Simultaneous Determination of Xanthine and Hypoxanthine Using Polyglycine/rGO-Modified Glassy Carbon Electrode
Abstract
:1. Introduction
2. Results
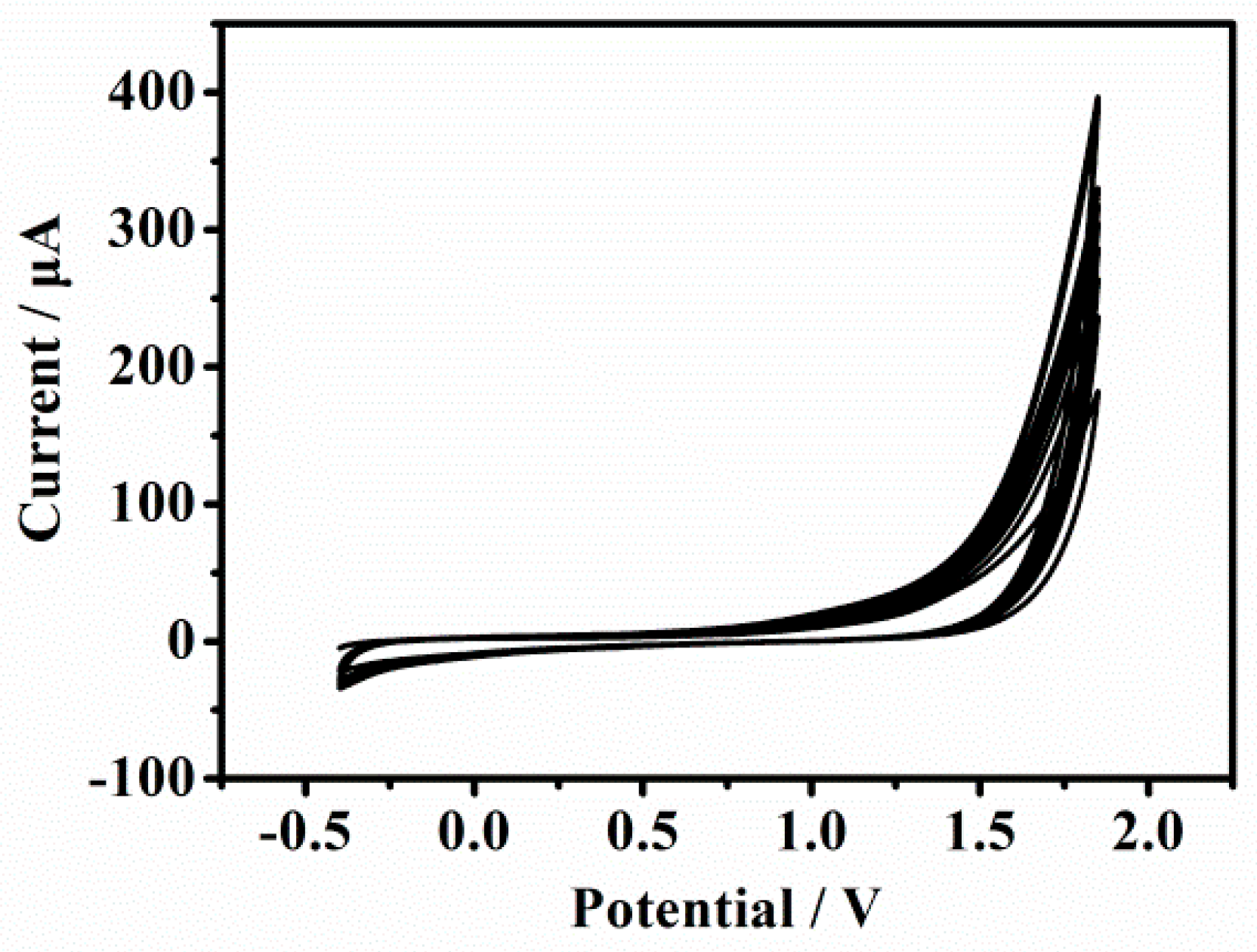
2.1. Characteristics of p-Gly/rGO Film on GCE
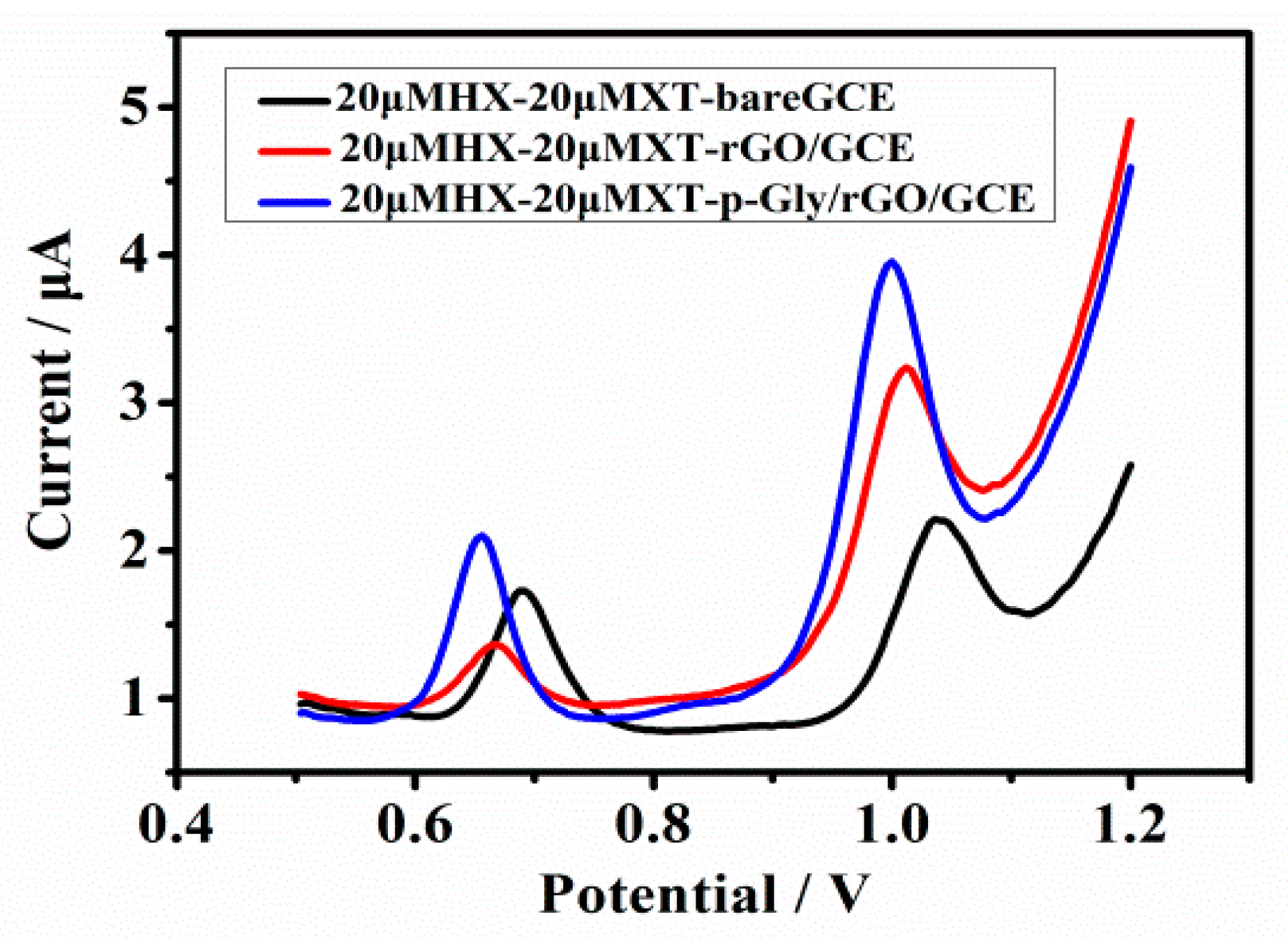
2.2. Electrochemical Behavior of XT and HX on p-Gly/rGO/GCE
2.3. Impact of Scanning Rates
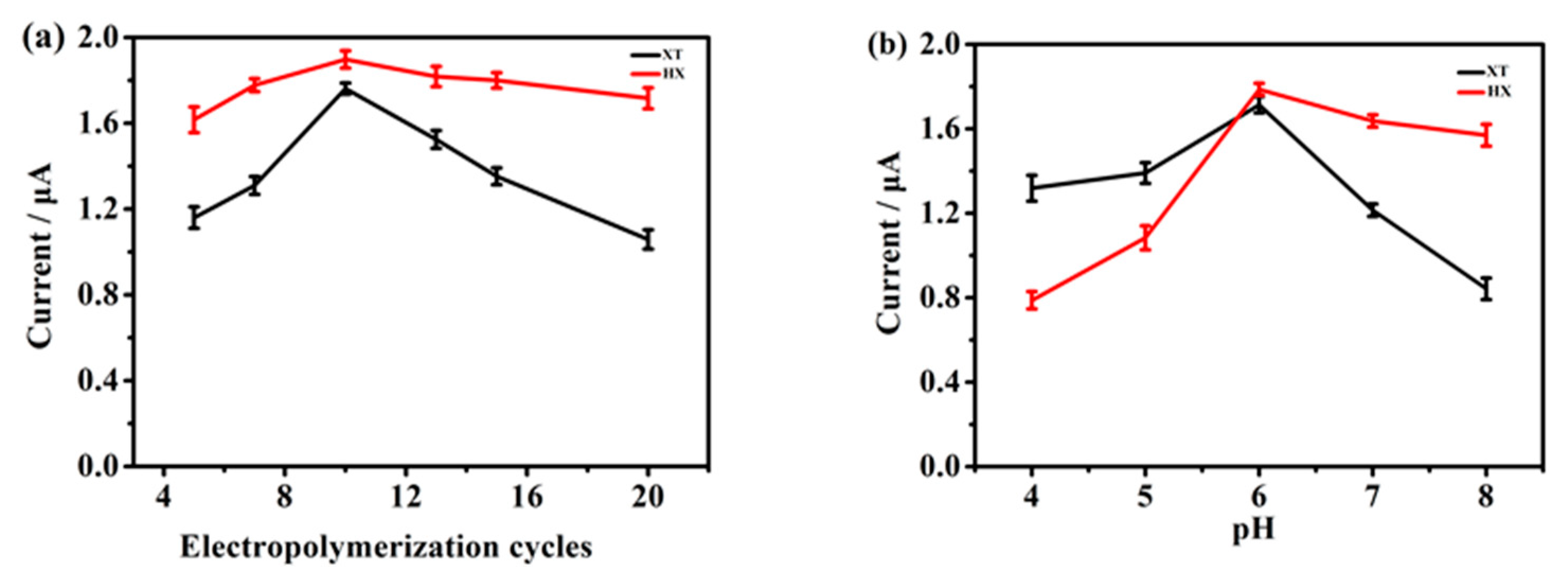
2.4. Optimization of the Experimental Conditions
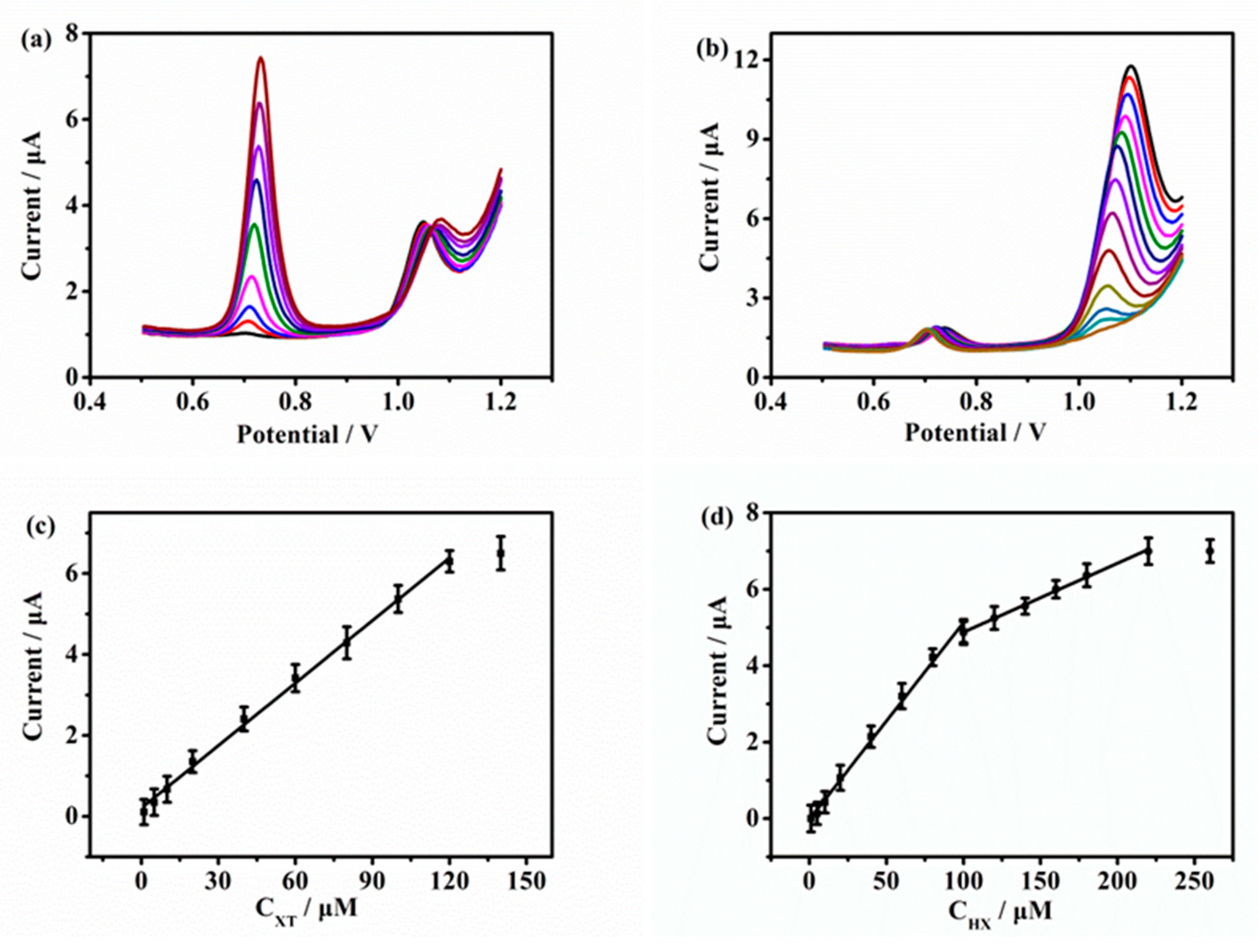
2.5. Determination of XT and HX
2.6. Reproducibility, Stability and Interference Tests
2.7. Real Sample Analyses
3. Materials and Methods
3.1. Reagents and Apparatus
3.2. Preparation of p-Gly/rGO/GCE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Amal Raj, M.; Abraham John, S. Simultaneous determination of uric acid, xanthine, hypoxanthine and caffeine in human blood serum and urine samples using electrochemically reduced graphene oxide modified electrode. Anal. Chim. Acta 2013, 771, 14–20. [Google Scholar]
- Zhu, D.; Guo, D.; Zhang, L.; Tan, L.; Pang, H.; Ma, H.; Zhai, M. Non-enzymatic xanthine sensor of heteropolyacids doped ferrocene and reduced graphene oxide via one-step electrodeposition combined with layer-by-layer self-assembly technology. Sens. Actuators B 2019, 281, 893–904. [Google Scholar] [CrossRef]
- Pierini, G.D.; Robledo, S.N.; Zon, M.A.; Di Nezio, M.S.; Granero, A.M.; Fernández, H. Development of an electroanalytical method to control quality in fish samples based on an edge plane pyrolytic graphite electrode. Simultaneous determination of hypoxanthine, xanthine and uric acid. Microchem. J. 2018, 138, 58–64. [Google Scholar] [CrossRef]
- Lavanya, N.; Sekar, C.; Murugan, R.; Ravi, G. An ultrasensitive electrochemical sensor for simultaneous determination of xanthine, hypoxanthine and uric acid based on Co doped CeO2 nanoparticles. Mater. Sci. Eng. C 2016, 65, 278–286. [Google Scholar] [CrossRef]
- Tohgi, H.; Abe, T.; Takahashi, S.; Kikuchi, T. The urate and xanthine concentrations in the cerebrospinal fluid in patients with vascular dementia of the Binswanger type, Alzheimer type dementia, and Parkinson’s disease. J. Neural Transm. Park. Dis. Dement. Sect. 1993, 6, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.; Khosravan, R.; Erdmann, C.; Fiene, J.; Lee, J.W. Quantification of uric acid, xanthine and hypoxanthine in human serum by HPLC for pharmacodynamic studies. J. Chromatogr. B 2006, 837, 1–10. [Google Scholar] [CrossRef]
- Farthing, D.; Sica, D.; Gehr, T.; Wilson, B.; Fakhry, I.; Larus, T.; Farthing, C.; Karnes, H.T. An HPLC method for determination of inosine and hypoxanthine in human plasma from healthy volunteers and patients presenting with potential acute cardiac ischemia. J. Chromatogr. B 2007, 854, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.S.; Saadallah, A.A.A.; Rahbeeni, Z.; Eyaid, W.; Seidahmed, M.Z.; Al-Shahwan, S.; Salih, M.A.M.; Osman, M.E.; Al-Amoudi, M.; Al-Ahaidib, L.; et al. Determination of urinary S-sulphocysteine, xanthine and hypoxanthine by liquid chromatography–electrospray tandem mass spectrometry. Biomed. Chromatogr. 2005, 19, 223–230. [Google Scholar] [CrossRef]
- Causse, E.; Pradelles, A.; Dirat, B.; Negre-Salvayre, A.; Salvayre, R.; Couderc, F. Simultaneous determination of allantoin, hypoxanthine, xanthine, and uric acid in serum/plasma by CE. Electrophoresis 2007, 28, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chu, Q.; Zhang, L.; Ye, J. Separation of six purine bases by capillary electrophoresis with electrochemical detection. Anal. Chim. Acta 2002, 457, 225–233. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Carbon-based functional nanomaterials: Preparation, properties and applications. Compos. Sci. Technol. 2019, 179, 10–40. [Google Scholar]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar]
- Das, S.K.; Kc, C.B.; Ohkubo, K.; Yamada, Y.; Fukuzumi, S.; D’Souza, F. Decorating single layer graphene oxide with electron donor and acceptor molecules for the study of photoinduced electron transfer. Chem. Commun. 2013, 49, 2013–2015. [Google Scholar] [CrossRef]
- Neklyudov, V.V.; Khafizov, N.R.; Sedov, I.A.; Dimiev, A.M. New insights into the solubility of graphene oxide in water and alcohols. Phys. Chem. Chem. Phys. 2017, 19, 17000–17008. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, L.; Thesing, A.; Noremberg, B.D.; Lopes, B.V.; Maron, G.K.; Machado, G.; Pope, M.A.; Carreno, N.L.V. Direct laser writing of poly(furfuryl alcohol)/graphene oxide electrodes for electrochemical determination of ascorbic acid. ChemElectroChem 2012, 9, e202200334. [Google Scholar]
- Sharma, D.; Kanchi, S.; Sabela, M.I.; Bisetty, K. Insight into the biosensing of graphene oxide: Present and future prospects. Arab. J. Chem. 2016, 9, 238–261. [Google Scholar]
- Devadas, B.; Rajkumar, M.; Chen, S.M.; Saraswathi, R. Electrochemically reduced graphene oxide/neodymium hexacyanoferrate modified electrodes for the electrochemical detection of paracetomol. Int. J. Electrochem. Sci. 2012, 7, 3339–3349. [Google Scholar]
- Xi, X.; Ming, L. A voltammetric sensor based on electrochemically reduced graphene modified electrode for sensitive determination of midecamycin. Anal. Methods 2012, 4, 3013–3018. [Google Scholar] [CrossRef]
- Palanisamy, S.; Thirumalraj, B.; Chen, S.M.; Wang, Y.T.; Velusamy, V.; Ramaraj, S.K. A facile electrochemical preparation of reduced graphene oxide@polydopamine composite: A novel electrochemical sensing platform for amperometric detection of chlorpromazine. Sci. Rep. 2016, 6, 33599. [Google Scholar] [CrossRef]
- Mutyala, S.; Mathiyarasu, J. A reagentless non-enzymatic hydrogen peroxide sensor presented using electrochemically reduced graphene oxide modified glassy carbon electrode. Mater. Sci. Eng. C 2016, 69, 398–406. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Ying, Y.; Wu, J. Application of electrochemically reduced graphene oxide on screen-printed ion-selective electrode. Anal. Chem. 2012, 84, 3473–3479. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.A.; John, S.A. Fabrication of electrochemically reduced graphene oxide films on glassy carbon electrode by self-assembly method and their electrocatalytic application. J. Phys. Chem. C 2013, 117, 4326–4335. [Google Scholar] [CrossRef]
- Erdogan Kablan, S.; Yilmaz, A.; Kervan, U.; Ozaltin, N.; Nemutlu, E. Electrochemically based targeted metabolomics for uric acid, xanthine, and hypoxanthine in plasma samples for early diagnosis of acute renal failure after cardiopulmonary bypass using rGO-GCE. Talanta 2023, 253, 124005. [Google Scholar] [CrossRef]
- Kumar, D.; Dhakal, G.; Shafi, P.; Sayed, M.; Lee, J.; Lee, Y.; Shim, J. Sulfite food additive electrochemical determination by nucleophilic addition on poly(4-aminodiphenylamine)-4-aminothiophenol-Au composite electrode. Microchem. J. 2022, 181, 107635. [Google Scholar] [CrossRef]
- Carreno-Vega, O.; Vargas-Zamarripa, M.; Salas, P.; Ramirez-Garcia, G. Poly(allylamine)-copper(II) coordination complex grafted on core@shell upconversion nanoparticles for ultrafast and sensitive determination of the phytohormone salicylic acid in plant extracts. Dalton Trans. 2022, 51, 11630–11640. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Morrin, A.; Li, M.; Liu, N.; Luo, X. Nanomaterial-doped conducting polymers for electrochemical sensors and biosensors. J. Mater. Chem. B 2018, 6, 4173–4190. [Google Scholar] [CrossRef]
- Jadon, N.; Jain, R.; Sharma, S.; Singh, K. Recent trends in electrochemical sensors for multianalyte detection: A review. Talanta 2016, 161, 894–916. [Google Scholar]
- Mathew, M.R.; Kumar, K.G. Poly(amino hydroxy naphthalene sulphonic acid) modified glassy carbon electrode: An effective sensing platform for the simultaneous determination of xanthine and hypoxanthine. J. Electrochem. Soc. 2020, 167, 047519. [Google Scholar] [CrossRef]
- Ouyang, X.X.; Luo, L.Q.; Ding, Y.P.; Liu, B.D.; Xu, D.; Huang, A.Q. Simultaneous determination of uric acid, dopamine and ascorbic acid based on poly(bromocresol green) modified glassy carbon electrode. J. Electroanal. Chem. 2015, 748, 1–7. [Google Scholar] [CrossRef]
- Lei, W.; He, P.; Wang, Y.; Zhang, S.; Dong, F.; Liu, H. Soft template interfacial growth of novel ultralong polypyrrole nanowires for electrochemical energy storage. Electrochimica Acta 2014, 132, 112–117. [Google Scholar] [CrossRef]
- Mîndroiu, M.; Ungureanu, C.; Ion, R.; Pîrvu, C. The effect of deposition electrolyte on polypyrrole surface interaction with biological environment. Appl. Surf. Sci. 2013, 276, 401–410. [Google Scholar] [CrossRef]
- Chen, J.; He, P.; Bai, H.; Lei, H.; Zhang, G.; Dong, F.; Ma, Y. A glassy carbon electrode modified with a nanocomposite consisting of carbon nanohorns and poly(2-aminopyridine) for non-enzymatic amperometric determination of hydrogen peroxide. Microchim. Acta 2016, 183, 3237–3242. [Google Scholar] [CrossRef]
- Koo, A.N.; Lee, H.J.; Kim, S.E.; Chang, J.H.; Park, C.; Kim, C.; Park, J.H.; Lee, S.C. Disulfide-cross-linked PEG-poly(amino acid)s copolymer micelles for glutathione-mediated intracellular drug delivery. Chem. Commun. 2008, 48, 6570–6572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, P.; Feng, W.; Ding, S.; Chen, J.; Li, L.; He, H.; Zhang, S.; Dong, F. Carbon nanohorns/poly(glycine) modified glassy carbon electrode: Preparation, characterization and simultaneous electrochemical determination of uric acid, dopamine and ascorbic acid. J. Electroanal. Chem. 2016, 760, 24–31. [Google Scholar] [CrossRef]
- Gilbert, O.; Kumara Swamy, B.E.; Chandra, U.; Sherigara, B.S. Simultaneous detection of dopamine and ascorbic acid using polyglycine modified carbon paste electrode: A cyclic voltammetric study. J. Electroanal. Chem. 2009, 636, 80–85. [Google Scholar] [CrossRef]
- Thomas, T.; Mascarenhas, R.J.; Swamy, B.E.K.; Martis, P.; Mekhalif, Z.; Sherigara, B.S. Multi-walled carbon nanotube/poly(glycine) modified carbon paste electrode for the determination of dopamine in biological fluids and pharmaceuticals. Colloids Surf. B 2013, 110, 458–465. [Google Scholar] [CrossRef]
- Harisha, K.V.; Kumara Swamy, B.E.; Ebenso, E.E. Poly(glycine) modified carbon paste electrode for simultaneous determination of catechol and hydroquinone: A voltammetric study. J. Electroanal. Chem. 2018, 823, 730–736. [Google Scholar] [CrossRef]
- Pashazadeh, S.; Habibi, B. Simultaneous determination of benzenediols isomers using copper nanoparticles/poly (glycine)/graphene oxide nanosheets modified glassy carbon electrode. J. Electrochem. Soc. 2020, 167, 167504–167514. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, L.L. Electrochemical sensor for simultaneous determination of uric acid, xanthine and hypoxanthine based on poly(bromocresol purple) modified glassy carbon electrode. Sens. Actuators B 2010, 150, 43–49. [Google Scholar] [CrossRef]
- Hoan, N.T.V.; Minh, N.N.; Trang, N.T.H.; Thuy, L.T.T.; Van Hoang, C.; Mau, T.X.; Vu, H.X.A.; Thu, P.T.K.; Phong, N.H.; Khieu, D.Q. Simultaneous voltammetric determination of uric acid, xanthine, and hypoxanthine using CoFe2O4/reduced graphene oxide-modified electrode. J. Nanomater. 2020, 2020, 9797509. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Zhang, Y.; Zheng, Z.; Wang, C.; Du, Y.; Ye, W. Simultaneous electrochemical determination of uric acid, xanthine and hypoxanthine based on poly(L-arginine)/graphene composite film modified electrode. Talanta 2012, 93, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Zen, J.M.; Lai, Y.Y.; Yang, H.H.; Kumar, A.S. Multianalyte sensor for the simultaneous determination of hypoxanthine, xanthine and uric acid based on a preanodized nontronite-coated screen-printed electrode. Sens. Actuators B 2002, 84, 237–244. [Google Scholar] [CrossRef]
- Pundir, C.S.; Devi, R. Biosensing methods for xanthine determination: A review. Enzym. Microb. Technol. 2014, 57, 55–62. [Google Scholar] [CrossRef] [PubMed]




| Electrode | Analyte | Linear Range (μM) | Detection Limit (μM) | Reference |
|---|---|---|---|---|
| rGO | XT HX | 10–60 10–60 | 0.11 0.32 | [1] |
| Poly(bromocresol purple) | XT HX | 0.1–100 20–80 | 0.06 0.12 | [39] |
| CoFe2O4/rGO | XT HX | 2–10 2–10 | 0.650 0.506 | [40] |
| Poly(L-arginine)/graphene | XT HX | 0.1–10 0.2–20 | 0.05 0.1 | [41] |
| Preanodized nontronite | XT HX | 2.0–40 4.0–30 | 0.07 0.34 | [42] |
| p-Gly/rGO | XT HX | 1–100 1–340 | 0.1 0.2 | This work |
| Serum Sample | Analyte | Added (μM) | Founded (μM) | Recovery (%) |
|---|---|---|---|---|
| 1 | XT HX | 20 | 18.7 19.1 | 93.5 95.5 |
| 2 | XT HX | 40 | 40.8 41.2 | 102 103 |
| 3 | XT HX | 60 | 58.5 60.8 | 97.5 101.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Zhang, L.; Zhang, C.; Deng, D.; Wang, D.; Luo, L. Simultaneous Determination of Xanthine and Hypoxanthine Using Polyglycine/rGO-Modified Glassy Carbon Electrode. Molecules 2023, 28, 1458. https://doi.org/10.3390/molecules28031458
Wang T, Zhang L, Zhang C, Deng D, Wang D, Luo L. Simultaneous Determination of Xanthine and Hypoxanthine Using Polyglycine/rGO-Modified Glassy Carbon Electrode. Molecules. 2023; 28(3):1458. https://doi.org/10.3390/molecules28031458
Chicago/Turabian StyleWang, Ting, Lin Zhang, Chengyu Zhang, Dongmei Deng, Dejia Wang, and Liqiang Luo. 2023. "Simultaneous Determination of Xanthine and Hypoxanthine Using Polyglycine/rGO-Modified Glassy Carbon Electrode" Molecules 28, no. 3: 1458. https://doi.org/10.3390/molecules28031458





