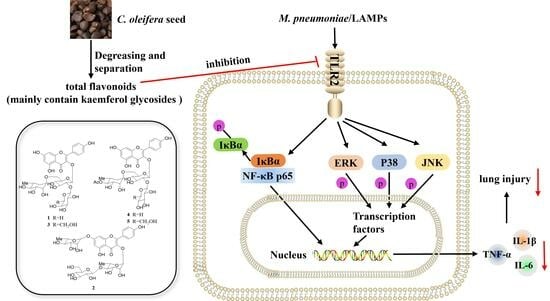
Total Flavonoids from Camellia oleifera Alleviated Mycoplasma pneumoniae-Induced Lung Injury via Inhibition of the TLR2-Mediated NF-κB and MAPK Pathways
Abstract
:1. Introduction
2. Results
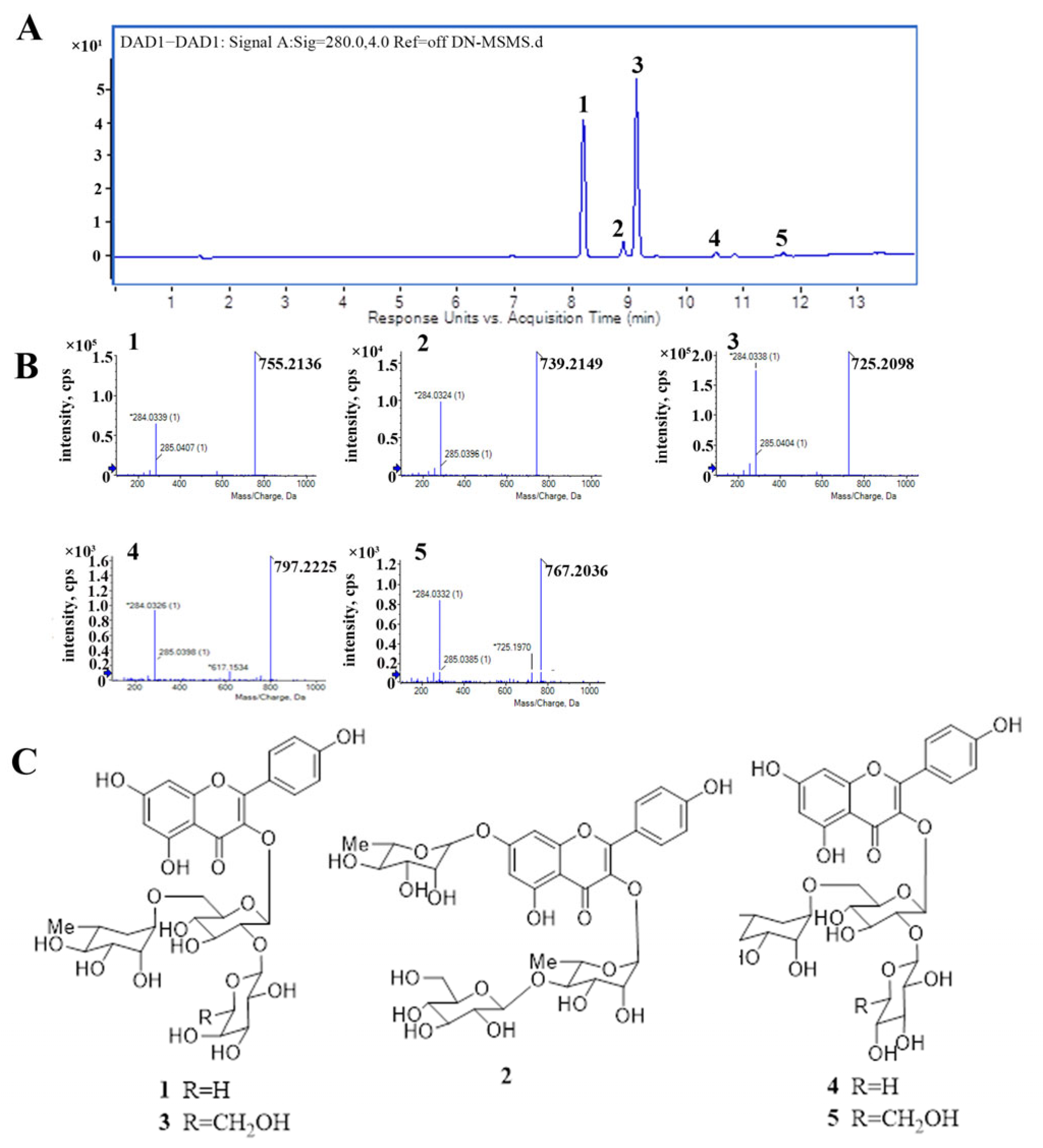
2.1. The Major Components of TFCO
2.2. TFCO Alleviated Pathological Changes in M. pneumoniae-Induced Lung Injury in Mice
2.3. TFCO Suppressed Pro-Inflammatory Cytokines Secretion in M. pneumoniae-Infected Mice and LAMPs-Stimulated RAW264.7 Cells without Affecting Cell Viability
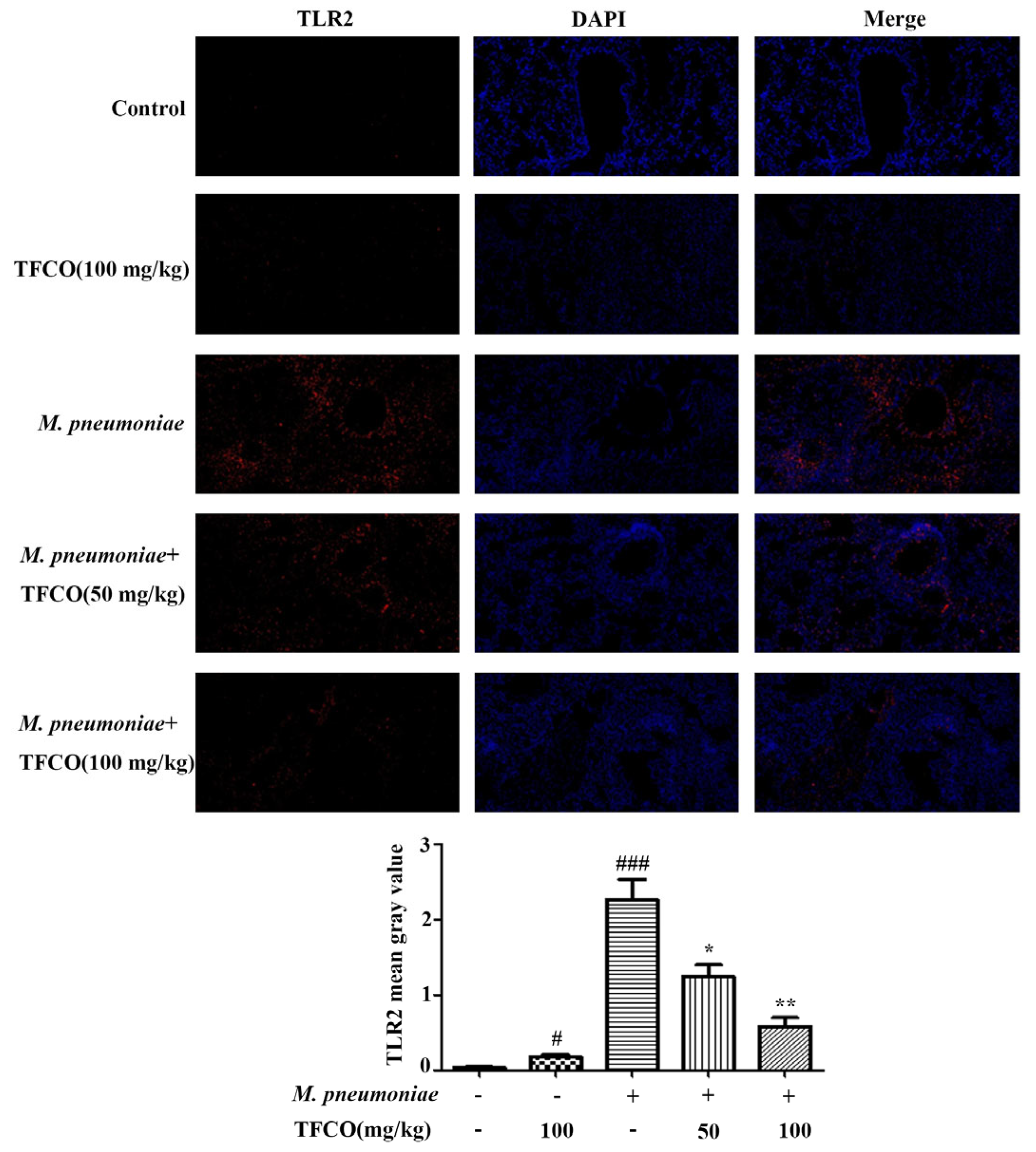
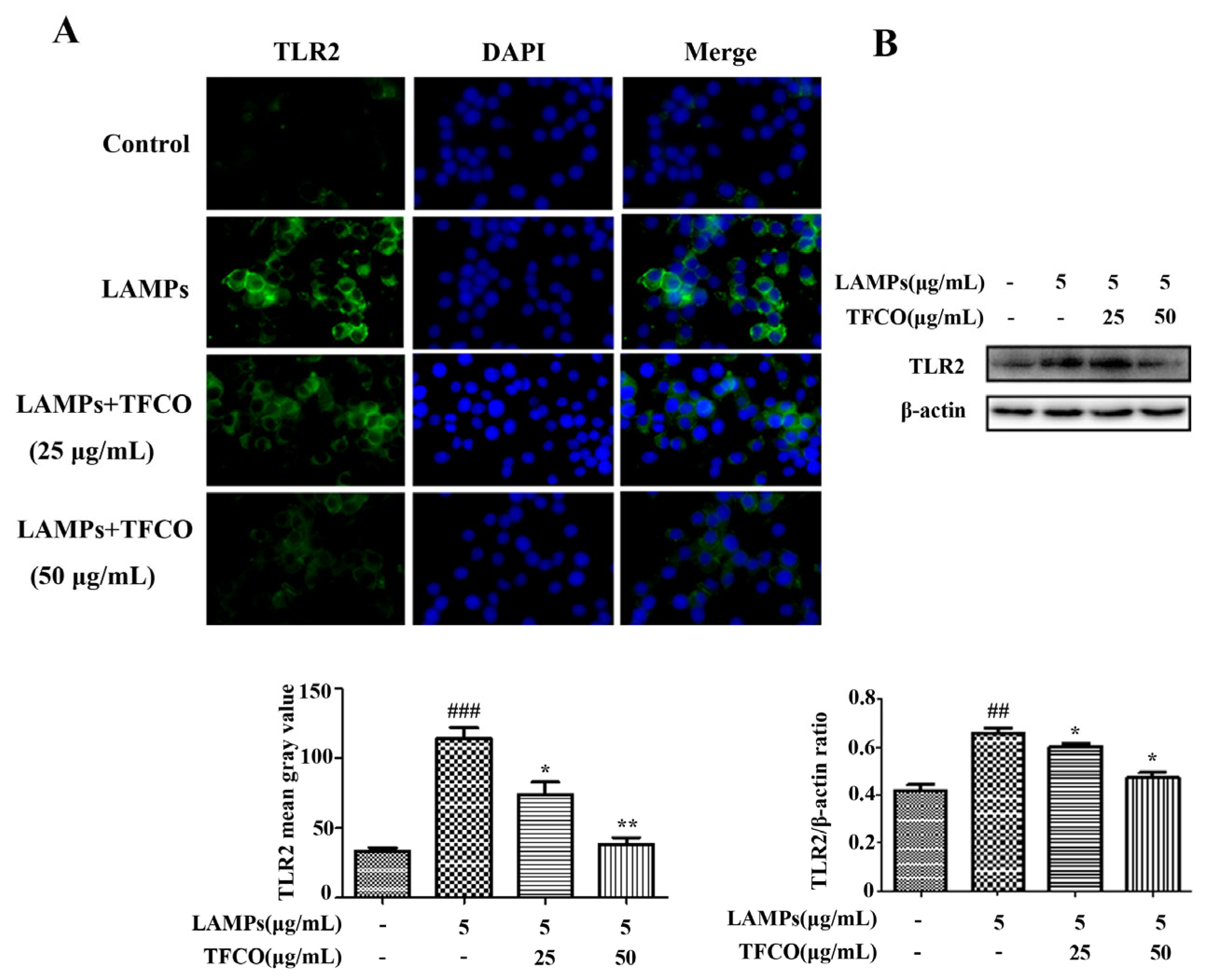
2.4. TFCO Suppressed TLR2 Expression in M. pneumoniae-Infected Mice and LAMPs-Stimulated RAW264.7 Cells
2.5. TFCO Attenuated the Activation of NF-κB Pathway in LAMPs-Stimulated RAW264.7 Cells
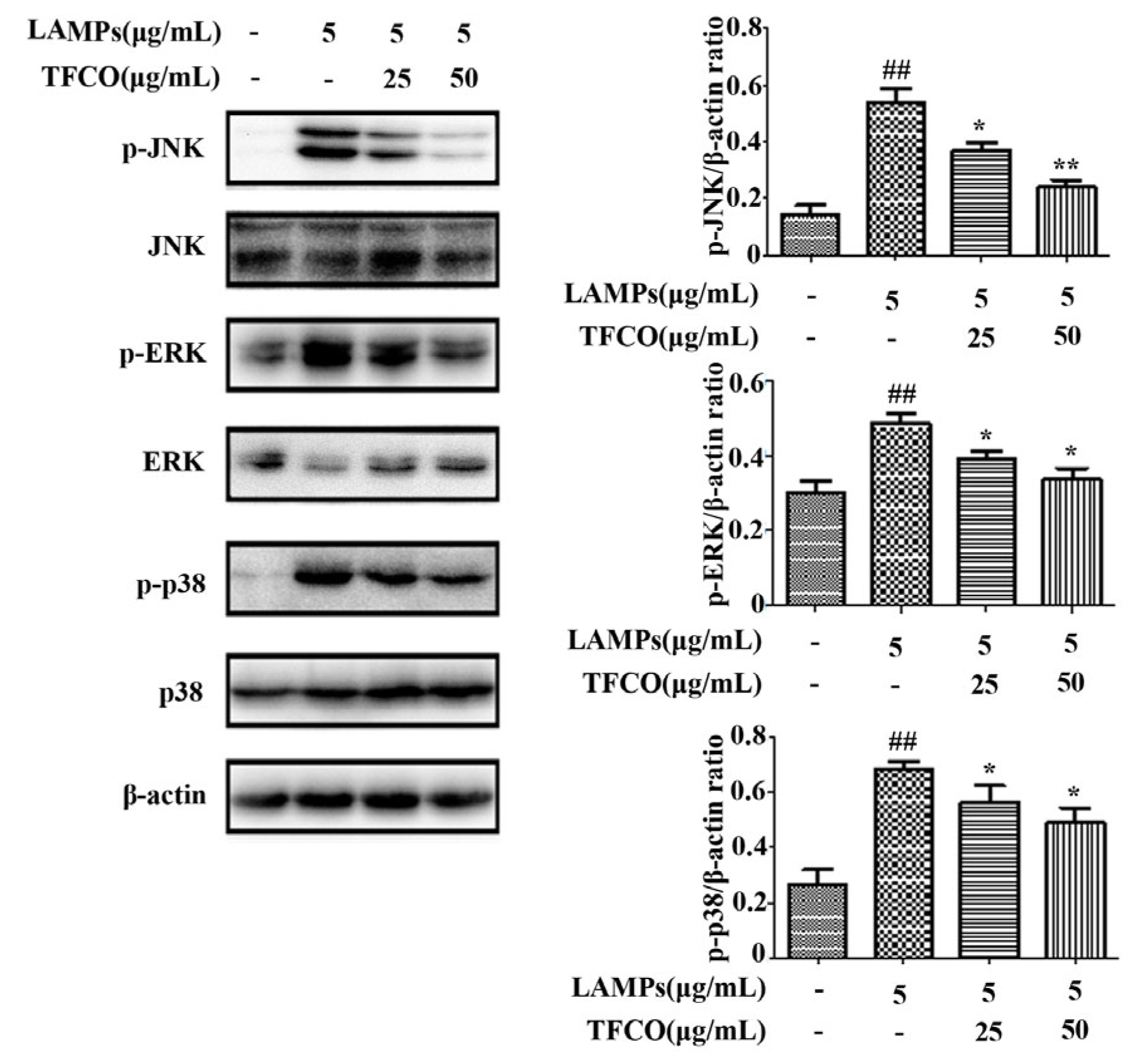
2.6. TFCO Inhibited the Activation of MAPK Pathway in LAMPs-Stimulated RAW264.7 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Preparation and UPLC-HRESIMS Analysis of TFCO
4.3. M. pneumoniae Culture and Extraction of LAMPs
4.4. Animals and Experimental Design
4.5. Cell Culture and Stimulation with LAMPs
4.6. Cell Viability Assay
4.7. HE Staining
4.8. Enzyme-Linked Immunosorbent Assay
4.9. Immunofluorescence Staining
4.10. Western Blot Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tong, L.; Huang, S.; Zheng, C.; Zhang, Y.; Chen, Z. Refractory Mycoplasma pneumoniae pneumonia in children: Early recognition and management. J. Clin. Med. 2022, 11, 2824. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Zhou, Z.F.; Jing, C.; Xian, L.S.; Ling, W.X.; Fang, T.L. Pseudomembranous necrotizing laryngotracheobronchitis due to Mycoplasma pneumoniae: A case report and literature review. BMC Infect. Dis. 2022, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.C.A.; Meyer Sauteur, P.M.; Unger, W.W.J.; van Rossum, A.M.C. Things that could be Mycoplasma pneumoniae. J. Infect. 2017, 74, S95–S100. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, S.; Zhu, C.; Zhou, R.; Leung, P.H.M. Mycoplasma pneumoniae infections: Pathogenesis and vaccine development. Pathogens 2021, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.L.; Lee, C.M.; Yang, T.L.; Yen, T.Y.; Chang, L.Y.; Chen, J.M.; Lee, P.I.; Huang, L.M.; Lu, C.Y. Severe Mycoplasma pneumoniae pneumonia requiring intensive care in children, 2010-2019. J. Formos. Med. Assoc. 2021, 120, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hooper, W.C.; Phillips, D.J.; Talkington, D.F. Cytokines in Mycoplasma pneumoniae infections. Cytokine Growth Factor Rev. 2004, 15, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T. Inflammation-inducing factors of Mycoplasma pneumoniae. Front. Microbiol. 2016, 7, 414–421. [Google Scholar] [CrossRef]
- Athamna, A.; Kramer, M.R.; Kahane, I. Adherence of Mycoplasma pneumoniae to human alveolar macrophages. FEMS Immunol. Med. Microbiol. 1996, 15, 135–141. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chang, C.H.; Lee, W.J.; Liu, T.Y.; Tsai, C.M.; Tsai, T.A.; Tsai, C.K.; Kuo, K.C.; Chen, C.C.; Niu, C.K.; et al. Altered chemokine profile in Refractory Mycoplasma pneumoniae pneumonia infected children. J. Microbiol. Immunol. Infect. 2021, 54, 673–679. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, M.; Han, H. Interaction between alveolar macrophages and epithelial cells during Mycoplasma pneumoniae infection. Front. Cell. Infect. Microbiol. 2023, 13, 1052020. [Google Scholar] [CrossRef]
- Tamiya, S.; Yoshikawa, E.; Ogura, M.; Kuroda, E.; Suzuki, K.; Yoshioka, Y. Neutrophil-mediated lung injury both via TLR2-dependent production of IL-1α and IL-12 p40, and TLR2-independent CARDS toxin after Mycoplasma pneumoniae infection in mice. Microbiol. Spectr. 2021, 9, e0158821. [Google Scholar] [CrossRef]
- Ma, C.; Hao, X.; Gao, L.; Wang, Y.; Shi, J.; Luo, H.; Li, M. Extracellular vesicles released from macrophages infected with Mycoplasma pneumoniae stimulate proinflammatory response via the TLR2-NF-κB/JNK signaling pathway. Int. J. Mol. Sci. 2023, 24, 8588. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; He, J.; Qin, L.; Chen, Y.; Chen, L.; Li, R.; Zeng, Y.; Zhu, C.; You, X.; Wu, Y. Mycoplasma pneumoniae lipids license TLR-4 for activation of NLRP3 inflammasome and autophagy to evoke a proinflammatory response. Clin. Exp. Immunol. 2021, 203, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kida, Y.; Kuwano, K. A dipalmitoylated lipoprotein from Mycoplasma pneumoniae activates NF-kappa B through TLR1, TLR2, and TLR6. J. Immunol. 2005, 175, 4641–4646. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Wang, A.; Gao, C.; Li, C. Applications of Chinese Camellia oleifera and its by-products: A review. Front. Chem. 2022, 10, 921246. [Google Scholar] [CrossRef]
- Teixeira, A.M.; Sousa, C. A review on the biological activity of Camellia species. Molecules 2021, 26, 2178. [Google Scholar] [CrossRef]
- Sekine, T.; Arita, J.; Yamaguchi, A.; Saito, K.; Okonogi, S.; Morisaki, N.; Iwasaki, S.; Murakoshi, I. Two flavonol glycosides from seeds of Camellia sinensis. Phytochemistry 1991, 30, 991–995. [Google Scholar] [CrossRef]
- Du, L.C.; Wu, B.L.; Chen, J.M. Flavonoid triglycosides from the seeds of Camellia oleifera Abel. Chin. Chem. Lett. 2008, 19, 1315–1318. [Google Scholar] [CrossRef]
- Gao, D.-F.; Xu, M.; Zhao, P.; Zhang, X.-Y.; Wang, Y.-F.; Yang, C.-R.; Zhang, Y.-J. Kaempferol acetylated glycosides from the seed cake of Camellia oleifera. Food Chem. 2011, 124, 432–436. [Google Scholar] [CrossRef]
- Liu, X.; Jia, L.; Gao, Y.; Li, B.; Tu, Y. Anti-inflammatory activity of total flavonoids from seeds of Camellia oleifera Abel. Acta Biochim. Biophys. Sin. 2014, 46, 920–922. [Google Scholar] [CrossRef]
- Bing, J.; Xu, C.-T.; Li, Q.; Qin, J.-K.; Luo, Y.-W.; Yang, W.G. Chemical constituents of the flavonoids from Camellia oleifera and their antiinflammatory activities in vitro. Chin. Tradit. Pat. Med. 2019, 41, 327–333. [Google Scholar]
- Zhang, X.F.; Han, Y.Y.; Di, T.M.; Gao, L.P.; Xia, T. Triterpene saponins from tea seed pomace (Camellia oleifera Abel) and their cytotoxic activity on MCF-7 cells in vitro. Nat. Prod. Res. 2021, 35, 2730–2733. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, S.; Wan, F.; Zhou, Y.; Wang, Z.; Fan, G.; Wang, P.; Luo, H.; Liao, S.; He, L.; et al. Quantitative analysis of Camellia oleifera seed saponins and aqueous two-phase extraction and separation. Molecules 2023, 28, 2132. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tang, M.; Zhao, X.R.; Feng, S.L.; Liu, L.; Zhou, L.J.; Cao, X.H.; Huang, Y.; Yang, H.Y.; Ding, C.B. Antioxidant potential evaluation of polysaccharides from Camellia oleifera Abel in vitro and in vivo. Int. J. Biol. Macromol. 2023, 248, 125726. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Tang, M.; Jiang, Z.; Ruan, Y.; Liu, L.; Kong, Q.; Xiang, Z.; Chen, T.; Zhou, L.; Yang, H.; et al. Optimization of extraction process, structure characterization, and antioxidant activity of polysaccharides from different parts of Camellia oleifera Abel. Foods 2022, 11, 3185. [Google Scholar] [CrossRef]
- Xiao, X.; He, L.; Chen, Y.; Wu, L.; Wang, L.; Liu, Z. Anti-inflammatory and antioxidative effects of Camellia oleifera Abel components. Future Med. Chem. 2017, 9, 2069–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.F.; Wang, C.L.; Ye, F.; Sun, H.P.; Ma, C.Y.; Liu, W.Y.; Feng, F.; Abe, M.; Akihisa, T.; Zhang, J. Chemical constituents of the seed cake of Camellia oleifera and their antioxidant and antimelanogenic activities. Chem. Biodivers. 2018, 15, e1800137. [Google Scholar] [CrossRef]
- Qiu, Y.; He, D.; Yang, J.; Ma, L.; Zhu, K.; Cao, Y. Kaempferol separated from Camellia oleifera meal by high-speed countercurrent chromatography for antibacterial application. Eur. Food Res. Technol. 2020, 246, 2383–2397. [Google Scholar] [CrossRef]
- Ko, J.; Yeh, W.J.; Huang, W.C.; Yang, H.Y. Camellia oleifera seed extract mildly ameliorates carbon tetrachloride-induced hepatotoxicity in rats by suppressing inflammation. J. Food Sci. 2019, 84, 1586–1591. [Google Scholar] [CrossRef]
- Yeh, W.J.; Ko, J.; Huang, W.C.; Cheng, W.Y.; Yang, H.Y. Crude extract of Camellia oleifera pomace ameliorates the progression of non-alcoholic fatty liver disease via decreasing fat accumulation, insulin resistance and inflammation. Br. J. Nutr. 2020, 123, 508–515. [Google Scholar] [CrossRef]
- Chen, L. Extraction and Separation, Chemical Structure Characterization and Biological Activity of Flavonoid Glycosides in Camellia oleifera Seed Cake. Maters’ Thesis, Nanchang University, Nanchang, China, 2011. [Google Scholar]
- Waites, K.B.; Xiao, L.; Liu, Y.; Balish, M.F.; Atkinson, T.P. Mycoplasma pneumoniae from the respiratory tract and beyond. Clin. Microbiol. Rev. 2017, 30, 747–809. [Google Scholar] [CrossRef]
- Chung, M.J.; Pandey, R.P.; Choi, J.W.; Sohng, J.K.; Choi, D.J.; Park, Y.I. Inhibitory effects of kaempferol-3-O-rhamnoside on ovalbumin-induced lung inflammation in a mouse model of allergic asthma. Int. Immunopharmacol. 2015, 25, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, K.C.; Faustino, L.; Borduchi, E.; Nascimento, R.J.; Silva, T.M.; Gomes, E.; Piuvezam, M.R.; Russo, M. Preventive and curative glycoside kaempferol treatments attenuate the TH2-driven allergic airway disease. Int. Immunopharmacol. 2009, 9, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, C.; Wang, L.F.; Kuang, X.; Liu, K.; Zhang, H.; Du, J.R. Neuroprotective effect of kaempferol glycosides against brain injury and neuroinflammation by inhibiting the activation of NF-kappaB and STAT3 in transient focal stroke. PLoS ONE 2013, 8, e55839. [Google Scholar] [CrossRef]
- Lin, Y.; Tan, D.; Kan, Q.; Xiao, Z.; Jiang, Z. The protective effect of naringenin on airway remodeling after Mycoplasma pneumoniae infection by inhibiting autophagy-mediated lung inflammation and fibrosis. Mediat. Inflamm. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Wang, J.; Wu, Q.; Yan, H. Polydatin suppresses the development of lung inflammation and fibrosis by inhibiting activation of the NACHT domain-, leucine-rich repeat-, and pyd-containing protein 3 inflammasome and the nuclear factor-κB pathway after Mycoplasma pneumoniae infection. J. Cell. Biochem. 2018, 120, 10137–10144. [Google Scholar] [CrossRef]
- Li, G.; Fan, L.; Wang, Y.; Huang, L.; Wang, M.; Zhu, C.; Hao, C.; Ji, W.; Liang, H.; Yan, Y.; et al. High co-expression of TNF-alpha and CARDS toxin is a good predictor for refractory Mycoplasma pneumoniae pneumonia. Mol. Med. 2019, 25, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chu, C.; Li, Y.; Li, G.; Lei, X.; Zhou, W.; Chen, Z. High expression of HMGB1 in children with refractory Mycoplasma pneumoniae pneumonia. BMC Infect. Dis. 2018, 18, 439. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, J. Changes and significance of serum sB7-H3 and cytokines in children with Mycoplasma pneumonae pneumonia. J. Coll. Physicians Surg. Pak. 2020, 30, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, H.; Xia, X.; Yang, L.; He, J. Kaempferol 3-O-(2G-glucosylrutinoside)-7-O-glucoside isolated from the flowers of Hosta plantaginea exerts anti-inflammatory activity via suppression of NF-κB, MAPKs and Akt pathways in RAW 264.7 cells. Biomed. Pharmacother. 2022, 153, 113295–113301. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.H.; Rao, Y.K.; Tzeng, Y.M. Inhibitory effects of flavonol glycosides from Cinnamomum osmophloeum on inflammatory mediators in LPS/IFN-gamma-activated murine macrophages. Bioorg. Med. Chem. 2005, 13, 2381–2388. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kida, Y.; Kuwano, K. Triacylated lipoproteins derived from Mycoplasma pneumoniae activate nuclear factor-?B through toll-like receptors 1 and 2. Immunology 2007, 121, 473–483. [Google Scholar] [CrossRef]
- Chu, H.W.; Jeyaseelan, S.; Rino, J.G.; Voelker, D.R.; Wexler, R.B.; Campbell, K.; Harbeck, R.J.; Martin, R.J. TLR2 signaling is critical for Mycoplasma pneumoniae-induced airway mucin expression. J. Immunol. 2005, 174, 5713–5719. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Kaufmann, A.; Grote, K.; Kawai, T.; Hoshino, K.; Morr, M.; Mühlradt, P.F.; Akira, S. Cutting edge: Preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 2000, 164, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Hirao, S.; Wada, H.; Nakagaki, K.; Saraya, T.; Kurai, D.; Mikura, S.; Yasutake, T.; Higaki, M.; Yokoyama, T.; Ishii, H.; et al. Inflammation provoked by Mycoplasma pneumoniae extract: Implications for combination treatment with clarithromycin and dexamethasone. FEMS Immunol. Med. Microbiol. 2011, 62, 182–189. [Google Scholar] [CrossRef]
- Ye, Y.; Fang, F.; Li, Y. Isolation of the sapogenin from defatted seeds of Camellia oleifera and its neuroprotective effects on dopaminergic neurons. J. Agric. Food Chem. 2014, 62, 6175–6182. [Google Scholar] [CrossRef]
- Ye, Y.; Xing, H.; Chen, X. Anti-inflammatory and analgesic activities of the hydrolyzed sasanquasaponins from the defatted seeds of Camellia oleifera. Arch. Pharm. Res. 2013, 36, 941–951. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.E.; Petri, W.A. TLR2 as a therapeutic target in bacterial infection. Trends Mol. Med. 2020, 26, 715–717. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, N.; Lei, A.; Shi, Z.; Xiang, L.; Wei, B.; Wu, Y. Total Flavonoids from Camellia oleifera Alleviated Mycoplasma pneumoniae-Induced Lung Injury via Inhibition of the TLR2-Mediated NF-κB and MAPK Pathways. Molecules 2023, 28, 7077. https://doi.org/10.3390/molecules28207077
Ding N, Lei A, Shi Z, Xiang L, Wei B, Wu Y. Total Flavonoids from Camellia oleifera Alleviated Mycoplasma pneumoniae-Induced Lung Injury via Inhibition of the TLR2-Mediated NF-κB and MAPK Pathways. Molecules. 2023; 28(20):7077. https://doi.org/10.3390/molecules28207077
Chicago/Turabian StyleDing, Nan, Aihua Lei, Zhisheng Shi, Lin Xiang, Bo Wei, and Yimou Wu. 2023. "Total Flavonoids from Camellia oleifera Alleviated Mycoplasma pneumoniae-Induced Lung Injury via Inhibition of the TLR2-Mediated NF-κB and MAPK Pathways" Molecules 28, no. 20: 7077. https://doi.org/10.3390/molecules28207077