; Cong, P.; Xu, J.; Xue, C. Lipidomics Approach in High-Fat-Diet-Induced Atherosclerosis Dyslipidemia Hamsters: Alleviation Using Ether-Phospholipids in Sea Urchin. J. Agric. Food Chem. 2021, 69, 9167–9177. [Google Scholar] [CrossRef]
- Nguma, E.; Tominaga, Y.; Yamashita, S.; Otoki, Y.; Yamamoto, A.; Nakagawa, K.; Miyazawa, T.; Kinoshita, M. Dietary PlsEtn Ameliorates Colon Mucosa Inflammatory Stress and ACF in DMH-Induced Colon Carcinogenesis Mice: Protective Role of Vinyl Ether Linkage. Lipids 2021, 56, 167–180. [Google Scholar] [CrossRef]
- Jang, J.E.; Park, H.S.; Yoo, H.J.; Baek, I.J.; Yoon, J.E.; Ko, M.S.; Kim, A.R.; Kim, H.S.; Park, H.S.; Lee, S.E.; et al. Protective role of endogenous plasmalogens against hepatic steatosis and steatohepatitis in mice. Hepatology 2017, 66, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, A.; Yang, D.; Vella, M.; Chiu, I.M. The intestinal neuro-immune axis: Crosstalk between neurons, immune cells, and microbes. Mucosal Immunol. 2021, 14, 555–565. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing Incidence and Prevalence of the Inflammatory Bowel Diseases With Time, Based on Systematic Review. Gastroenterology 2012, 142, 46–54.e42. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Jaszewski, R. Aging and cancer of the stomach and colon. Front. Biosci. 1999, 4, 322–328. [Google Scholar] [CrossRef]
- Triantafillidis, J.K.; Nasioulas, G.; Kosmidis, P.A. Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009, 29, 2727–2737. [Google Scholar]
- Lopez, D.H.; Bestard-Escalas, J.; Garate, J.; Maimó-Barceló, A.; Fernández, R.; Reigada, R.; Khorrami, S.; Ginard, D.; Okazaki, T.; Fernández, J.A.; et al. Tissue-selective alteration of ethanolamine plasmalogen metabolism in dedifferentiated colon mucosa. Biochim. Biophys. Acta 2018, 1863, 928–938. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, L.; Liu, J.; Ma, K.; Liu, C.; Zhang, Y.; Zou, W. Choline Plasmalogens Isolated from Swine Liver Inhibit Hepatoma Cell Proliferation Associated with Caveolin-1/Akt Signaling. PLoS ONE 2013, 8, e77387. [Google Scholar] [CrossRef] [PubMed]
- Nguma, E.; Yamashita, S.; Han, K.H.; Otoki, Y.; Yamamoto, A.; Nakagawa, K.; Fukushima, M.; Miyazawa, T.; Kinoshita, M. Dietary Ethanolamine Plasmalogen Alleviates DSS-Induced Colitis by Enhancing Colon Mucosa Integrity, Antioxidative Stress, and Anti-inflammatory Responses via Increased Ethanolamine Plasmalogen Molecular Species: Protective Role of Vinyl Ether Linkages. J. Agric. Food Chem. 2021, 69, 13034–13044. [Google Scholar] [CrossRef]
- Nguma, E.; Yamashita, S.; Kumagai, K.; Otoki, Y.; Yamamoto, A.; Eitsuka, T.; Nakagawa, K.; Miyazawa, T.; Kinoshita, M. Ethanolamine Plasmalogen Suppresses Apoptosis in Human Intestinal Tract Cells in Vitro by Attenuating Induced Inflammatory Stress. ACS Omega 2021, 6, 3140–3148. [Google Scholar] [CrossRef]
- Misawa, N.; Osaki, T.; Takeuchi, S. Membrane protein-based biosensors. J. R. Soc. Interface 2018, 15, 20170952. [Google Scholar] [CrossRef]
- Tabata, M.; Miyahara, Y. From new materials to advanced biomedical applications of solid-state biosensor: A review. Sens. Actuators B Chem. 2022, 352, 131033. [Google Scholar] [CrossRef]
- Abe, C.; Bhaswant, M.; Miyazawa, T.; Miyazawa, T. The Potential Use of Exosomes in Anti-Cancer Effect Induced by Polarized Macrophages. Pharmaceutics 2023, 15, 1024. [Google Scholar] [CrossRef] [PubMed]
- Nikoleli, G.-P.; Nikolelis, D.P.; Siontorou, C.G.; Karapetis, S.; Nikolelis, M.-T. Application of Biosensors Based on Lipid Membranes for the Rapid Detection of Toxins. Biosensors 2018, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Naaman, R.; Capua, E.; Bavli, D.; Tkachev, M. Protein Detector Based on Molecular Controlled Semiconductor Resistor. U.S. Patent No. 8,957,460, 17 February 2015. [Google Scholar]
- Khashab, N.M.; Caruso, F. The Future of Healthcare Materials. Chem. Mater. 2023, 35, 364–365. [Google Scholar] [CrossRef]
- Thompson, D.H.; Gerasimov, O.V.; Wheeler, J.J.; Rui, Y.; Anderson, V.C. Triggerable plasmalogen liposomes: Improvement of system efficiency. Biochim. Biophys. Acta 1996, 1279, 25–34. [Google Scholar] [CrossRef]
- Rani, V.; Venkatesan, J.; Prabhu, A. Liposomes- A promising strategy for drug delivery in anticancer applications. J. Drug Deliv. Sci. Technol. 2022, 76, 103739. [Google Scholar] [CrossRef]
- Lajunen, T.; Nurmi, R.; Kontturi, L.; Viitala, L.; Yliperttula, M.; Murtomäki, L.; Urtti, A. Light activated liposomes: Functionality and prospects in ocular drug delivery. J. Control. Release 2016, 244, 157–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ma, J.; Wang, X.; Yuan, Z.; Wang, W. Convenient preparation of charge-adaptive chitosan nanomedicines for extended blood circulation and accelerated endosomal escape. Nano Res. 2018, 11, 4278–4292. [Google Scholar] [CrossRef]
- Rietwyk, S.; Peer, D. Next-Generation Lipids in RNA Interference Therapeutics. ACS Nano 2017, 11, 7572–7586. [Google Scholar] [CrossRef]
- Fay, F.; Quinn, D.J.; Gilmore, B.F.; McCarron, P.A.; Scott, C.J. Gene delivery using dimethyldidodecylammonium bromide-coated PLGA nanoparticles. Biomaterials 2010, 31, 4214–4222. [Google Scholar] [CrossRef] [PubMed]
- Ma, D. Enhancing endosomal escape for nanoparticle mediated siRNA delivery. Nanoscale 2014, 6, 6415–6425. [Google Scholar] [CrossRef]
- Jayaraman, M.; Ansell, S.M.; Mui, B.L.; Tam, Y.K.; Chen, J.; Du, X.; Butler, D.; Eltepu, L.; Matsuda, S.; Narayanannair, J.K.; et al. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. Engl. 2012, 51, 8529–8533. [Google Scholar] [CrossRef] [PubMed]



| Species | Organs and Tissues | PlsEtn (% EtnGpl) | PlsEtn (% Total PL) | PlsCho (% ChoGpl) | PlsCho (% Total PL) | Plasmalogen (% Total PL) | Plasmalogen (% Wet Weight) | Refs. |
|---|---|---|---|---|---|---|---|---|
| Human | ||||||||
| Whole brain | 58 | 20, 22 | 1 | 0.8, 0.9 | 22 | [17,18] | ||
| Brain prefrontal cortex | 49 | 0.7 | [19] | |||||
| Brain temporal cortex | 46 | 0.5 | ||||||
| Brain gray matter | 49 | 19 | 0.1 | 19 | 0.4, 0.7 | [20,21] | ||
| Brain gray matter—frontal cortex | 57 | 20 | 0.7 | [10,22] | ||||
| Brain gray matter—parietal cortex | 58 | 19 | 0.7 | |||||
| Brain gray matter—temporal cortex | 56 | |||||||
| Brain gray matter—cerebellum | 63 | |||||||
| Brain white matter | 83, 86 | 33 | 2.6, 0.4 | 0.1 | 37 | 1.1, 1.8 | [19,20,21] | |
| Brain white matter—frontal cortex | 84 | 29 | 2 | [10,22] | ||||
| Brain white matter—parietal cortex | 81 | 36 | 2.7 | |||||
| Brain white matter—temporal cortex | 83 | |||||||
| Brain white matter—cerebellum | 78 | |||||||
| Myelin | 91 | 36 | 0.5 | 0.1 | 43 | 7.0 | [20] | |
| Heart | 53, 51 | 17, 14, 15 | 26, 36 | 14, 11, 15 | 32, 29 | [17,18,23] | ||
| Skeletal muscle | 48, 63 | 14, 15 | 19, 16 | 6.5, 9.7 | 0.2 | [17,18,22,23] | ||
| Cultured artery endothelial cells | 48 | 13 | 1.9 | 0.9 | [24] | |||
| Cultured vein endothelial cells | 34 | 8.7 | 0.2 | 0.1 | ||||
| Kidney | 46 | 14 | 5 | 4.7 | [17,18] | |||
| Liver | 8 | 4.7 | 3 | 3.4 | ||||
| Colon | 36 | 11 | 9 | 3.9 | 19, 15 | 0.1, 0.1 | [22,25] | |
| Lens | 70 | 14 | [26] | |||||
| Neutrophils | 68 | 3.6 | [27] | |||||
| Eosinophils | 72 | 4 | [28] | |||||
| Red blood cells | 58, 48, 46 | 17,14, 9 | 8 | 2.8 | 20, 14, 9 | [29,30,31] | ||
| Plasma | 53 | 2.5 | 5.5 | 5.2 | 7.7 | [32] | ||
| Serum | 5.9 | 4.5 | 10 | [33] | ||||
| HDL | 55 | 6.4, 5 | 4.7 | 4.5, 4 | 11, 10 | [32,34] | ||
| LDL | 60 | 5.9, 4 | 4.4 | 4.2, 4 | 10, 10 | |||
| Platelet | 54 | 0.8 | [35] | |||||
| Cultured macrophage | 62 | 8.8 | [36] | |||||
| Spermatozoa | 9 | 3 | 12 | [37] | ||||
| Rat | ||||||||
| Whole brain | 66 | 1.7 | 1 | [19,22] | ||||
| Cortex | 22 | [38] | ||||||
| Cerebellum | 26 | |||||||
| Hippocampus | 23 | |||||||
| Brainstem | 32 | |||||||
| Midbrain | 24 | |||||||
| Brain synaptic vesicles | 16 | [39] | ||||||
| Heart | 28 | 9.8 | 5.7 | 2.4 | 12 | 0.2 | [22,40] | |
| Cultured heart sacrolemma | 43 | 19 | [41] | |||||
| Lung | 42 | 1.6 | 16 | 0.3 | [22,42] | |||
| Kidney | 20 | 2.3 | 12 | 0.3 | ||||
| Liver | 3.3 | 0.4 | 3.4 | 0.07 | ||||
| Red blood cells | 65 | 16 | 0.9 | 0.5 | 16 | [13] | ||
| Plasma | 51, 36 | 0.4 | 0.5 | 0.4 | 0.8 | [13,43] | ||
| HDL | 46 | 2.4 | [43] | |||||
| LDL | 32 | 1.9 | ||||||
| VLDL | 14 | 1.6 | ||||||
| Mature spermatozoa | 42 | 52 | 38 | [44] | ||||
| Mouse | ||||||||
| Whole brain | 47 | 1.2 | [19] | |||||
| Cortex | 46 | [10,22] | ||||||
| Cerebellum | 53 | |||||||
| Heart | 24 | 8.2 | 62 | 31 | 39 | [45] | ||
| Dog | ||||||||
| Heart sarcolemma | 73 | 16 | 57 | 37 | 53 | [46] | ||
| Rabbit | ||||||||
| Whole brain | 26 | 1.3 | [22] | |||||
| Heart | 32 | 0.6 | ||||||
| Lung | 14 | 0.3 | ||||||
| Kidney | 14 | 0.3 | ||||||
| Liver | 2 | 0.06 | ||||||
| Macrophage | 61 | 13 | 6 | 2 | 15 | [47] | ||
| Guinea pig | ||||||||
| Whole brain | 0.9 | [21] |
| Species | Organs and Tissues | Predominant Plasmalogen Species | Refs. |
|---|---|---|---|
| Human | |||
| Brain gray matter—frontal cortex | 16:0/18:1-PlsEtn, 16:0/22:4-PlsEtn, 16:0/22:5-PlsEtn, 18:0/18:2-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:4-PlsEtn, 18:0/22:5-PlsEtn, 18:0/22:6-PlsEtn, 18:1/18:1-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:4-PlsEtn | [10] | |
| Brain gray matter—parietal cortex | 16:0/18:1-PlsEtn, 16:0/22:4-PlsEtn, 16:0/22:5-PlsEtn, 18:0/18:2-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:4-PlsEtn, 18:0/22:5-PlsEtn, 18:0/22:6-PlsEtn, 18:1/18:1-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:4-PlsEtn | ||
| Brain gray matter—temporal cortex | 16:0/22:4-PlsEtn, 16:0/22:5-PlsEtn, 16:0/22:6-PlsEtn, 18:0/18:2-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:4-PlsEtn, 18:0/22:5-PlsEtn, 18:0/22:6-PlsEtn, 18:1/18:1-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:4-PlsEtn | ||
| Brain gray matter—cerebellum | 16:0/18:1-PlsEtn, 16:0/20:1-PlsEtn, 16:0/22:4-PlsEtn, 16:0/22:5-PlsEtn, 16:0/22:6-PlsEtn, 18:0/18:1-PlsEtn, 18:0/18:2-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:4-PlsEtn, 18:0/22:5-PlsEtn, 18:0/22:6-PlsEtn, 18:1/18:1-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:4-PlsEtn | ||
| Brain white matter—frontal cortex | 16:0/18:1-PlsEtn, 16:0/20:1-PlsEtn, 16:0/22:4-PlsEtn, 16:0/22:5-PlsEtn, 18:0/18:1-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:4-PlsEtn, 18:0/22:5-PlsEtn, 18:1/18:1-PlsEtn, 18:1/20:1-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:4-PlsEtn | ||
| Brain white matter—parietal cortex | 16:0/18:1-PlsEtn, 16:0/20:1-PlsEtn, 16:0/22:4-PlsEtn, 16:0/22:5-PlsEtn, 18:0/18:1-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:4-PlsEtn, 18:0/22:5-PlsEtn, 18:1/18:1-PlsEtn, 18:1/20:1-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:4-PlsEtn | ||
| Brain white matter—temporal cortex | 16:0/18:1-PlsEtn, 16:0/20:1-PlsEtn, 16:0/22:4-PlsEtn, 16:0/22:5-PlsEtn, 18:0/18:1-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:4-PlsEtn, 18:0/22:5-PlsEtn, 18:1/18:1-PlsEtn, 18:1/20:1-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:4-PlsEtn | ||
| Brain white matter—cerebellum | 16:0/18:1-PlsEtn, 16:0/20:1-PlsEtn, 16:0/22:4-PlsEtn, 16:0/22:5-PlsEtn, 18:0/18:1-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:4-PlsEtn, 18:0/22:5-PlsEtn, 18:1/18:1-PlsEtn, 18:1/20:1-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:4-PlsEtn | ||
| Cultured artery endothelial cells | 16:0/20:4-PlsEtn, 16:0/22:4-PlsEtn, 16:0/22:5-PlsEtn, 16:0/22:6-PlsEtn, 18:0/20:4-PlsEtn, 18:1/20:4-PlsEtn | [24] | |
| Cultured vein endothelial cells | 16:0/20:4-PlsEtn, 16:0/22:4-PlsEtn, 16:0/22:5-PlsEtn, 16:0/22:6-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:5-PlsEtn, 18:0/22:6-PlsEtn | ||
| Neutrophils | 16:0/18:1-PlsEtn, 16:0/18:2-PlsEtn, 16:0/20:4-PlsEtn, 18:0/18:1-PlsEtn, 18:0/18:2-PlsEtn, 18:0/20:4-PlsEtn, 18:1/18:1-PlsEtn, 18:1/18:2-PlsEtn, 18:1/20:4-PlsEtn, 20:0/18:1-PlsEtn, 20:0/18:2-PlsEtn, 20:0/20:4-PlsEtn, 22:0/18:1-PlsEtn, 22:1/18:1-PlsEtn, 22:1/18:2-PlsEtn, 22:1/20:4-PlsEtn, 24:1/18:1-PlsEtn, 24:1/18:2-PlsEtn, 24:1/20:4-PlsEtn | [48] | |
| Lymphocytes | 16:0/18:1-PlsEtn, 16:0/18:2-PlsEtn, 16:0/20:4-PlsEtn, 16:0/22:6-PlsEtn | [49] | |
| Serum | 16:0/18:1-PlsEtn, 16:0/18:2-PlsEtn, 16:0/20:4-PlsEtn, 16:0/22:6-PlsEtn, 18:0/18:1-PlsEtn, 18:0/18:2-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:6-PlsEtn | [50] | |
| HDL | 16:0/18:2-PlsEtn, 16:0/20:4-PlsEtn, 16:0/22:6-PlsEtn, 18:0/18:2-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:6-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:6-PlsEtn 16:0/18:1-PlsCho, 16:0/18:2-PlsCho, 16:0/20:4-PlsCho, 16:0/22:6-PlsCho, 18:0/18:2-PlsCho, 18:0/20:4-PlsCho, 18:1/20:4-PlsCho | [34] | |
| LDL | 16:0/18:2-PlsEtn, 16:0/20:4-PlsEtn, 16:0/22:6-PlsEtn, 18:0/18:2-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:6-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:6-PlsEtn 16:0/18:1-PlsCho, 16:0/18:2-PlsCho, 16:0/20:4-PlsCho, 16:0/22:6-PlsCho, 18:0/18:2-PlsCho, 18:0/20:4-PlsCho | ||
| VLDL | 16:0/18:2-PlsEtn, 16:0/20:4-PlsEtn, 16:0/22:6-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:6-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:6-PlsEtn 16:0/18:1-PlsCho, 16:0/18:2-PlsCho, 16:0/20:4-PlsCho, 16:0/22:6-PlsCho, 18:0/18:2-PlsCho, 18:0/20:4-PlsCho, 18:1/20:4-PlsCho | ||
| Platelet | 16:0/18:2-PlsEtn, 16:0/20:4-PlsEtn, 18:0/20:4-PlsEtn 16:0/20:4-PlsCho, 18:0/20:4-PlsCho, 18:1/20:4-PlsCho | [35] | |
| Rat | |||
| Cardiac sarcolemma | 16:0/18:0-PlsEtn, 16:0/18:3-PlsEtn, 18:0/18:0-PlsEtn, 18:0/22:4-PlsEtn, 18:1/18:1-PlsEtn, 18:2/16:0-PlsEtn, 18:2/18:0-PlsEtn 16:0/18:0-PlsCho, 16:0/18:2-PlsCho, 16:0/18:3-PlsCho, 18:0/18:0-PlsCho, 18:0/20:4-PlsCho, 18:0/22:6-PlsCho, 18:1/22:6-PlsCho | [51] | |
| Mouse | |||
| Cerebral cortex | 16:0/18:1-PlsEtn, 16:0/20:1-PlsEtn, 16:0/22:6-PlsEtn, 18:0/18:1-PlsEtn, 18:0/20:4-PlsEtn, 18:0/22:6-PlsEtn, 18:1/16:0-PlsEtn, 18:1/18:1-PlsEtn, 18:1/20:4-PlsEtn, 18:1/22:6-PlsEtn | [52] | |
| Hippocampus | 18:1/20:3-PlsEtn, 18:1/20:4-PlsEtn, 18:0/22:5-PlsEtn, 18:1/22:4-PlsEtn 16:0/18:1-PlsCho, 16:0/20:5-PlsCho, 16:0/22:5-PlsCho, 18:0/16:0-PlsCho | [53] | |
| Macrophage cell line RAW264.7 | 16:0/18:1-PlsEtn, 16:0/20:3-PlsEtn, 16:0/20:4-PlsEtn, 18:0/18:1-PlsEtn, 18:0/20:3-PlsEtn, 18:0/20:4-PlsEtn, 18:1/16:0-PlsEtn, 18:1/18:1-PlsEtn, 18:1/20:3-PlsEtn, 18:1/20:4-PlsEtn | [54] | |
| Rabbit | |||
| Proximal tubules | 16:0/18:1-PlsEtn, 16:0/20:4-PlsEtn, 18:0/18:2-PlsEtn, 18:0/20:4-PlsEtn, 18:1/18:2-PlsEtn, 18:1/20:4-PlsEtn | [55] | |
| Dog | |||
| Heart sarcolemma | 16:0/20:4-PlsEtn, 18:0/20:4-PlsEtn, 18:1/20:4-PlsEtn 16:0/18:1-PlsCho, 16:0/18:2-PlsCho, 16:0/20:4-PlsCho, 16:0/18:2-PlsCho, 18:1/18:1-PlsCho | [46] |
| Resource and Dose | Subject | Effects | Refs. |
|---|---|---|---|
| Scallop Plasmalogen composition: unclear 1.0 mg/day for 24 weeks | Mild AD and MCI Placebo: n = 140, age 76.5, MMSE 24.2 1.0 mg: n = 145, age 76.4, MMSE 24.0 | Not affected in whole In mild AD (MMSE 20–23) Female: WMS-R improvement compared with the placebo Younger than 78: WMS-R improvement compared with the placebo | [11] |
| Scallop Plasmalogen composition: unclear 1.0 mg/day for 24 weeks | MCI Placebo: n = 88, age 75.9, MMSE 25.6 1.0 mg: n = 90, age 75.8, MMSE 25.6 | Improvement in total MMSE compared with before the intake Improvement in domain “orientation to place” compared with the placebo and before the intake Maintenance of domain “orientation to time” compared with before the intake | [143] |
| Scallop Plasmalogen composition: unclear 0.5 or 1.0 mg/day for 12 weeks | Moderate-to-severe AD 0.5 mg: n = 68, age 78.5, MMSE 13.0 1.0 mg: n = 74, age 76.6, MMSE 13.4 | Improvement in total MMSE compared with before the intake Increase in plasma and RBC levels of PlsEtn compared with before the intake Increase in plasma PlsEtn levels in the 0.5 mg group compared with the 1.0 mg group Correlation between changes in MMSE and RBC PlsEtn | [144] |
| Scallop Ether lipid composition: 52% PlsEtn, 2% PlsCho, 4% PakEtn, 42% PakCho 1.0 mg/day for 24 weeks | PD PD: n = 10, age 67.8, MMSE 28.6 | Improvement in total PDQ-39 compared with immediately before trial Deterioration of total PDQ-39 at 4 weeks later of the final administration Increase in plasma and RBC levels of PlsEtn compared with levels immediately before trial Decrease in RBC PlsEtn levels after 4 weeks without administration | [142] |
| Ascidian Plasmalogen composition: unclear 1.0 mg/day for 12 weeks | Healthy subjects with mild forgetfulness Placebo: n = 24, age 46.4, MMSE 29.0 1.0 mg: n = 25, age 45.6, MMSE 28.5 | Improvement in Cognitrax domain “composite memory” at 8 and 12 weeks compared with the placebo | [145] |
| Ascidian Plasmalogen composition: 100% PlsEtn, trace PlsCho 0.5 or 1.0 mg/day for 12 weeks | MCI Placebo: n = 44, age 51.4, MMSE 25.3 0.5 mg: n = 45, age 51.5, MMSE 25.4 1.0 mg: n = 49, age 52.7, MMSE 25.7 | Improvement in CogEvo domain “working memory performance” in the 1.0 mg group compared with the placebo In subjects older than 49 Improvement in total MMSE and “working memory performance” in the 1.0 mg group compared with the placebo | [12] |
| Chicken breast Plasmalogen composition: unclear 1.0, 10, or 100 mg/day for 12 weeks | Healthy subjects with mild forgetfulness Placebo: n = 17, age 58.7 1.0 mg: n = 15, age 57.2 10 mg: n = 16, age 59.9 100 mg: n = 15, age 59.0 | Improvement in total RBANS in the 1.0 and 100 mg groups compared with before the intake Improvement in RBANS domain “attention” in all the groups, including the placebo, compared with before the intake Improvement in some Cognitrax domains in the plasmalogen groups compared with before the intake | [146] |
| Chicken breast Plasmalogen composition: unclear 0.5 or 1.0 mg/day for 12 weeks | Healthy subjects with mild forgetfulness age 50 to 79, MSSE > 26 Placebo: n = 24 0.5 mg: n = 25 1.0 mg: n = 25 | Improvement in some CogEvo domains in all the groups, including the placebo, compared with before the intake In subjects older than 59 Improvement in some CogEvo domains in the 1.0 mg group compared with before the intake Improvement in CogEvo domain “verbal memory” in the 1.0 mg group compared with the placebo Improvement in CogEvo domain “psychomotor speed” in the 0.5 mg group compared with the placebo | [147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, S.; Miyazawa, T.; Higuchi, O.; Kinoshita, M.; Miyazawa, T. Marine Plasmalogens: A Gift from the Sea with Benefits for Age-Associated Diseases. Molecules 2023, 28, 6328. https://doi.org/10.3390/molecules28176328
Yamashita S, Miyazawa T, Higuchi O, Kinoshita M, Miyazawa T. Marine Plasmalogens: A Gift from the Sea with Benefits for Age-Associated Diseases. Molecules. 2023; 28(17):6328. https://doi.org/10.3390/molecules28176328
Chicago/Turabian StyleYamashita, Shinji, Taiki Miyazawa, Ohki Higuchi, Mikio Kinoshita, and Teruo Miyazawa. 2023. "Marine Plasmalogens: A Gift from the Sea with Benefits for Age-Associated Diseases" Molecules 28, no. 17: 6328. https://doi.org/10.3390/molecules28176328