A Novel Fluorescence Sensor for Iodide Detection Based on the 1,3-Diaryl Pyrazole Unit with AIE and Mechanochromic Fluorescence Behavior
Abstract
:1. Introduction
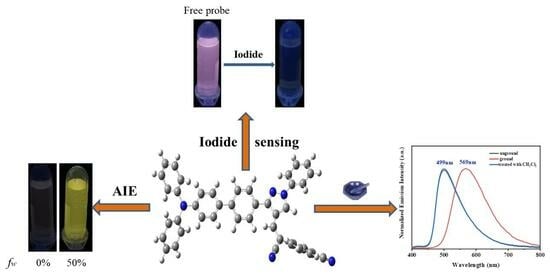
2. Results and Discussion
2.1. Optical Response of the TPA-CDP Probe
2.2. Aggregation−Induced Emission (AIE) Characteristics of TPA-CDP
2.3. Crystal Solid−State Emission of TPA-CDP Compounds
2.4. Contrasting Mechanochromic Fluorescence Behavior of TPA-CDP Compounds
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mansha, M.; Akram Khan, S.; Aziz, M.A.; Zeeshan Khan, A.; Ali, S.; Khan, M. Optical Chemical Sensing of Iodide Ions: A Comprehensive Review for The Synthetic Strategies of Iodide Sensing Probes, Challenges, and Future Aspects. Chem. Rec. 2022, 22, e202200059. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.H.; Liu, S.G.; Ling, Y.; Li, N.B.; Luo, H.Q. Facile Method for Iodide Ion Detection Via the Fluorescence Decrease of Dihydrolipoic Acid/Beta-Cyclodextrin Protected Ag Nanoclusters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 212, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, R.; Feng, S.; Wang, D.; Liu, H. Porosity-Induced Selective Sensing of Iodide in Aqueous Solution by A Fluorescent Imidazolium-Based Ionic Porous Framework. ACS Appl. Mater. Interface 2020, 12, 11104–11114. [Google Scholar] [CrossRef]
- Xie, H.F.; Wu, C.; Zou, J.; Yang, Y.-X.; Xu, H.; Zhang, Q.-L.; Redshaw, C.; Yamato, T. A Pyrenyl-Appended C3v-Symmetric Hexahomotrioxacalix Arene for Selective Fluorescence Sensing of Iodide. Dye. Pigment. 2020, 178, 108340. [Google Scholar] [CrossRef]
- Petersen, M.; Bülow Pedersen, I.; Knudsen, N.; Andersen, S.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Banke Rasmussen, L.; Thuesen, B.H.; Carlé, A. Changes in Subtypes of Overt Thyrotoxicosis and Hypothyroidism Following Iodine Fortification. Clin. Endocrinol. 2019, 91, 652–659. [Google Scholar] [CrossRef]
- Hussain, S.; De, S.; Iyer, P.K. Thiazole-Containing Conjugated Polymer as A Visual and Fluorometric Sensor for Iodide and Mercury. ACS Appl. Mater. Interfaces 2013, 5, 2234–2240. [Google Scholar] [CrossRef]
- Huang, X.; Lu, Z.; Wang, Z.; Fan, C.; Fan, W.; Shi, X.; Zhang, H.; Pei, M. A Colorimetric and Turn-On Fluorescent Chemosensor for Selectively Sensing Hg2+ and Its Resultant Complex for Fast Detection of I− Over S2−. Dye. Pigment. 2016, 128, 33–40. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Q.; Zhang, Y.M.; Wei, T.B. A Reversible and Highly Selective Fluorescent Probe for Monitoring Hg2+ and Iodide in Aqueous Solution. Sens. Actuators B Chem. 2014, 196, 619–623. [Google Scholar] [CrossRef]
- Mahapatra, A.K.; Hazra, G.; Roy, J.; Sahoo, P. A Simple Coumarin-Based Colorimetric and Ratiometric Chemosensor for Acetate and A Selective Fluorescence Turn-On Probe for Iodide. J. Lumin. 2011, 131, 1255–1259. [Google Scholar] [CrossRef]
- Mitra, A.; Pariyar, A.; Bose, S.; Bandyopadhyay, P.; Sarkar, A. First Phenalenone Based Receptor for Selective Iodide Ion Sensing. Sens. Actuators B Chem. 2015, 210, 712–718. [Google Scholar] [CrossRef]
- Hou, X.; Hansen, V.; Aldahan, A.; Possnert, G.; Lind, O.C.; Lujaniene, G. A Review on Speciation of Iodin in the Environmental and Biological Samples. Anal. Chim. Acta 2009, 632, 181–196. [Google Scholar] [CrossRef] [PubMed]
- Flores, É.M.M.; Mesko, M.F.; Moraes, D.P.; Pereira, J.S.F.; Mello, P.A.; Barin, J.S.; Knapp, G. Determination of Halogens in Coal After Digestion Using the Microwave-Induced Combustion Technique. Anal. Chem. 2008, 80, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Odenigbo, C.; Makonnen, Y.; Asfaw, A.; Anastassiades, T.; Beauchemin, D. Towards the Use of ICP-OES for the Elemental Analysis of Organic Compounds such as Glucosamine. J. Anal. At. Spectrom. 2014, 29, 454–457. [Google Scholar] [CrossRef]
- Singh, A.; Sinha, S.; Kaur, R.; Kaur, N.; Singh, N. Rhodamine Based Organic Nanoparticles for Sensing of Fe3+ with High Selectivity in Aqueous Medium: Application to Iron Supplement Analysis. Sens. Actuat. B Chem. 2014, 204, 617–621. [Google Scholar] [CrossRef]
- Ge, L.; Guo, C.; Li, H.; Xia, X.; Chen, L.; Ning, D.; Liu, X.; Li, F. Direct-Laser-Writing of Electrochemiluminescent Electrode on Glassy Carbon for Iodide Sensing in Aqueous Solution. Sens. Actuators B Chem. 2021, 337, 129766. [Google Scholar] [CrossRef]
- Mendy, J.S.; Saeed, M.A.; Fronczek, F.R.; Powell, D.R.; Hossain, M.A. Anion Recognition and Sensing by a New Macrocyclic Dinuclear Copper(II) Complex: A Selective Receptor for Iodide. Inorg. Chem. 2010, 49, 7223–7225. [Google Scholar] [CrossRef]
- Amirjani, A.; Tsoulos, T.V.; Sajjadi, S.H.; Antonucci, A.; Wu, S.J.; Tagliabue, G.; Haghshenas, D.F.; Boghossian, A.A. Plasmon-induced near-infrared fluorescence enhancement of single-walled carbon nanotubes. Carbon 2022, 194, 162–175. [Google Scholar] [CrossRef]
- Cao, X.; Gao, A.; Hou, J.T.; Yi, T. Fluorescent supramolecular self-assembly gels and their application as sensors: A review. Coord. Chem. Rev. 2021, 434, 213792. [Google Scholar]
- Zhang, X.Y.; Yang, Y.S.; Wang, W.; Jiao, Q.C.; Zhu, H.L. Fluorescent sensors for the detection of hydrazine in environmental and biological systems: Recent advances and future prospects. Coord. Chem. Rev. 2020, 417, 213367. [Google Scholar]
- Li, Z.; Liu, R.; Xing, G.; Wang, T.; Liu, S. A Novel Fluorometric and Colorimetric Sensor for Iodide Determination Using DNA-Templated Gold/Silver Nanoclusters. Biosens. Bioelectron. 2017, 96, 44–48. [Google Scholar] [CrossRef]
- Jiang, R.; Zhang, Y.; Zhang, Q.; Li, L.; Yang, L. Carbon Dot/Gold Nanocluster-Based Fluorescent Colorimetric Paper Strips for Quantitative Detection of Iodide Ions in Urine. ACS Appl. Nano Mater. 2021, 4, 9760–9767. [Google Scholar] [CrossRef]
- Rani, P.; Kiran; Priyanka; Sindhu, J.; Kumar, S. 5-Hydroxydibenzo[a,i]phenazine-8,13-dione: A Selective and Sensitive Colorimetric and Fluorescent ‘Turn-Off’ Sensor for Iodide Ion. J. Mol. Struct. 2023, 1275, 134621. [Google Scholar] [CrossRef]
- Zou, X.; Hu, J.; Zhu, H.H.; Chen, Q.M.; Gong, Z.J. Ultrasensitive Turn-Off Fluorescence Detection of Iodide Using Carbon Dots/Gold Nanocluster as Fluorescent Nanoprobe. Microchem. J. 2023, 185, 108275. [Google Scholar] [CrossRef]
- Patel, A.M.; Ray, D.; Aswal, V.K.; Ballabh, A. Probing the Supramolecular Assembly in Solid, Solution and Gel Phase in Uriede based Thiazole Derivatives and Its Potential Application as Iodide Ion Sensor. J. Mol. Liq. 2022, 362, 119763. [Google Scholar] [CrossRef]
- Liu, H.W.; Li, K.; Hu, X.X.; Zhu, L.M.; Rong, Q.M.; Liu, Y.C.; Zhang, X.B.; Hasserodt, J.; Qu, F.L.; Tan, W.H. In Situ Localization of Enzyme Activity in Live Cells by a Molecular Probe Releasing a Precipitating Fluorochrome. Angew. Chem. Int. Ed. 2017, 56, 11788–11792. [Google Scholar] [CrossRef]
- Gao, X.; Feng, G.; Manghnani, P.N.; Hu, F.; Jiang, N.; Liu, J.; Liu, B.; Sun, J.; Tang, B.A. Two-Channel Responsive Fluorescent Probe with AIE Characteristics and Its Application for Selective Imaging of Superoxide Anions in Living Cells. Chem. Commun. 2017, 53, 1653–1656. [Google Scholar] [CrossRef]
- Niu, J.; Fan, J.; Wang, X.; Xiao, Y.; Xie, X.; Jiao, X.; Sun, C.; Tang, B. Simultaneous Fluorescence and Chemiluminescence Turned on by Aggregation-Induced Emission for Real-Time Monitoring of Endogenous Superoxide Anion in Live Cells. Anal. Chem. 2017, 89, 7210–7215. [Google Scholar] [CrossRef]
- Mei, J.; Hong, Y.; Lam, W.Y.J.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by Luminogens with Aggregation-Induced Emission Characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, P.; Gong, Y.; Ding, C.F. Two-Photon AIE Based Fluorescent Probe with Large Stokes Shift for Selective and Sensitive Detection and Visualization of Hypochlorite. Sens. Actuators B Chem. 2019, 278, 73–81. [Google Scholar] [CrossRef]
- He, H.F.; Li, T.; Yao, L.F.; Liu, M.J.; Xia, H.Y.; Shen, L. Aggregation-Induced Emission Enhancement (AIEE)-Active Donor-Acceptor Type Fluorophores Based on the 1,3-Diaryl Pyrazole Unit: Conspicuous Mechanochromic Fluorescence and Biosensing of Iodide Ions. Dye. Pigment. 2022, 203, 110309. [Google Scholar] [CrossRef]
- Yoon, S.J.; Chung, J.W.; Gierschner, J.; Kim, K.S.; Choi, M.G.; Kim, D. Multistimuli Two-Color Luminescence Switching via Different Slip-Stacking of Highly Fluorescent Molecular Sheets. J. Am. Chem. Soc. 2010, 132, 13675–13683. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Han, S.H.; Kim, J.B.; Kim, J.H.; Lee, J.M.; Kim, S.H. Colloidal Photonic Inks for Mechanochromic Films and Patterns with Structural Colors of High Saturation. Chem. Mater. 2019, 31, 8154–8162. [Google Scholar] [CrossRef]
- Zhang, T.; Han, Y.; Liang, M.; Bian, W.; Zhang, Y.; Li, X. Substituent Effect on Photophysical Properties, Crystal Structures and Mechanochromism of D-π-A Phenothiazine Derivatives. Dye. Pigment. 2019, 171, 107692. [Google Scholar] [CrossRef]
- Zare, E.N.; Khorsandi, D.; Zarepour, A.; Yilmaz, H.; Agarwal, T.; Hooshmand, S.; Mohammadinejad, R.; Ozdemir, F.; Sahin, O.; Adiguzel, S.; et al. Biomedical applications of engineered heparin-based materials. Bioact. Mater. 2024, 31, 87–118. [Google Scholar] [CrossRef]
- Tan, S.; Yin, Y.; Chen, W.Z.; Chen, Z.; Tian, W.; Pu, S.Z. Carbazole-Based Highly Solid-State Emissive Fluorene Derivatives with Various Mechanochromic Fluorescence Characteristics. Dye. Pigment. 2020, 177, 108302–108312. [Google Scholar] [CrossRef]
- Guerlin, A.; Dumur, F.; Dumas, E.; Miomandre, F.; Wantz, G.; Mayer, C.R. Tunable Optical Properties of Chromophores Derived from Oligo (P-Phenylene Vinylene). Org. Lett. 2010, 12, 2382–2385. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, S.; Xue, Z.; Li, Y.; Liu, H.; Yang, W. Donor-Acceptor Molecules based on Benzothiadiazole: Synthesis, X-ray Crystal Structures, Linear and Third-Order Nonlinear Optical Properties. Dye. Pigment. 2016, 125, 100–105. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, J.; Luo, W.; Wu, H.; Zeng, C.; Zhang, K. Synergistic Tuning of The Optical and Electrical Performance of AIEgens with a Hybridized Local and Charge-Transfer Excited State. J. Mater. Chem. C 2019, 7, 6359–6368. [Google Scholar] [CrossRef]
- Xue, P.; Zhang, C.; Wang, K.; Liang, M.; Zhang, T. Alkyl Chain-Dependent Cyano-Stilbene Derivative’s Molecular Stacking, Emission Enhancement and Fluorescent Response to the Mechanical Force and Thermal Stimulus. Dye. Pigment. 2019, 163, 516–524. [Google Scholar] [CrossRef]
- Huang, Y.; Mei, J.; Ma, X. A Novel Simple Red Emitter Characterized with AIE Plus Intramolecular Charge Transfer Effects and Its Application for Thiol-Containing Amino Acids Detection. Dye. Pigment. 2019, 165, 499–507. [Google Scholar] [CrossRef]
- Tang, A.; Chen, Z.; Liu, G.; Pu, S. 1, 8-Naphthalimide-Based Highly Emissive Luminogen with Reversible Mechanofluorochromism and Good Cell Imaging Characteristics. Tetrahedron Lett. 2018, 59, 3600–3604. [Google Scholar] [CrossRef]
- Gu, J.; Xu, Z.; Ma, D.; Qin, A.; Tang, B.Z. Aggregation-Induced Emission Polymers for High Performance PLEDs with Low Efficiency Roll-Off. Mater. Chem. Front. 2020, 4, 1206–1211. [Google Scholar] [CrossRef]
- Wu, Y.; Jin, P.; Gu, K.; Shi, C.; Guo, Z.; Yu, Z.Q. Broadening AIEgen Application: Rapid and Portable Sensing of Foodstuff Hazards in Deep-Frying Oil. Chem. Commun. 2019, 55, 4087–4090. [Google Scholar] [CrossRef]
- Ahumada, M.; Lissi, E.; Montagut, A.M.; Valenzuela-Henríquez, F.; Pacioni, N.L.; Alarcon, E.I. Association models for binding of molecules to nanostructures. Analyst. 2017, 142, 2067–2089. [Google Scholar] [CrossRef]
- Shortreed, M.; Kopelman, R.; Kuhn, M.; Hoyland, B. Fluorescent fiberoptic calcium sensor for physiological measurements. Anal. Chem. 1996, 68, 1414–1418. [Google Scholar] [CrossRef]
- Leung, C.W.T.; Hong, Y.; Chen, S.; Zhao, E.; Lam, J.W.Y.; Tang, B.Z. A Photostable AIE Luminogen for Specific Mitochondrial Imaging and Tracking. J. Am. Chem. Soc. 2013, 135, 62–65. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Hua, J.; Tang, J.; Li, B.; Qian, S. Multibranched Triarylamine End-Capped Triazines with Aggregation-Induced Emission and Large Two-Photon Absorption Cross-Sections. Chem. Commun. 2010, 46, 4689–4691. [Google Scholar] [CrossRef]
- Osawa, M.; Kawata, I.; Igawa, S.; Hoshino, M.; Fukunaga, T.; Hashizume, D. Vapochromic and Mechanochromic Tetrahedral Gold(I) Complexes based on the 1,2-Bis (diphenylphosphino) Benzene Ligand. Chem. Eur. J. 2010, 16, 12114–12126. [Google Scholar] [CrossRef]
- Ito, H.; Saito, T.; Oshima, N.; Kitamura, N.; Ishizaka, S.; Hinatsu, Y. Reversible Mechanochromic Luminescence of [(C6F5Au) 2 (μ-1, 4-diisocyanobenzene)]. J. Am. Chem. Soc. 2008, 130, 10044–10045. [Google Scholar] [CrossRef] [PubMed]
- Frisch, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G. Gaussian 09, Revision B. 01; Gaussian Inc.: Wallingford, CT, USA, 2010. [Google Scholar]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Xiong, J.; Liu, W.; Wu, L.; Hu, H.; Wu, J.; Liu, Y.; Yu, L.; Zhou, Y.; Gao, W.; et al. A Novel Fluorescence Sensor for Iodide Detection Based on the 1,3-Diaryl Pyrazole Unit with AIE and Mechanochromic Fluorescence Behavior. Molecules 2023, 28, 7111. https://doi.org/10.3390/molecules28207111
Deng L, Xiong J, Liu W, Wu L, Hu H, Wu J, Liu Y, Yu L, Zhou Y, Gao W, et al. A Novel Fluorescence Sensor for Iodide Detection Based on the 1,3-Diaryl Pyrazole Unit with AIE and Mechanochromic Fluorescence Behavior. Molecules. 2023; 28(20):7111. https://doi.org/10.3390/molecules28207111
Chicago/Turabian StyleDeng, Lili, Jian Xiong, Wenqin Liu, Lixue Wu, Huiyi Hu, Jiaqing Wu, Yue Liu, Lide Yu, Yuling Zhou, Wenjun Gao, and et al. 2023. "A Novel Fluorescence Sensor for Iodide Detection Based on the 1,3-Diaryl Pyrazole Unit with AIE and Mechanochromic Fluorescence Behavior" Molecules 28, no. 20: 7111. https://doi.org/10.3390/molecules28207111