Ag–NHC Complexes in the π-Activation of Alkynes
Abstract
:1. Introduction
2. Intramolecular Cyclizations
3. CO2 Fixation
4. Hydrofunctionalization Reactions
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arduengo, A.J., III; Dias, H.R.; Calabrese, J.C.; Davidson, F. Homoleptic Carbene-Silver (I) and Carbene-Copper (I) Complexes. Organometallics 1993, 12, 3405–3409. [Google Scholar] [CrossRef]
- Wang, H.M.; Lin, I.J. Facile Synthesis of Silver (I)−Carbene Complexes. Useful Carbene Transfer Agents. Organometallics 1998, 17, 972–975. [Google Scholar] [CrossRef]
- Nolan, S.P. N-Heterocyclic Carbenes in Synthesis, 1st ed.; Wiley: New York, NY, USA, 2006. [Google Scholar]
- Glorius, F. N-Heterocyclic Carbenes in Transition Metal Catalysis, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Nolan, S.P. N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis, 1st ed.; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Díez-Gonzalez, S. N-Heterocyclic Carbenes: From Laboratory to Curiosities to Efficient Synthetic Tools, 2nd ed.; Royal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Nolan, S.; Cazin, C. N-Heterocyclic Carbenes in Catalytic Organic Synthesis, 1st ed.; Thieme: Stuttgart, Germany, 2017. [Google Scholar]
- Huynh, H.V. The Organometallic Chemistry of N-Heterocyclic Carbenes; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, H.D.; Verpoort, F. N-Heterocyclic Carbene Transition Metal Complexes for Catalysis in Aqueous Media. Chem. Soc. Rev. 2012, 41, 7032–7060. [Google Scholar] [CrossRef]
- Liu, W.K.; Gust, R. Metal N-Heterocyclic Carbene Complexes as Potential Antitumor Metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An Overview of N-Heterocyclic Carbenes. Nature 2014, 510, 485. [Google Scholar] [CrossRef]
- Visbal, R.; Gimeno, M.C. N-Heterocyclic Carbene Metal Complexes: Photoluminescence and Applications. Chem. Soc. Rev. 2014, 43, 3551–3574. [Google Scholar] [CrossRef]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef]
- Huynh, H.V. Electronic Properties of N-Heterocyclic Carbenes and Their Experimental Determination. Chem. Rev. 2018, 118, 9457–9492. [Google Scholar] [CrossRef]
- Shi, S.; Nolan, S.P.; Szostak, M. Well-Defined Palladium(II)-NHC (NHC = N-Heterocyclic Carbene) Precatalysts for Cross-Coupling Reactions of Amides and Esters by Selective Acyl CO–X (X = N, O) Cleavage. Acc. Chem. Res. 2018, 51, 2589–2599. [Google Scholar] [CrossRef]
- Zhao, Q.; Meng, G.; Nolan, S.P.; Szostak, M. N-Heterocyclic Carbene Complexes in C–H Activation Reactions. Chem. Rev. 2020, 120, 1981–2048. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, F.S.; Szostak, M. BIAN-NHC Ligands in Transition-Metal-Catalysis: A Perfect Union of Sterically Encumbered, Electronically Tunable N-Heterocyclic Carbenes? Chem. Eur. J. 2021, 27, 4478–4499. [Google Scholar] [CrossRef] [PubMed]
- Melaiye, A.; Simons, R.S.; Milsted, A.; Pingitore, F.; Wesdemiotis, C.; Tessier, C.A.; Youngs, W.J. Formation of water-soluble pincer silver(I)-carbene complexes: A novel antimicrobial agent. J. Med. Chem. 2004, 47, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Garrison, J.C.; Youngs, W.J. Ag(I) N-Heterocyclic Carbene Complexes: Synthesis, Structure, and Application. Chem. Rev. 2005, 105, 3978–4008. [Google Scholar] [CrossRef]
- Ray, S.; Mohan, R.; Singh, J.K.; Samantaray, M.K.; Shaikh, M.M.; Panda, D.; Ghosh, P. Anticancer and Antimicrobial Metallopharmaceutical Agents Based on Palladium, Gold, and Silver N-Heterocyclic Carbene Complexes. J. Am. Chem. Soc. 2007, 129, 15042–15053. [Google Scholar] [CrossRef]
- Johnson, N.A.; Southerland, M.R.; Youngs, W.J. Recent Developments in the Medicinal Applications of Silver-NHC Complexes and Imidazolium Salts. Molecules 2017, 22, 1263. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Luan, S.; Yin, Z.; He, M.; He, C.; Yin, L.; Zou, Y.; Yuan, Z.; Li, L.; Song, X.; et al. Recent advances in the medical use of silver complex. Eur. J. Med. Chem. 2018, 157, 62–80. [Google Scholar] [CrossRef]
- Hussaini, S.Y.; Haque, R.A.; Razali, M.R. Recent Progress in Silver(I)-, Gold(I)/(III)- and Palladium(II)-N-Heterocyclic Carbene Complexes: A Review Towards Biological Perspectives. J. Organomet. Chem. 2019, 882, 96–111. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Yamamoto, Y. Introduction: Coinage metals in organic synthesis. Chem. Rev. 2008, 108, 2793–2795. [Google Scholar] [CrossRef]
- Patil, N.T.; Yamamoto, Y. Coinage metal-assisted synthesis of heterocycles. Chem. Rev. 2008, 108, 3395–3442. [Google Scholar] [CrossRef]
- Lin, J.C.Y.; Huang, R.T.W.; Lee, C.S.; Bhattacharyya, A.; Hwang, W.S.; Lin, I.J.B. Coinage Metal–N-Heterocyclic Carbene Complexes. Chem. Rev. 2009, 109, 3561–3598. [Google Scholar] [CrossRef] [PubMed]
- Jazzar, R.; Soleilhavoup, M.; Bertrand, G. Cyclic (Alkyl)- and (Aryl)-(Amino)Carbene Coinage Metal Complexes and Their Applications. Chem. Rev. 2020, 120, 4141–4168. [Google Scholar] [CrossRef]
- Metal Prices: Gold, Silver, Copper and More. Available online: https://thestockmarketwatch.com/metal/prices.aspx (accessed on 30 December 2022).
- Haynes, W.M. Handbook of Chemistry and Physics, 96th ed.; Haynes, W.M., Bruno, T.J., Lide, D.R., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Weibel, J.-M.; Blanc, A.; Pale, P. Ag-Mediated Reactions: Coupling and Heterocyclization Reactions. Chem. Rev. 2008, 108, 3149–3173. [Google Scholar] [CrossRef]
- Muñoz, M.P. Silver and Platinum-Catalysed Addition of O-H and N-H Bonds to Allenes. Chem. Soc. Rev. 2014, 43, 3164–3183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, G.; Bi, X. Silver-Catalyzed Reactions of Alkynes: Recent Advances. Chem. Soc. Rev. 2015, 44, 8124–8173. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.-Z.; Jiao, N. Ag-Catalyzed C–H/C–C Bond Functionalization. Chem. Soc. Rev. 2016, 45, 4590–4627. [Google Scholar] [CrossRef]
- Pellissier, H. Enantioselective silver-catalyzed transformations. Chem. Rev. 2016, 116, 14868–14917. [Google Scholar] [CrossRef]
- Sekine, K.; Yamada, T. Silver-catalyzed carboxylation. Chem. Soc. Rev. 2016, 45, 4524–4532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Tzouras, N.V.; Nolan, S.P.; Bi, X. Silver N-Heterocyclic Carbenes: Emerging Powerful Catalysts. Trends Chem 2021, 3, 674–685. [Google Scholar] [CrossRef]
- Wanniarachchi, Y.A.; Khan, M.A.; Slaughter, L.G.M. An Unusually Static, Sterically Hindered Silver Bis(N-heterocyclic carbene) Complex and Its Use in Transmetalation. Organometallics 2004, 23, 5881–5884. [Google Scholar] [CrossRef]
- Green, S.P.; Jones, C.; Mills, D.P.; Stasch, A. Group 9 and 11 Metal(I) Gallyl Complexes Stabilized by N-Heterocyclic Carbene Coordination: First Structural Characterization of Ga–M (M = Cu or Ag) Bonds. Organometallics 2007, 26, 3424–3430. [Google Scholar] [CrossRef]
- Gaillard, S.; Bantreil, X.; Slawin, A.W.; Nolan, S.P. Synthesis and Characterization IPrMe-containing Silver(I), Gold(I) and Gold(III) Complexes. Dalton Trans. 2009, 35, 6967–6971. [Google Scholar] [CrossRef] [PubMed]
- de Frémont, P.; Scott, N.M.; Stevens, E.D.; Ramnial, T.; Lightbody, O.C.; Macdonald, C.L.B.; Clyburne, J.A.C.; Abernethy, C.D.; Nolan, S.P. Synthesis of Well-Defined N-Heterocyclic Carbene Silver(I) Complexes. Organometallics 2005, 24, 6301–6309. [Google Scholar] [CrossRef]
- Ramnial, T.; Abernethy, C.D.; Spicer, M.D.; McKenzie, I.D.; Gay, I.D.; Clyburne, J.A.C. A monomeric imidazol-2-ylidene–silver(I) chloride complex: Synthesis, structure, and solid-state 109Ag and 13C CP/MAS NMR characterization. Inorg. Chem. 2003, 42, 1391–1393. [Google Scholar] [CrossRef]
- Gorin, D.J.; Toste, F.D. Relativistic Effects in Homogeneous Gold Catalysis. Nature 2007, 446, 395–403. [Google Scholar] [CrossRef]
- Fürstner, A.; Davies, P.W. Catalytic Carbophilic Activation: Catalysis by Platinum and Gold π-Acids. Angew. Chem. Int. Ed. 2007, 46, 3410–3449. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide–Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. Recent Advances in Sonogashira Reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. Chemicals from Alkynes with Palladium Catalysts. Chem. Rev. 2014, 114, 1783–1826. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y. Synthesis of Heterocycles via Transition-Metal Catalyzed Hydroarylation of Alkynes. Chem. Soc. Rev. 2014, 43, 1575–1600. [Google Scholar] [CrossRef]
- Kumar, R.K.; Bi, X. Catalytic σ-Activation of Carbon–Carbon Triple Bonds: Reactions of Propargylic Alcohols and Alkynes. Chem. Commun. 2016, 52, 853–868. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Masters, J.T. Transition Metal-Catalyzed Couplings of Alkynes to 1,3-Enynes: Modern Methods and Synthetic Applications. Chem. Soc. Rev. 2016, 45, 2212–2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyarskiy, V.P.; Ryabukhin, D.S.; Bokach, N.A.; Vasilyev, A.V. Alkenylation of Arenes and Heteroarenes with Alkynes. Chem. Rev. 2016, 116, 5894–5986. [Google Scholar] [CrossRef] [PubMed]
- Nechaev, M.S.; Rayon, V.M.; Frenking, G. Energy Partitioning Analysis of the Bonding in Ethylene and Acetylene Complexes of Group 6, 8, and 11 Metals: (CO)5TM–C2Hx and Cl4TM–C2Hx (TM = Cr, Mo, W), (CO)4TM–C2Hx (TM = Fe, Ru, Os), and TM+–C2Hx (TM = Cu, Ag, Au). J. Phys. Chem. A 2004, 108, 3134–3142. [Google Scholar] [CrossRef]
- Shapiro, N.D.; Toste, F.D. Synthesis and Structural Characterization of Isolable Phosphine Coinage Metal π-Complexes. Proc. Natl. Acad. Sci. USA 2008, 105, 2779–2782. [Google Scholar] [CrossRef] [Green Version]
- Fournier, D.; Hoogenboom, R.; Schubert, U.S. Clicking polymers: A straightforward approach to novel macromolecular architectures. Chem. Soc. Rev. 2007, 36, 1369–1380. [Google Scholar] [CrossRef]
- Lutz, J.F.; Zarafshani, Z. Efficient Construction of Therapeutics, Bioconjugates, Biomaterials and Bioactive Surfaces using Azide-Alkyne “Click” Chemistry. Adv. Drug Delivery Rev. 2008, 60, 958–970. [Google Scholar] [CrossRef]
- Debets, M.F.; van Berkel, S.S.; Dommerholt, J.; Dirks, A.J.; Rutjes, F.P.J.T.; van Delft, F.L. Bioconjugation with Strained Alkenes and Alkynes. Acc. Chem. Res. 2011, 44, 805–815. [Google Scholar] [CrossRef]
- Godoi, B.; Schumacher, R.F.; Zeni, G. Synthesis of Heterocycles via Electrophilic Cyclization of Alkynes Containing Heteroatom. Chem. Rev. 2011, 111, 2937–2980. [Google Scholar] [CrossRef]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef]
- Wong, V.H.L.; Vummaleti, S.V.C.; Cavallo, L.; White, A.J.P.; Nolan, S.P.; Hii, K.K. Synthesis, Structure and Catalytic Activity of NHC-Ag-I Carboxylate Complexes. Chem. Eur. J. 2016, 22, 13320–13327. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.; Muller-Bunz, H.; Albrecht, M. Silver(I) NHC Mediated C-C Bond Activation of Alkyl Nitriles and Catalytic Efficiency in Oxazoline Synthesis. Chem. Commun. 2015, 51, 8699–8701. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Reyes, A.; Saxl, T.; Stein, P.M.; Rudolph, M.; Rominger, F.; Asiri, A.M.; Hashmi, A.S.K. Expanded Ring NHC Silver Carboxylate Complexes as Efficient and Reusable Catalysts for Carboxylative Cyclization of Unsubstituted Propargylic Derivatives. ChemSusChem 2021, 14, 2367–2374. [Google Scholar] [CrossRef]
- Ramírez, J.; Corberán, R.; Sanaú, M.; Peris, E.; Fernandez, E. Unprecedented Use of Silver (I) N-Heterocyclic Carbene Complexes for the Catalytic Preparation of 1,2-Bis (Boronate) Esters. Chem. Commun. 2005, 24, 3056–3058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunananda, M.K.; Mankad, N.P. E-Selective Semi-Hydrogenation of Alkynes by Heterobimetallic Catalysis. J. Am. Chem. Soc. 2015, 137, 14598–14601. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Li, X.; Lv, A.; Li, X.; Wang, Z.; Wang, R.; Ma, Y.; Fang, R.; Szostak, R.; et al. Thiazol-2-ylidenes as N-Heterocyclic carbene ligands with enhanced electrophilicity for transition metal catalysis. Commun Chem. 2022, 5, 60. [Google Scholar] [CrossRef]
- Corberan, R.; Ramírez, J.; Poyatos, M.; Peris, E.; Fernández, E. Coinage Metal Complexes with N-Heterocyclic Carbene Ligands as Selective Catalysts in Diboration Reaction. Tetrahedron Asymmetry 2006, 17, 1759–1762. [Google Scholar] [CrossRef]
- Weyrauch, J.P.; Hashmi, A.S.K.; Schuster, A.; Hengst, T.; Schetter, S.; Littmann, A.; Rudolph, M.; Hamzic, M.; Visus, J.; Rominger, F.; et al. Cyclization of propargylic amides: Mild access to oxazole derivatives. Chem. Eur. J. 2010, 16, 956–963. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Schuster, A.M.; Gaillard, S.; Cavallo, L.; Poater, A.; Nolan, S.P. Selectivity Switch in the Synthesis of Vinylgold(I) Intermediates. Organometallics 2011, 30, 6328–6337. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Schuster, A.M.; Schmuck, M.; Rominger, F. Gold-catalyzed cyclization of nonterminal propargylic amides to substituted alkylideneoxazolines and -oxazines. Eur. J. Org. Chem. 2011, 2011, 4595–4602. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Li, T.; Zhang, G.; Wan, K.; Ma, Y.; Fang, S.; Szostak, R.; Szostak, M. Copper(I)–Thiazol-2-ylidenes: Highly Reactive N-Heterocyclic Carbenes for the Hydroboration of Terminal and Internal Alkynes. Ligand Development, Synthetic Utility, and Mechanistic Studies. ACS Catal. 2022, 12, 15323–15333. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, X.; Wu, J. Silver Triflate and N-Heterocyclic Carbene Co-Catalyzed Reaction of N′-(2-Alkynylbenzylidene)Hydrazide, Methanol with α,β- Unsaturated Aldehyde. Chem. Commun. 2010, 46, 6356–6358. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, A.; Heinrich, N.F.; Kohl, S.R.; Hokamp, M.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. A Silver-Catalyzed Modular Intermolecular Access to 6,6-Spiroketals. Adv. Synth. Catal. 2019, 361, 5605–5615. [Google Scholar] [CrossRef]
- Sarbajna, A.; Sadhukhan, N.; Saha, S.; Bera, J.K. Ferrocene-appended anionic N-heterocyclic carbene and its complex with silver (I): Synthesis, structure and catalytic evaluation. Indian J. Chem. 2013, 52, 1072–1077. [Google Scholar]
- Tortajada, A.; Juliá-Hernández, F.; Börjesson, M.; Moragas, T.; Martin, R. Transition-Metal-Catalyzed Carboxylation Reactions with Carbon Dioxide. Angew. Chem., Int. Ed. 2018, 57, 15948–15982. [Google Scholar] [CrossRef]
- Dabral, S.; Schauba, T. The Use of Carbon Dioxide (CO2) as a Building Block in Organic Synthesis from an Industrial Perspective. Adv. Synth. Catal. 2019, 361, 223–246. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Qi, C.; He, H.; Jiang, H.; Ren, Y.; Yuan, G. Polystyrene-Supported N-Heterocyclic Carbene-Silver Complexes as Robust and Efficient Catalysts for the Reaction of Carbon Dioxide and Propargylic Alcohols. Adv. Synth. Catal. 2013, 355, 2019–2028. [Google Scholar] [CrossRef]
- Yamashita, K.; Hase, S.; Kayaki, Y.; Ikariya, K. Highly selective carboxylative cyclization of allenylmethylamines with carbon dioxide Using N-heterocyclic carbene-silver(I) catalysts. Org. Lett. 2015, 17, 2334–2337. [Google Scholar] [CrossRef]
- Whyte, A.; Torelli, A.; Mirabi, B.; Zhang, A.; Lautens, M. Copper-Catalyzed Borylative Difunctionalization of π-Systems. ACS Catal. 2020, 10, 11578–11622. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, J.; Lu, Z. Recent advances in metal-catalysed asymmetric sequential double hydrofunctionalization of alkynes. Chem. Commun. 2020, 56, 2229–2239. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, Z.; Chen, J.; Chen, X.; Lu, Z. Iron- and Cobalt-Catalyzed Asymmetric Hydrofunctionalization of Alkenes and Alkynes. Acc. Chem. Res. 2021, 54, 2701–2716. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Terao, J.; Kambe, N. Silver-Catalyzed Carbomagnesiation of Terminal Aryl and Silyl Alkynes and Enynes in the Presence of 1,2-Dibromoethane. Chem. Commun. 2009, 9, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kageyuki, I.; Takaki, K. Silver-Catalyzed Highly Regioselective Formal Hydroboration of Alkynes. Org. Lett. 2014, 16, 3512–3515. [Google Scholar] [CrossRef] [PubMed]
- Farley, C.M.; Uyeda, C. Organic Reactions Enabled by Catalytically Active Metal-Metal Bonds. Trends Chem. 2019, 1, 497–509. [Google Scholar] [CrossRef]
- Lee, M.T.; Goodstein, M.B.; Lalic, G. Synthesis of Isomerically Pure (Z)-Alkenes from Terminal Alkynes and Terminal Alkenes: Silver-Catalyzed Hydroalkylation of Alkynes. J. Am. Chem. Soc. 2019, 141, 17086–17091. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, X.-S.; Fu, Y.; Ji, P. Comprehensive Basicity Scales for N-Heterocyclic Carbenes in DMSO: Implications on the Stabilities of N-Heterocyclic Carbene and CO2 Adducts. Chem. Asian J. 2020, 15, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Pandey, A.K.; Sharma, S.; Pandey, M.; Alam, M.M.; Shaquiquzzaman, M.; Akhter, M. 4,5-Dihydrooxazole-pyrazoline hybrids: Synthesis and their evaluation as potential antimalarial agents. Eur. J. Med. Chem. 2016, 123, 476–486. [Google Scholar] [CrossRef]
- Chen, J.J.; Lin, W.J.; Liao, C.H.; Shieh, P.C. Anti-inflammatory benzenoids from Antrodia camphorata. J. Nat. Prod. 2007, 70, 989–992. [Google Scholar] [CrossRef]
- Jandu, K.S.; Barrett, V.; Brockwell, M.; Cambridge, D.; Farrant, D.R.; Foster, C.; Giles, H.; Glen, R.C.; Hill, A.P.; Hobbs, H.; et al. Discovery of 4-[3-(trans-3-Dimethylaminocyclobutyl)-1H-indol-5-ylmethyl]- (4S)-oxazolidin-2-one (4991W93), a 5HT1B/1D Receptor Partial Agonist and a Potent Inhibitor of Electrically Induced Plasma Extravasation. J. Med. Chem. 2001, 44, 681–693. [Google Scholar] [CrossRef]
- Matada, B.S.; Pattanashettar, R.; Yernale, N.G. Comprehensive Review on the Biological Interest of Quinoline and Its Derivatives. Bioorg. Med. Chem. 2021, 32, 115973. [Google Scholar] [CrossRef]
- Bodor, N.; Farag, H.H.; Barros, M.D.C.; Wu, W.-M.; Buchwald, P. In Vitro and In Vivo Evaluations of Dihydroquinoline- and Dihydroisoquinoline-based Targetor Moieties for Brain-specific Chemical Delivery Systems. J. Drug Target. 2002, 10, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, T.; Milburn, R.; Yates, J.; Hinnant, E.; Luzzio, M.J.; Noble, S.A.; Attardo, G. Novel 1,3-disubstituted-5,10-dihydro-5,10-dioxo-1H-mbenzo[g]isochromene-3-carboxamides as potent antitumor agents. Bioorg. Med. Chem. Lett. 1998, 8, 1579–1584. [Google Scholar] [CrossRef] [PubMed]



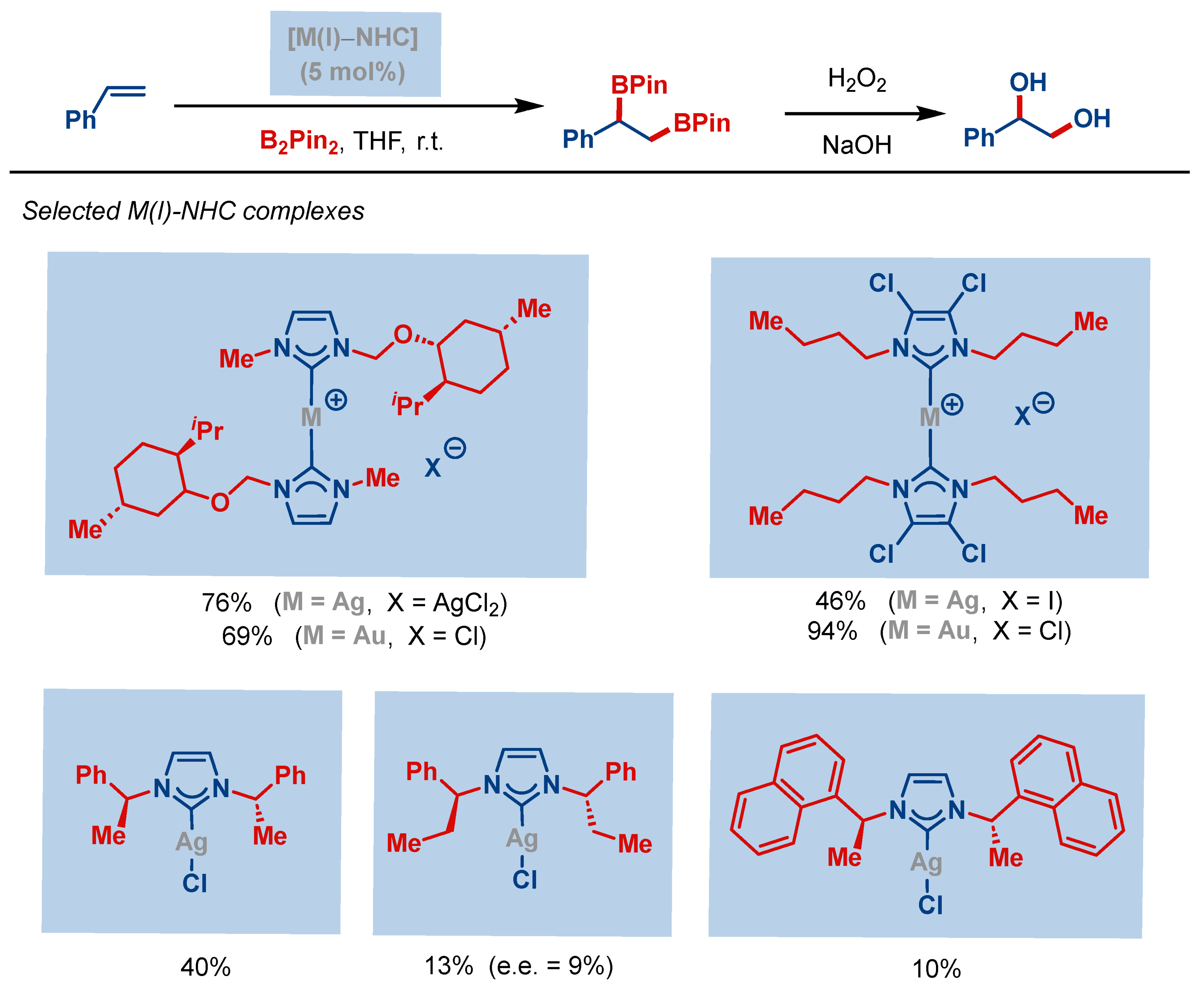
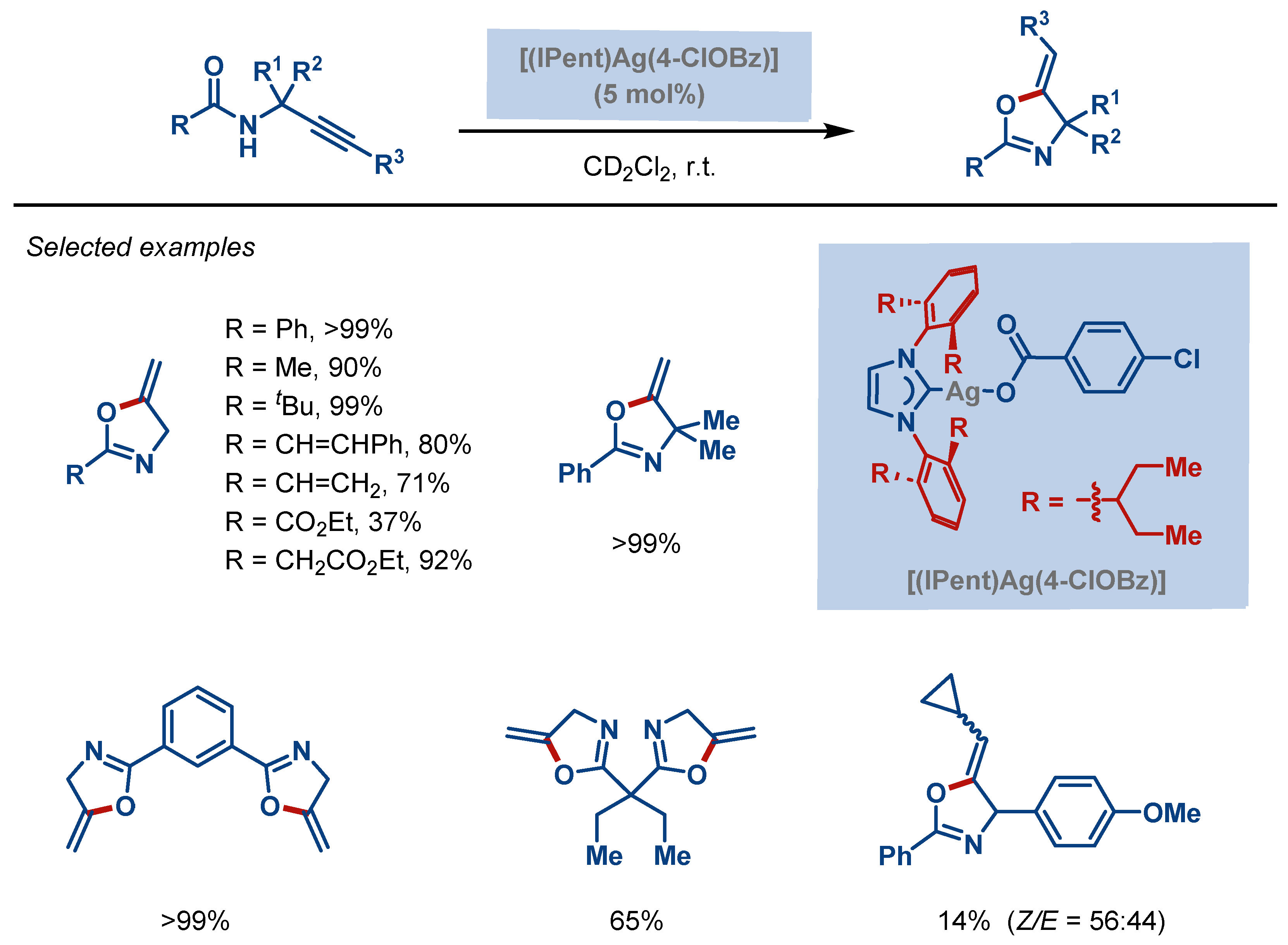
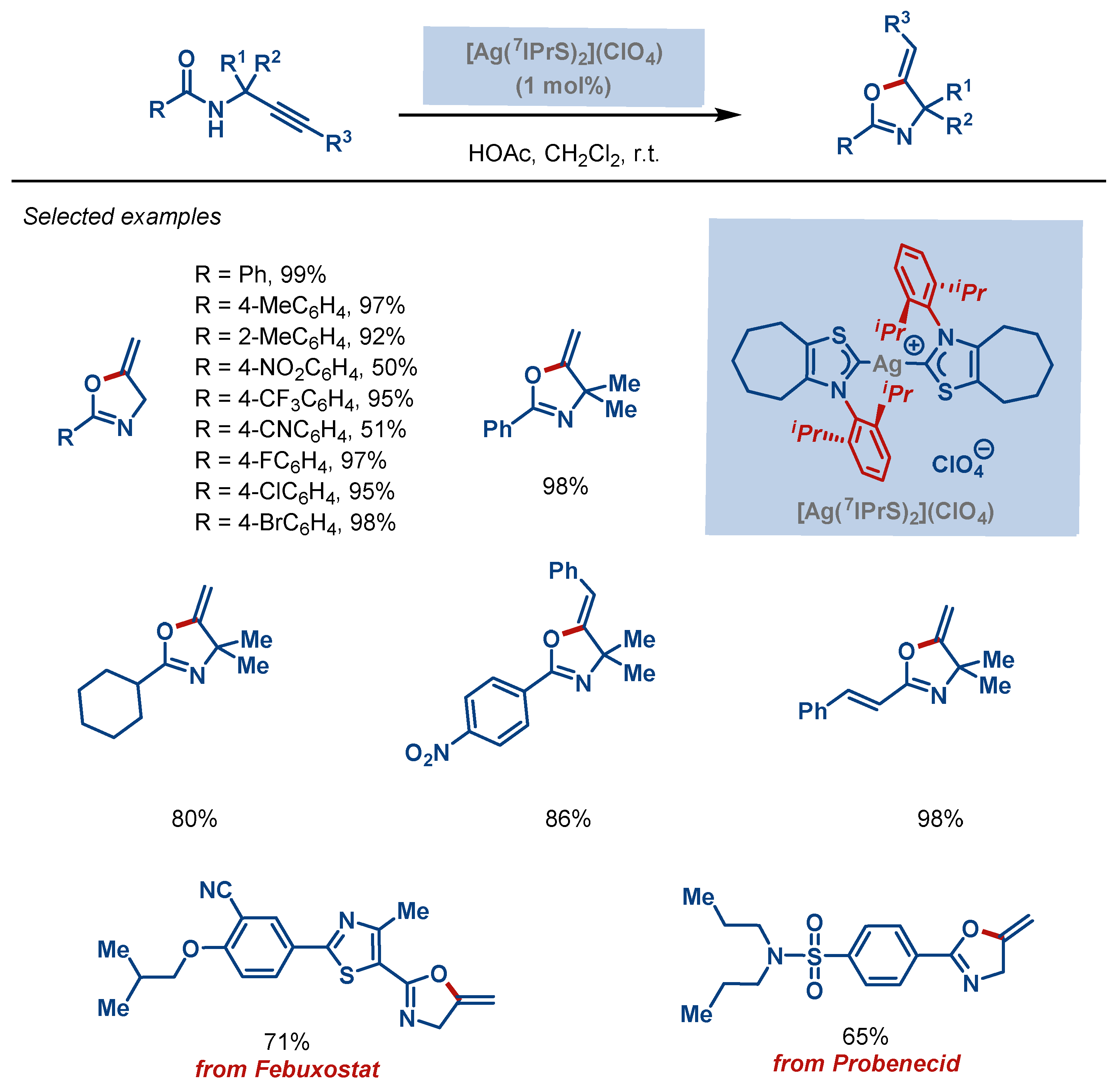

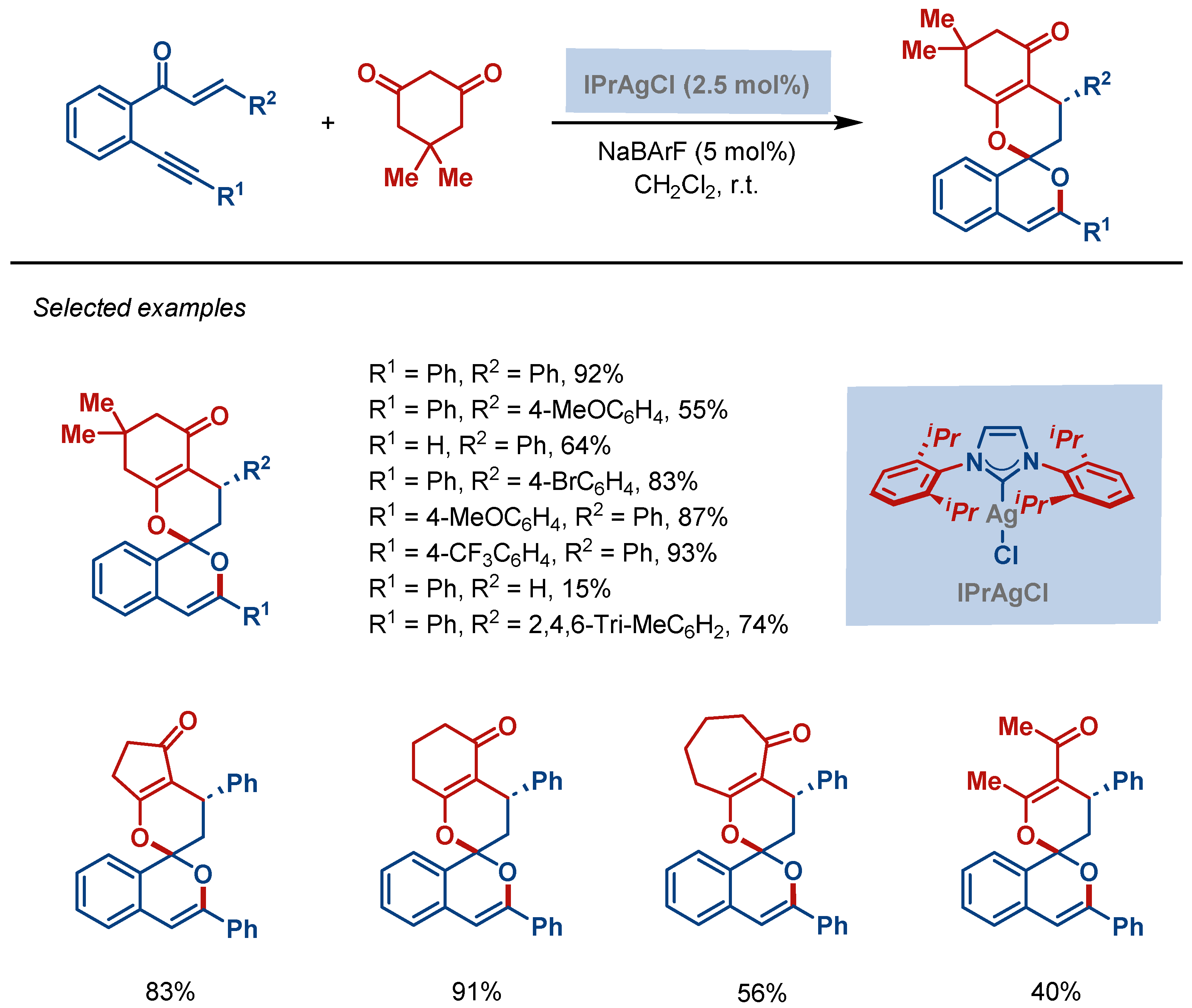
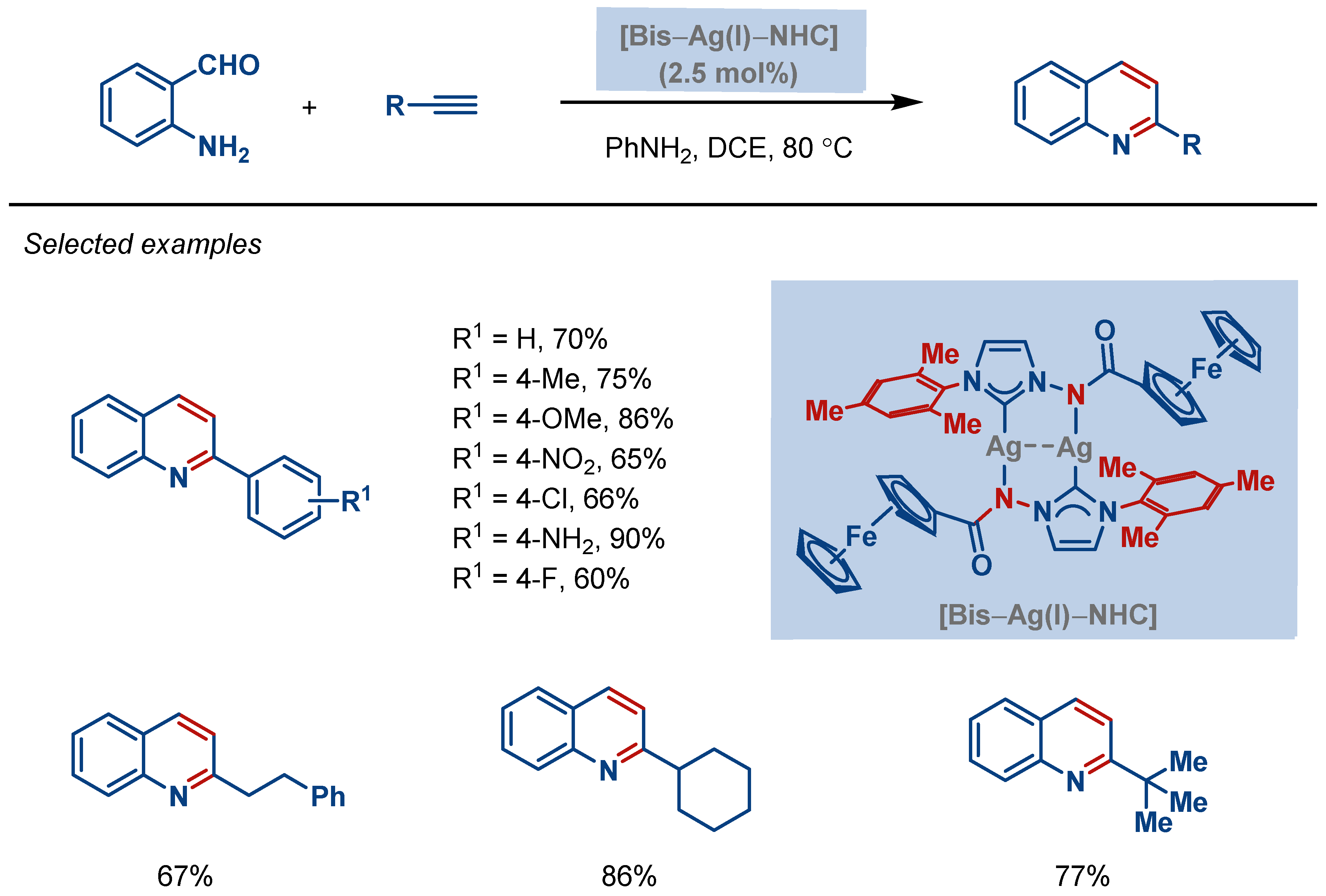


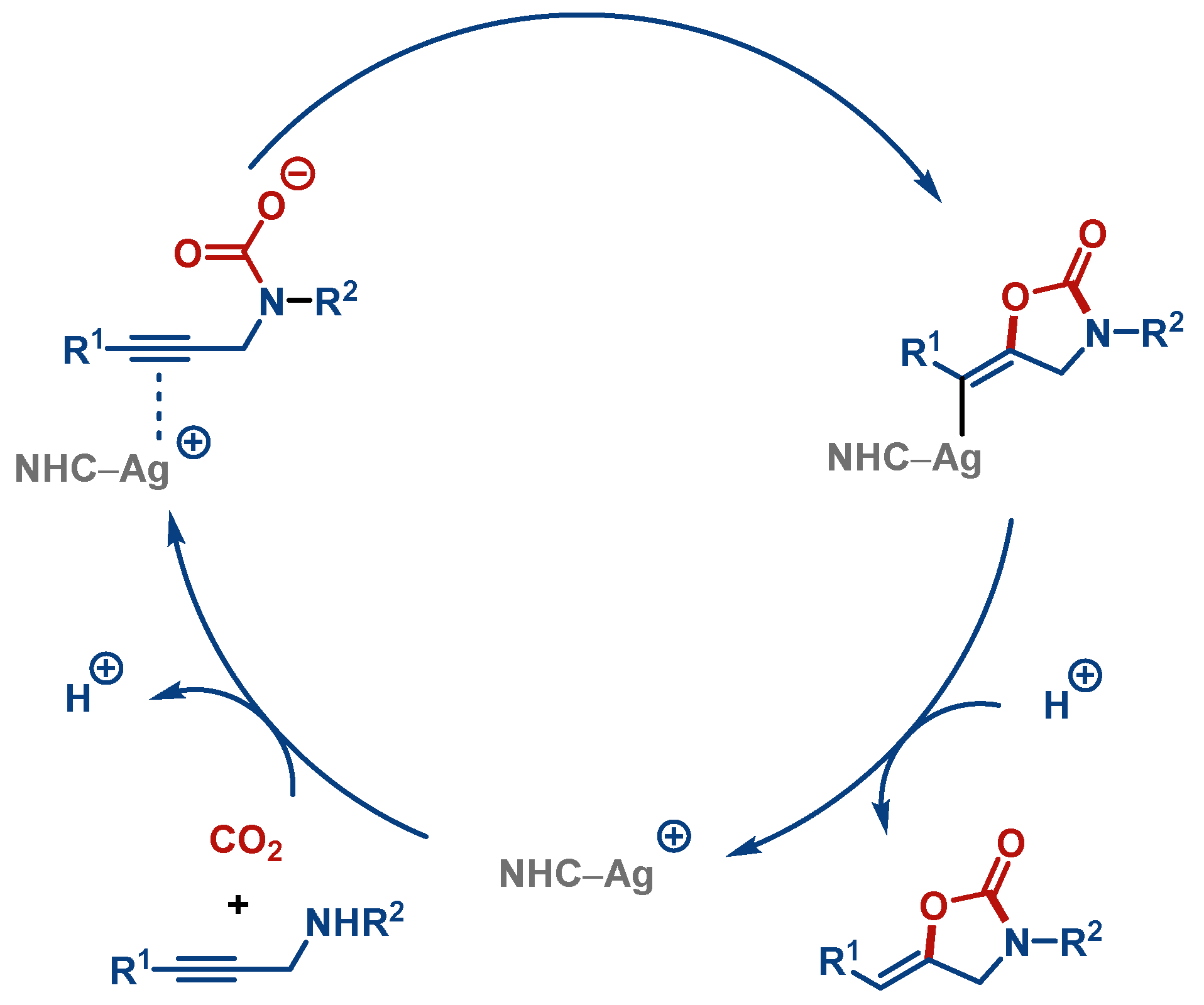


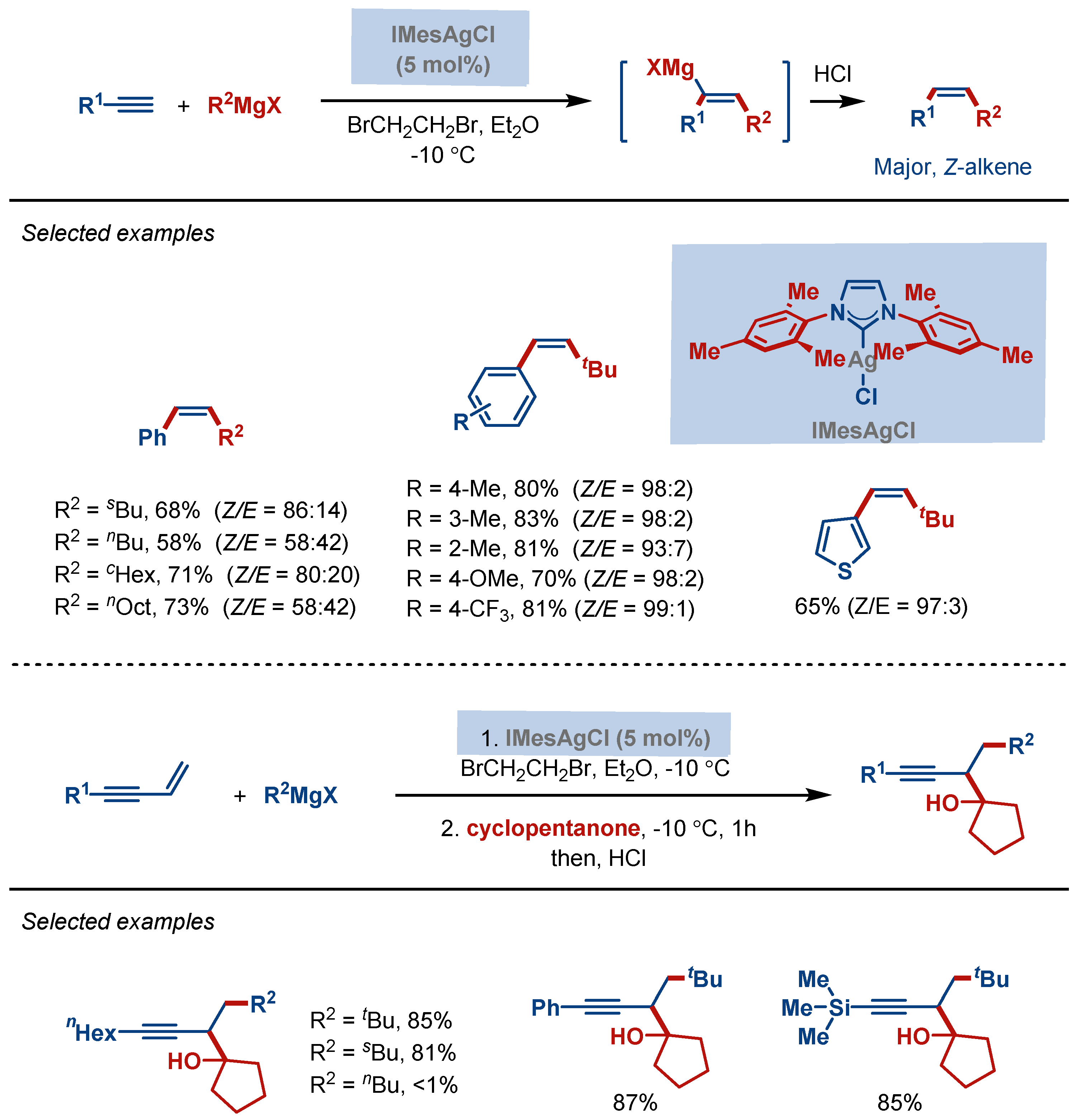
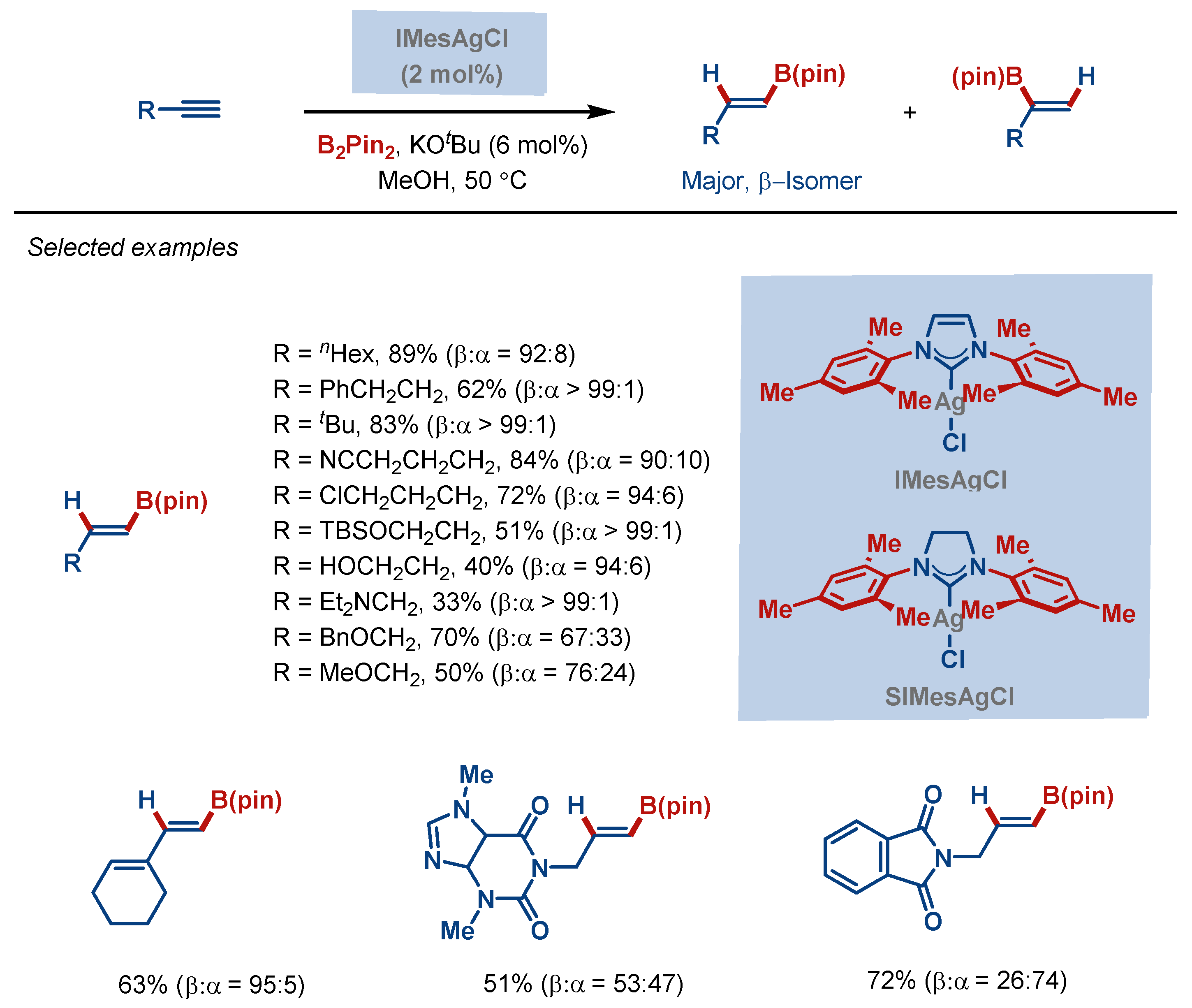

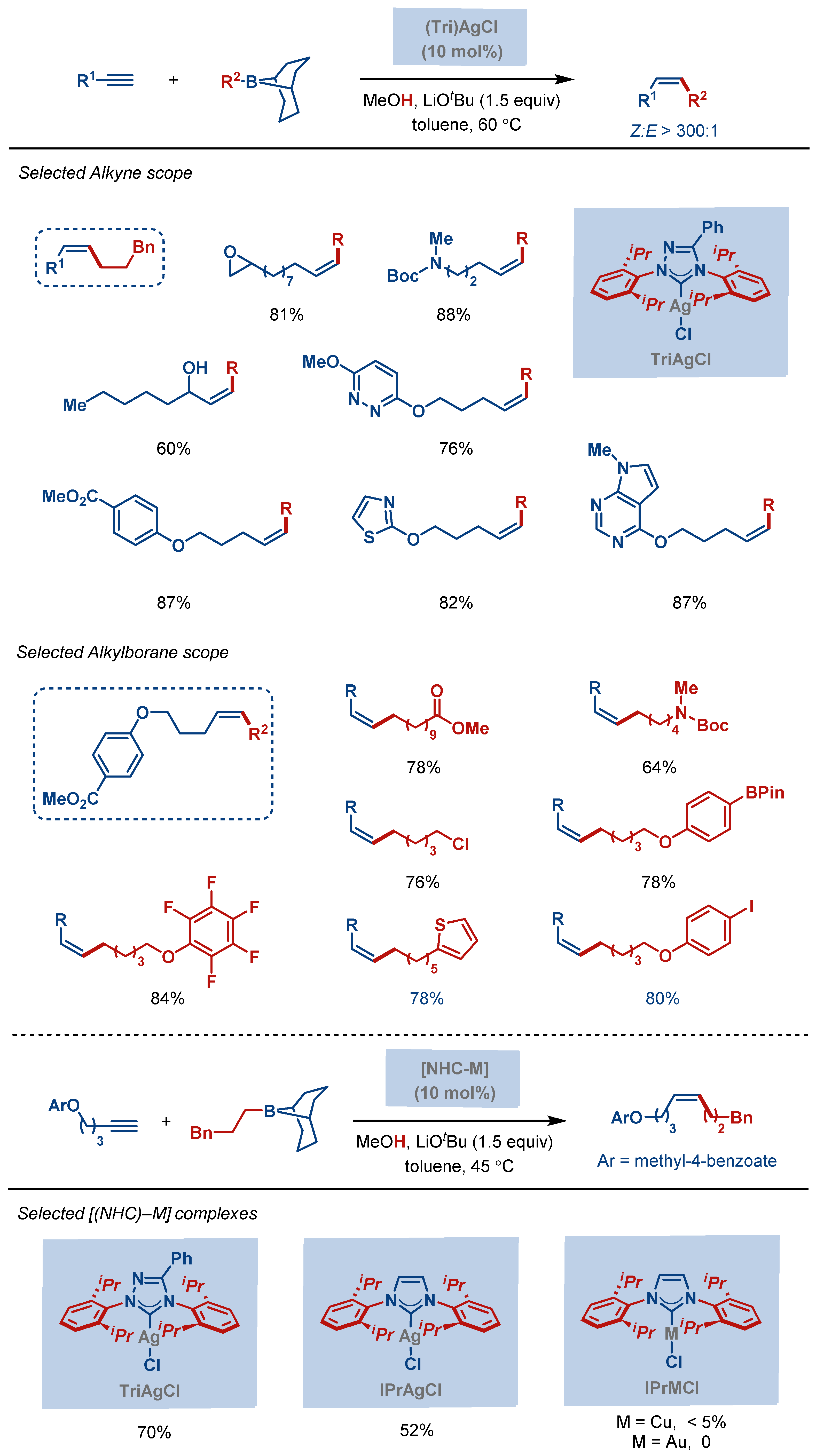

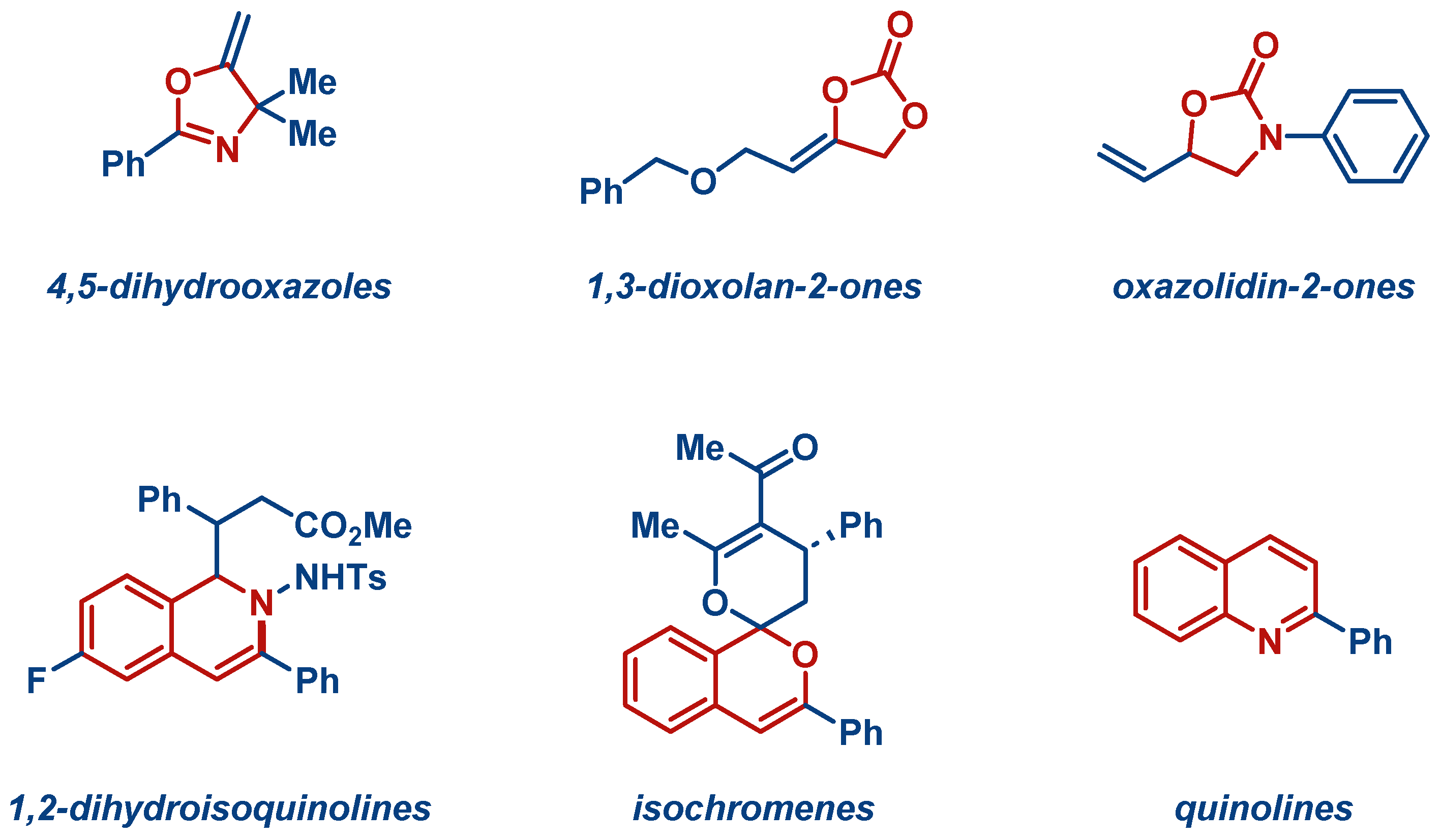
| Entry | Complex No. | [(NHC)Ag(X)] | Ag–C Bond Length (Å) | Ag–X Bond Length (Å) | Reference |
|---|---|---|---|---|---|
| 1 | 1 | [(IPr)Ag(Cl)] | 2.056 | 2.313 (X = Cl) | [41] |
| 2 | 2 | [(IMes)Ag(Cl)] | 2.056 | 2.314 (X = Cl) | [42] |
| 3 | 3a | [(IPent)Ag(OAc)] | 2.067 | 2.111 (X = OAc) | [59] |
| 4 | 3b | [(IPent)Ag(OBz)] | 2.059 | 2.100 (X = OBz) | [59] |
| 5 | 3c | [(IPent)Ag(4-ClOBz)] | 2.064 | 2.100 (X = 4-ClOBz) | [59] |
| 6 | 4a | [(Trz)Ag(CN)] | 2.087 | 2.073 (X = CN) | [60] |
| 7 | 4b | [(Trz)Ag(I)] | 2.091 | 2.636 (X = I) | [60] |
| 8 | 7a | [(BPDPr)Ag(OAc)] | 2.089 | 2.112 (X = OAc) | [61] |
| 9 | 7b | [(BPDPr)Ag(OBz)] | 2.089 | 2.122 (X = OBz) | [61] |
| 10 | 8 | [(mentimid)2Ag(AgCl2)] | 2.102 | 2.952 (X = AgCl2) | [62] |
| 11 | 9 | [(IMes)Ag(RuCp(CO)2)] | 2.111 | 2.617 (X = RuCp(CO)2) | [63] |
| 12 | 10 | [Ag(7IPrS)2]+[ClO4]− | 2.081 | - | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhou, T.; Yu, X.; Szostak, M. Ag–NHC Complexes in the π-Activation of Alkynes. Molecules 2023, 28, 950. https://doi.org/10.3390/molecules28030950
Yang S, Zhou T, Yu X, Szostak M. Ag–NHC Complexes in the π-Activation of Alkynes. Molecules. 2023; 28(3):950. https://doi.org/10.3390/molecules28030950
Chicago/Turabian StyleYang, Shiyi, Tongliang Zhou, Xiang Yu, and Michal Szostak. 2023. "Ag–NHC Complexes in the π-Activation of Alkynes" Molecules 28, no. 3: 950. https://doi.org/10.3390/molecules28030950





