The Effects of Honeysuckle (Lonicera caerulea L.) Berry Iridoid-Anthocyanin Extract on the Intestinal and Muscle Histopathology in Mice during Experimental Trichinellosis
Abstract
:1. Introduction
2. Results
2.1. Morphological Analysis (HE)
2.1.1. Effect of LC on Morphology of the Jejunum
2.1.2. Morphometry
2.1.3. Effect of LC on the Morphology of the Masseter Muscles
3. Discussion
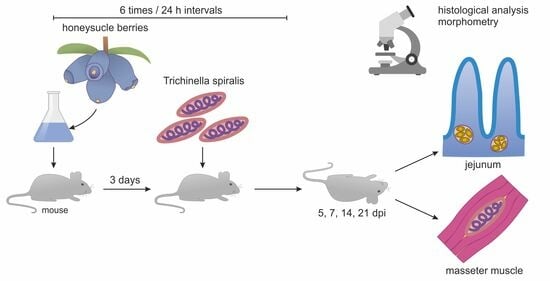
4. Materials and Methods
4.1. Preparation of the LC Extract
4.2. Animals and Treatment
4.3. Collection of Tissue Samples and Histological Analyses
Morphometry
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gołba, M.; Sokół-Łętowska, A.; Kucharska, A.Z. Health Properties and Composition of Honeysuckle Berry Lonicera caerulea L. An Update on Recent Studies. Molecules 2020, 25, 749. [Google Scholar] [CrossRef]
- Negreanu-Pirjol, B.S.; Oprea, O.C.; Negreanu-Pirjol, T.; Roncea, F.N.; Prelipcean, A.M.; Craciunescu, O.; Iosageanu, A.; Artem, V.; Ranca, A.; Motelica, L.; et al. Health Benefits of Antioxidant Bioactive Compounds in the Fruits and Leaves of Lonicera caerulea L. and Aronia melanocarpa (Michx.) Elliot. Antioxidants 2023, 12, 951. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castillo, M.; Pacheco-Yepez, J.; Flores-Huerta, N.; Guzmán-Téllez, P.; Jarillo-Luna, R.A.; Cárdenas-Jaramillo, L.M.; Campos-Rodríguez, R.; Shibayama, M. Flavonoids as a natural treatment against Entamoeba histolytica. Front. Cell. Infect. Microbiol. 2018, 8, 209. [Google Scholar] [CrossRef] [PubMed]
- Tshisekedi Tshibangu, P.; Mutwale Kapepula, P.; Kabongo Kapinga, M.J.; Tujibikila Mukuta, A.; Kalenda, D.T.; Tchinda, A.T.; Mouithys-Mickalad, A.A.; Jansen, O.; Cieckiewicz, E.; Tits, M.; et al. Antiplasmodial activity of Heinsia crinita (Rubiaceae) and identification of new iridoids. J. Ethnopharmacol. 2017, 196, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Nekoei, S.; Khamesipour, F.; Habtemariam, S.; de Souza, W.; Mohammadi Pour, P.; Hosseini, S.R. The anti-Trypanosoma activities of medicinal plants: A systematic review of the literature. Vet. Med. Sci. 2022, 8, 2738–2772. [Google Scholar] [CrossRef]
- El-Kady, A.M.; Abdel-Rahman, I.A.M.; Sayed, E.; Wakid, M.H.; Alobaid, H.M.; Mohamed, K.; Alshehri, E.A.; Elshazly, H.; Al-Megrin, W.A.I.; Iqbal, F.; et al. A potential herbal therapeutic for trichinellosis. Front. Vet. Sci. 2022, 23, 970327. [Google Scholar] [CrossRef]
- Panda, S.K.; Daemen, M.; Sahoo, G.; Luyten, W. Essential oils as novel anthelmintic drug candidates. Molecules 2022, 27, 8327. [Google Scholar] [CrossRef]
- El-Wakil, E.S.; Shaker, S.; Aboushousha, T.; Abdel-Hameed, E.S.; Osman, E.E.A. In vitro and in vivo anthelmintic and chemical studies of Cyperus rotundus L. extracts. BMC Complement. Med. Ther. 2023, 23, 15. [Google Scholar] [CrossRef]
- Piekarska, J.; Szczypka, M.; Kucharska, A.Z.; Gorczykowski, M. Effects of iridoid-anthocyanin extract of Cornus mas L. on hematological parameters, population and proliferation of lymphocytes during experimental infection of mice with Trichinella spiralis. Exp. Parasitol. 2018, 188, 58–64. [Google Scholar] [CrossRef]
- Piekarska, J.; Szczypka, M.; Gorczykowski, M.; Króliczewska, B.; Miśta, D.; Oszmiański, J. Effect of aqueous extract from Scutellaria baicalensis Georgi roots on CD4+ and CD8+ T cell responses during experimental infection with Trichinella spiralis in mice. Pol. J. Vet. Sci. 2020, 23, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Piekarska, J.; Szczypka, M.; Gorczykowski, M.; Sokół-Łętowska, A.; Kucharska, A.Z. Evaluation of immunotropic activity of iridoid-anthocyanin extract of honeysuckle berries (Lonicera caerulea L.) in the course of experimental trichinellosis in mice. Molecules 2022, 27, 1949. [Google Scholar] [CrossRef]
- Pozio, E. World distribution of Trichinella spp. infections in animals and humans. Vet. Parasitol. 2007, 149, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Kocięcka, W. Trichinellosis: Human disease, diagnosis and treatment. Vet. Parasitol. 2000, 93, 365–383. [Google Scholar] [CrossRef]
- Romaris, F.; North, S.J.; Gagliardo, L.F.; Butcher, B.A.; Ghosh, K.; Beiting, D.P.; Panico, M.; Arasu, P.; Dell, A.; Morris, H.R.; et al. A putative serine protease among the excretory-secretory glycoproteins of L1 Trichinella spiralis. Mol. Biochem. Parasitol. 2002, 122, 149–160. [Google Scholar] [CrossRef]
- Gottstein, B.; Pozio, E.; Nöckler, K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009, 22, 127–145. [Google Scholar] [CrossRef]
- Sofronic-Milosavljevic, L.; Ilic, N.; Pinelli, E.; Gruden-Movsesijan, A. Secretory products of Trichinella spiralis muscle larvae and immunomodulation: Implication for autoimmune diseases, allergies, and malignancies. J. Immunol. Res. 2015, 2015, 523875. [Google Scholar] [CrossRef]
- Bany, J.; Zdanowska, D.; Zdanowski, R.; Skopińska-Rózewska, E. The effect of herbal remedy on the development of Trichinella spiralis infection in mice. Pol. J. Vet. Sci. 2003, 6, 6–8. [Google Scholar] [PubMed]
- Shalaby, M.A.; Moghazy, F.M.; Shalaby, H.A.; Nasr, S.M. Effect of methanolic extract of Balanites aegyptiaca fruits on enteral and parenteral stages of Trichinella spiralis in rats. Parasitol. Res. 2010, 107, 17–25. [Google Scholar] [CrossRef]
- Salama, M.A.M.; Mostafa, N.E.; Abd El-Aal, N.F.; Moawad, H.S.F.; Hammad, S.K.; Adel, R.; Eman, M.M. Efficacy of Zingiber officinale and Cinnamomum zeylanicum extracts against experimental Trichinella spiralis infection. J. Parasit. Dis. 2022, 46, 24–36. [Google Scholar] [CrossRef]
- El-Wakil, E.S.; Abdelmaksoud, H.F.; AbouShousha, T.S.; Ghallab, M.M.I. Evaluation of Annona muricata (Graviola) leaves activity against experimental trichinellosis: In vitro and in vivo studies. J. Helminthol. 2021, 95, e53. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, S.H.; Mahmoud, S.S.; El-Shenawy, A.M.; Yousof, H.S.A. Anti-helminthic effect of Punica granatum peel extract on Trichinella spiralis worms and muscle larvae: In vitro and in vivo studies. J. Parasit. Dis. 2023, 47, 416–424. [Google Scholar] [CrossRef]
- Birchenough, G.M.; Johansson, M.E.; Gustafsson, J.K.; Bergstrom, J.H.; Hansson, G.C. New developments in goblet cell mucus secretion and function. Mucosal Immunol. 2015, 8, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Songwei, Y.; Min, Y. Role of goblet cells in intestinal barrier and mucosal immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef]
- Khan, W.I.; Blennerhasset, P.A.; Ma, C.; Collins, S.M. Stat6 dependent goblet cell hyperplasia during intestinal nematode infection. Parasite Immunol. 2001, 23, 39–42. [Google Scholar] [CrossRef]
- Miśta, D.; Piekarska, J.; Houszka, M.; Zawadzki, W.; Gorczykowski, M. The influence of orally administered short chain fatty acids on intestinal histopathological changes and intensity of Trichinella spiralis infection in mice. Vet. Med. Czech. 2010, 55, 264–274. [Google Scholar] [CrossRef]
- Piekarska, J.; Miśta, D.; Houszka, M.; Króliczewska, B.; Zawadzki, W.; Gorczykowski, M. Trichinella spiralis: The influence of short chain fatty acids on the proliferation of lymphocytes, the goblet cell count and apoptosis in the mouse intestine. Exp. Parasitol. 2011, 128, 419–426. [Google Scholar] [CrossRef]
- Dehlawi, M.S.; Mahida, Y.R.; Hughes, K.; Wakelin, D. Effects of Trichinella spiralis infection on intestinal pathology in mice lacking interleukin-4 (IL-4) or intestinal trefoil factor (ITF/TFF3). Parasitol. Int. 2006, 55, 207–211. [Google Scholar] [CrossRef]
- Birchenough, G.; Nystrom, E.E.; Johansson, M.E.; Hansson, G.C. A sentinel goblet cell guards the colonic crypt by triggering Nlrp6-dependent Muc2 secretion. Science 2016, 352, 1535–1542. [Google Scholar] [CrossRef]
- Ishikawa, N.; Wakelin, D.; Mahida, Y.R. Role of T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology 1997, 113, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Pyo, M.Y.; Yoon, S.J.; Yu, Y.; Park, S.; Jin, M. Cyanidin-3-glucoside suppresses Th2 cytokines and GATA-3 transcription factor in EL-4 T cells. Biosci. Biotechnol. Biochem. 2014, 78, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Rupasinghe, H.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-O-glucoside. J. Funct. Foods. 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, S.I.; Choi, S.H.; Song, C.H.; Park, S.J.; Shin, Y.K.; Han, C.H.; Lee, Y.J.; Ku, S.K. Single oral dose toxicity test of blue honeysuckle concentrate in mice. Toxicol. Res. 2015, 31, 61–68. [Google Scholar] [CrossRef] [PubMed]



| Variable | C | LC | T | T+LC |
|---|---|---|---|---|
| Villus height (µm) | ||||
| 5 dpi | 492 ± 46 ab | 517 ± 69 a | 450 ± 75 b | 463 ± 49 ab |
| 7 dpi | 471 ± 74 a | 460 ± 52 ab | 253 ± 74 c | 402 ± 59 ad |
| 14 dpi | 427 ± 72 a | 524 ± 44 b | 458 ± 88 a | 540 ± 77 b |
| 21 dpi | 395 ± 86 a | 423 ± 75 a | 419 ± 77 a | 440 ± 94 a |
| Crypt depth (µm) | ||||
| 5 dpi | 110 ± 21 a | 129 ± 28 a | 217 ± 33 b | 240 ± 34 b |
| 7 dpi | 97 ± 17 a | 104 ± 17 a | 259 ± 76 b | 233 ± 47 b |
| 14 dpi | 101 ± 16 a | 114 ± 28 a | 281 ± 99 b | 191 ± 51 c |
| 21 dpi | 108 ± 23 a | 118 ± 29 a | 133 ± 32 a | 140 ± 30 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piekarska, J.; Madej, J.P.; Gorczykowski, M.; Szczypka, M. The Effects of Honeysuckle (Lonicera caerulea L.) Berry Iridoid-Anthocyanin Extract on the Intestinal and Muscle Histopathology in Mice during Experimental Trichinellosis. Molecules 2023, 28, 7067. https://doi.org/10.3390/molecules28207067
Piekarska J, Madej JP, Gorczykowski M, Szczypka M. The Effects of Honeysuckle (Lonicera caerulea L.) Berry Iridoid-Anthocyanin Extract on the Intestinal and Muscle Histopathology in Mice during Experimental Trichinellosis. Molecules. 2023; 28(20):7067. https://doi.org/10.3390/molecules28207067
Chicago/Turabian StylePiekarska, Jolanta, Jan P. Madej, Michał Gorczykowski, and Marianna Szczypka. 2023. "The Effects of Honeysuckle (Lonicera caerulea L.) Berry Iridoid-Anthocyanin Extract on the Intestinal and Muscle Histopathology in Mice during Experimental Trichinellosis" Molecules 28, no. 20: 7067. https://doi.org/10.3390/molecules28207067