Emerging Immunotherapeutic and Diagnostic Modalities in Carcinoid Tumors
Abstract
:1. Introduction
2. Carcinoid
3. Carcinoid Biomarkers
3.1. Carcinoid Biomarkers
3.2. Multianalyte Biomarkers
3.3. Carcinoid Immune Biomarkers
3.3.1. Gastrointestinal Carcinoids
3.3.2. Pulmonary Carcinoids
4. Non-Immune Therapy for Carcinoid
4.1. Advantages and Disadvantages of Surgical Intervention
4.2. Advantages and Disadvantages of Pharmacotherapy
| Intervention | Results |
|---|---|
| STZ with cyclophosphamide [55] | Carcinoids primary to small bowel: overall response rate (ORR) 37% Carcinoids of pulmonary or unknown region: ORR 17% |
| STZ with 5-flourouracil (5-FU) [56] | Metastatic carcinoid tumors: ORR 22% |
| STZ with doxorubicin [57] | Advanced carcinoid tumors: ORR 16% |
| Recombinant IFN-alpha-2a [58] | Metastatic carcinoid tumors: Progression-free survival median of 14.1 months |
| Capecitabine paired with temozolomide [49] | Pancreatic, lung, and small bowel-origin NETs: ORR 21% |
| Immunotherapy | Target | Carcinoid Typed |
|---|---|---|
| Combined Ipilimumab/Nivolumab | CTLA-4/PD-1 | Locations 32 patients: 18 with high-grade disease, 10 with intermediate-grade disease, and 4 with low-grade disease. Gastrointestinal (GI): 15, Lung: 6 NCT02834013 [59,60] |
| Pembrolizumab | PD-1 | 25 PD-1-positive advanced or metastatic carcinoid tumors. Lung: 9, GI: 7, Other: 9 NCT02054806 [61] |
| Pembrolizumab | PD-1 | GI tumors: 14, Pancreatic NETs: 8 NCT03043664 [62] |
| Peptide Receptor Radionuclide Therapy (PRRT) 177 Lu-Dotatate | SSTR (somatostatin receptor) | Midgut carcinoid tumors NCT01578239 [63,64] |
| Spartalizumab (PDR001) | PD-1 | Advanced NETs from pancreatic, GI, and thoracic origins including 116 pts with well-differentiated NETs NCT02955069 [65] |
| Tidutamab (previously XmAb18087) | SSTR2 and CD3 | Advanced NETs including 41 participants comprised of the following: 46% pancreas, 22% intestine, 20% lung, and 12% GEP-NET/unknown NCT03411915 [66] |
| AdVince | Recombinant Adenovirus | Treat liver metastases from NETs including metastatic midgut carcinoids that express Chromogranin A NCT02749331 [67] |
5. Immunotherapy for Carcinoid
5.1. Overview of Immunotherapy and Carcinoid [27]
5.2. Active Immunotherapy for Carcinoid
5.2.1. Ipilimumab/Nivolumab
5.2.2. Pembrolizumab
5.2.3. Spartalizumab
5.3. Passive Immunotherapy for Carcinoid
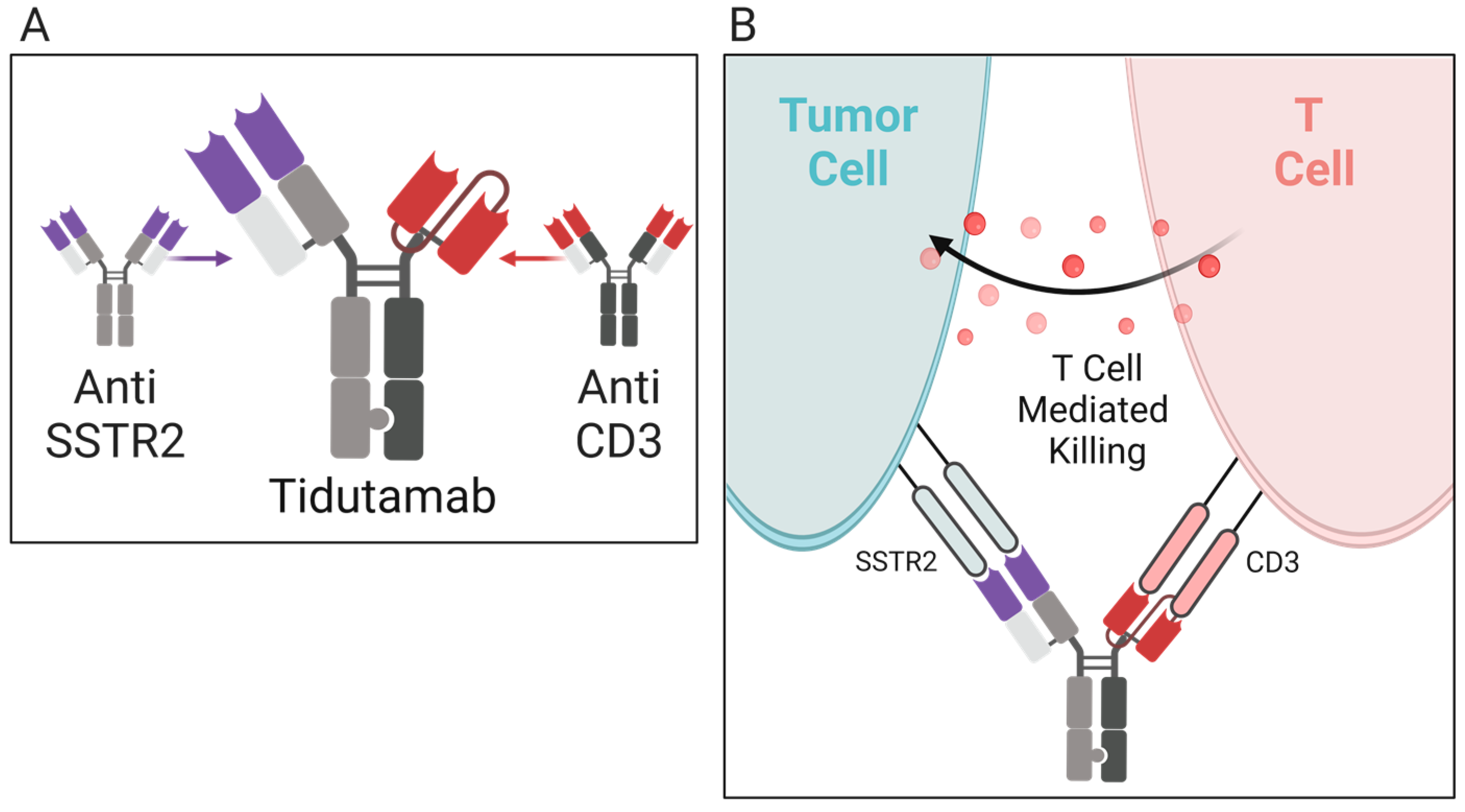
5.3.1. Tidutamab
5.3.2. 177Lu-Dotatate
6. Conclusions
7. Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breasted, J.H. (Ed.) The Edwin Smith Surgical Papyrus: Published in Facsimile and Hieroglyphic Transliteration with Translation and Commentary in Two Volumes; University of Chicago Press: Chicago, IL, USA, 1930. [Google Scholar]
- Hajdu, S.I. A note from history: Landmarks in history of cancer, part 1. Cancer 2011, 117, 1097–1102. [Google Scholar] [CrossRef]
- Di Lonardo, A.; Nasi, S.; Pulciani, S. Cancer: We Should Not Forget The Past. J. Cancer 2015, 6, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Haridy, Y.; Witzmann, F.; Asbach, P.; Schoch, R.R.; Fröbisch, N.; Rothschild, B.M. Triassic Cancer—Osteosarcoma in a 240-Million-Year-Old Stem-Turtle. JAMA Oncol. 2019, 5, 425–426. [Google Scholar] [CrossRef]
- Inthagard, J.; Edwards, J.; Roseweir, A.K. Immunotherapy: Enhancing the efficacy of this promising therapeutic in multiple cancers. Clin. Sci. 2019, 133, 181–193. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.M.; Phan, A.T.; Dagohoy, C.G.; Leary, C.C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [Green Version]
- Cingam, S.R.; Kashyap, S.; Karanchi, H. Carcinoid Tumors. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kuracinová, K.M.; Janega, P.; Janegová, A.; Čierna, Z. Histopathology of Neuroendocrine Neoplasms of the Gastrointestinal System. Klin. Onkol. 2018, 31, 167–177. [Google Scholar] [CrossRef]
- Manrow, R.E.; Beckwith, M.; Johnson, L.E. NCI’s Physician Data Query (PDQ®) Cancer Information Summaries: History, Editorial Processes, Influence, and Reach. J. Cancer Educ. 2013, 29, 198–205. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Oiseth, S.J.; Aziz, M.S. Cancer immunotherapy: A brief review of the history, possibilities, and challenges ahead. J. Cancer Metastasis Treat. 2017, 3, 250. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Coley, W.B. The treatment of malignant tumors by repeated inoculations of erysipelas: With a report of ten original cases. Am. J. Med. Sci. 1893, 105, 487. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buqué, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.-P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Gu, Z.; Chen, Y.; Chen, B.; Chen, W.; Weng, L.; Liu, X. Application of PD-1 Blockade in Cancer Immunotherapy. Comput. Struct. Biotechnol. J. 2019, 17, 661–674. [Google Scholar] [CrossRef]
- Weber, M.M.; Fottner, C. Immune Checkpoint Inhibitors in the Treatment of Patients with Neuroendocrine Neoplasia. Oncol. Res. Treat. 2018, 41, 306–312. [Google Scholar] [CrossRef]
- Linsley, P.S.; Wallace, P.M.; Johnson, J.; Gibson, M.G.; Greene, J.L.; Ledbetter, J.A.; Singh, C.; Tepper, M.A. Immunosuppression in Vivo by a Soluble Form of the CTLA-4 T Cell Activation Molecule. Science 1992, 257, 792–795. [Google Scholar] [CrossRef]
- Iwai, Y.; Ishida, M.; Tanaka, Y.; Okazaki, T.; Honjo, T.; Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 2002, 99, 12293–12297. [Google Scholar] [CrossRef] [Green Version]
- Thakur, A.; Huang, M.; Lum, L.G. Bispecific antibody based therapeutics: Strengths and challenges. Blood Rev. 2018, 32, 339–347. [Google Scholar] [CrossRef]
- Shim, H. Bispecific Antibodies and Antibody–Drug Conjugates for Cancer Therapy: Technological Considerations. Biomolecules 2020, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; De Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A common classification framework for neuroendocrine neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M. Gastrointestinal neuroendocrine tumors in 2020. World J. Gastrointest. Oncol. 2020, 12, 791–807. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.M.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Gade, A.K.; Olariu, E.; Douthit, N.T. Carcinoid Syndrome: A Review. Cureus 2020, 12, e7186. [Google Scholar] [CrossRef] [Green Version]
- Vinik, A.; Hughes, M.S.; Feliberti, E.; Perry, R.R.; Casellini, C.; Sinesi, M.; Vingan, H.; Johnson, L. Carcinoid Tumors. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Strosberg, J.R. Clinical Characteristics of Well-Differentiated Neuroendocrine (Carcinoid) Tumors Arising in the Gastrointestinal and Genitourinary Tracts; Post, T.W., Ed.; UpToDate: Waltham, MA, USA, 2021. [Google Scholar]
- Al-Toubah, T.; Cives, M.; Strosberg, J. Novel immunotherapy strategies for treatment of neuroendocrine neoplasms. Transl. Gastroenterol. Hepatol. 2020, 5, 54. [Google Scholar] [CrossRef]
- Krishnan, M.; Tuma, F. Intestinal Carcinoid Cancer. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Rossi, R.E.; Elvevi, A.; Citterio, D.; Coppa, J.; Invernizzi, P.; Mazzaferro, V.; Massironi, S. Gastrinoma and Zollinger Ellison syndrome: A roadmap for the management between new and old therapies. World J. Gastroenterol. 2021, 27, 5890–5907. [Google Scholar] [CrossRef]
- Biomarkers Definitions Working Group; Atkinson, A.J., Jr.; Colburn, W.A.; DeGruttola, V.G.; DeMets, D.L.; Downing, G.J.; Hoth, D.F.; Oates, J.A.; Peck, C.C.; Spilker, B.A.; et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar] [CrossRef]
- Capdevila, J.; Meeker, A.; García-Carbonero, R.; Pietras, K.; Astudillo, A.; Casanovas, O.; Scarpa, A. Molecular biology of neuroendocrine tumors: From pathways to biomarkers and targets. Cancer Metastasis Rev. 2013, 33, 345–351. [Google Scholar] [CrossRef]
- Modlin, I.M.; Bodei, L.; Kidd, M. Neuroendocrine tumor biomarkers: From monoanalytes to transcripts and algorithms. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 59–77. [Google Scholar] [CrossRef]
- Malczewska, A.; Kos-Kudła, B.; Kidd, M.; Drozdov, I.; Bodei, L.; Matar, S.; Oberg, K.; Modlin, I.M. The clinical applications of a multigene liquid biopsy (NETest) in neuroendocrine tumors. Adv. Med Sci. 2019, 65, 18–29. [Google Scholar] [CrossRef]
- Modlin, I.M.; Kidd, M.; Malczewska, A.; Drozdov, I.; Bodei, L.; Matar, S.; Chung, K.-M. The NETest. Endocrinol. Metab. Clin. N. Am. 2018, 47, 485–504. [Google Scholar] [CrossRef]
- Liu, E.; Paulson, S.; Gulati, A.; Freudman, J.; Grosh, W.; Kafer, S.; Wickremesinghe, P.C.; Salem, R.R.; Bodei, L. Assessment of NETest Clinical Utility in a U.S. Registry-Based Study. Oncologist 2018, 24, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Oberg, K.; Modlin, I.M.; De Herder, W.; Pavel, M.; Klimstra, D.; Frilling, A.; Metz, D.C.; Heaney, A.; Kwekkeboom, D.J.; Strosberg, J.R.; et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015, 16, e435–e446. [Google Scholar] [CrossRef] [Green Version]
- Onaitis, M.W.; Kirshbom, P.M.; Hayward, T.Z.; Quayle, F.J.; Feldman, J.M.; Seigler, H.F.; Tyler, D.S. Gastrointestinal Carcinoids: Characterization by Site of Origin and Hormone Production. Ann. Surg. 2000, 232, 549–556. [Google Scholar] [CrossRef]
- Ito, T.; Igarashi, H.; Jensen, R.T. Serum Pancreastatin. Pancreas 2012, 41, 505–507. [Google Scholar] [CrossRef] [Green Version]
- Cui, T.; Hurtig, M.; Elgue, G.; Li, S.-C.; Veronesi, G.; Essaghir, A.; Demoulin, J.-B.; Pelosi, G.; Alimohammadi, M.; Öberg, K.; et al. Paraneoplastic Antigen Ma2 Autoantibodies as Specific Blood Biomarkers for Detection of Early Recurrence of Small Intestine Neuroendocrine Tumors. PLoS ONE 2010, 5, e16010. [Google Scholar] [CrossRef]
- Kidd, M.; Drozdov, I.; Modlin, I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr.-Relat. Cancer 2015, 22, 561–575. [Google Scholar] [CrossRef]
- Vikman, S.; Sommaggio, R.; De La Torre, M.; Öberg, K.; Essand, M.; Giandomenico, V.; Loskog, A.; Tötterman, T.H. Midgut carcinoid patients display increased numbers of regulatory T cells in peripheral blood with infiltration into tumor tissue. Acta Oncol. 2009, 48, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J.; Al Diffalha, S.; Coppola, D. Analysis of the immune landscape of small bowel neuroendocrine tumors. Endocr.-Relat. Cancer 2019, 26, 119–130. [Google Scholar] [CrossRef]
- Stankovic, B.; Aamodt, H.; Bjørhovde, H.A.K.; Müller, E.; Hammarström, C.; Brustugun, O.T.; Helland, D.; Øynebråten, I.; Corthay, A. The immune microenvironment in typical carcinoid lung tumour, a brief report of four cases. Scand. J. Immunol. 2020, 92, e12893. [Google Scholar] [CrossRef]
- Vesterinen, T.; Kuopio, T.; Ahtiainen, M.; Knuuttila, A.; Mustonen, H.K.; Salmenkivi, K.; Arola, J.; Haglund, C. PD-1 and PD-L1 expression in pulmonary carcinoid tumors and their association to tumor spread. Endocr. Connect. 2019, 8, 1168–1175. [Google Scholar] [CrossRef] [Green Version]
- Reuling, E.; Dickhoff, C.; Plaisier, P.; Bonjer, H.; Daniels, J. Endobronchial and surgical treatment of pulmonary carcinoid tumors: A systematic literature review. Lung Cancer 2019, 134, 85–95. [Google Scholar] [CrossRef] [Green Version]
- Filosso, P.L.; Ruffini, E.; Di Gangi, S.; Guerrera, F.; Bora, G.; Ciccone, G.; Galassi, C.; Solidoro, P.; Lyberis, P.; Oliaro, A.; et al. Prognostic factors in neuroendocrine tumours of the lung: A single-centre experience †. Eur. J. Cardio-Thorac. Surg. 2013, 45, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Thomas, K.; Voros, B.A.; Meadows-Taylor, M.; Smeltzer, M.P.; Griffin, R.; Boudreaux, J.P.; Thiagarajan, R.; Woltering, E.A.; Ramirez, R.A. Outcomes of Capecitabine and Temozolomide (CAPTEM) in Advanced Neuroendocrine Neoplasms (NENs). Cancers 2020, 12, 206. [Google Scholar] [CrossRef] [Green Version]
- Pusceddu, S.; Verzoni, E.; Prinzi, N.; Mennitto, A.; Femia, D.; Grassi, P.; Concas, L.; Vernieri, C.; Russo, G.L.; Procopio, G. Everolimus treatment for neuroendocrine tumors: Latest results and clinical potential. Ther. Adv. Med. Oncol. 2017, 9, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2015, 387, 968–977. [Google Scholar] [CrossRef]
- Larouche, V.; Akirov, A.; Alshehri, S.; Ezzat, S. Management of Small Bowel Neuroendocrine Tumors. Cancers 2019, 11, 1395. [Google Scholar] [CrossRef] [Green Version]
- Comaru-Schally, A.M.; Schally, A.V. A clinical overview of carcinoid tumors: Perspectives for improvement in treatment using peptide analogs (review). Int. J. Oncol. 2005, 26, 301–309. [Google Scholar] [CrossRef]
- Das, S.; Al-Toubah, T.; Strosberg, J. Chemotherapy in Neuroendocrine Tumors. Cancers 2021, 13, 4872. [Google Scholar] [CrossRef]
- Moertel, C.G.; Hanley, J.A. Combination chemotherapy trials in metastatic carcinoid tumor and the malignant carcinoid syndrome. Cancer Clin. Trials 1979, 2, 327–334. [Google Scholar]
- Engstrom, P.F.; Lavin, P.T.; Moertel, C.G.; Folsch, E.; Douglass, H.O. Streptozocin plus fluorouracil versus doxorubicin therapy for metastatic carcinoid tumor. J. Clin. Oncol. 1984, 2, 1255–1259. [Google Scholar] [CrossRef]
- Sun, W.; Lipsitz, S.; Catalano, P.; Mailliard, J.A.; Haller, D.G. Phase II/III Study of Doxorubicin With Fluorouracil Compared With Streptozocin With Fluorouracil or Dacarbazine in the Treatment of Advanced Carcinoid Tumors: Eastern Cooperative Oncology Group Study E1281. J. Clin. Oncol. 2005, 23, 4897–4904. [Google Scholar] [CrossRef]
- Dahan, L.; Bonnetain, F.; Rougier, P.; Raoul, J.-L.; Gamelin, E.; Etienne, P.-L.; Cadiot, G.; Mitry, E.; Smith, D.; Cvitkovic, F.; et al. Phase III trial of chemotherapy using 5-fluorouracil and streptozotocin compared with interferon α for advanced carcinoid tumors: FNCLCC–FFCD 9710. Endocr.-Relat. Cancer 2009, 16, 1351–1361. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.P.; Othus, M.; Chae, Y.K.; Giles, F.J.; Hansel, D.E.; Singh, P.P.; Fontaine, A.; Shah, M.H.; Kasi, A.; Al Baghdadi, T.; et al. A Phase II Basket Trial of Dual Anti–CTLA-4 and Anti–PD-1 Blockade in Rare Tumors (DART SWOG 1609) in Patients with Nonpancreatic Neuroendocrine Tumors. Clin. Cancer Res. 2020, 26, 2290–2296. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.P.; Mayerson, E.; Chae, Y.K.; Strosberg, J.; Wang, J.; Konda, B.; Hayward, J.; McLeod, C.M.; Chen, H.X.; Sharon, E.; et al. A phase II basket trial of Dual Anti–CTLA–4 and Anti–PD–1 Blockade in Rare Tumors (DART) SWOG S1609: High-grade neuroendocrine neoplasm cohort. Cancer 2021, 127, 3194–3201. [Google Scholar] [CrossRef]
- Mehnert, J.M.; Bergsland, E.; O’Neil, B.H.; Santoro, A.; Schellens, J.H.M.; Cohen, R.B.; Doi, T.; Ott, P.A.; Pishvaian, M.J.; Puzanov, I.; et al. Pembrolizumab for the treatment of programmed death–ligand 1–positive advanced carcinoid or pancreatic neuroendocrine tumors: Results from the KEYNOTE-028 study. Cancer 2020, 126, 3021–3030. [Google Scholar] [CrossRef]
- Morse, M.; Halperin, D.M.; Uronis, H.E.; Hsu, D.S.; Hurwitz, H.; Bolch, E.; Warren, D.; Haley, S.; John, L.; Moyer, A.; et al. Phase Ib/II study of pembrolizumab with lanreotide depot for advanced, progressive gastroenteropancreatic neuroendocrine tumors (PLANET). J. Clin. Oncol. 2021, 39, 369. [Google Scholar] [CrossRef]
- Strosberg, J.R.; E Caplin, M.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; E Pavel, M.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Mandriani, B.; Pelle’, E.; Mannavola, F.; Ingravallo, G.; Cazzato, G.; Ramello, M.; Porta, C.; Strosberg, J.; Abate-Daga, D.; Cives, M. 1101MO Development of CAR T-cells for future treatment of NETs. Ann. Oncol. 2021, 32, S911. [Google Scholar] [CrossRef]
- Yao, J.; Strosberg, J.; Fazio, N.; Pavel, M.; Ruszniewski, P.; Bergsland, E.; Li, D.; Tafuto, S.; Raj, N.; Campana, D.; et al. Activity & safety of spartalizumab (PDR001) in patients (pts) with advanced neuroendocrine tumors (NET) of pancreatic (Pan), gastrointestinal (GI), or thoracic (T) origin, & gastroenteropancreatic neuroendocrine carcinoma (GEP NEC) who have progressed on prior treatment (Tx). Ann. Oncol. 2018, 29, viii467–viii468. [Google Scholar] [CrossRef]
- El-Rayes, B.F.; Hendifar, A.E.; Pant, S.; Wilky, B.A.; Reilley, M.J.; Benson, A.B.; Chow, W.A.; Konda, B.; Starr, J.; Ahn, D.H.; et al. Safety, Pharmacodynamic, and Antitumor Activity of Tidutamab, an SSTR2 x CD3 Bispecific Antibody, in Subjects with Advanced Neuroendocrine Tumors. In Proceedings of the NANETS Multidisciplinary NET Symposium, Virtual, 3–6 November 2021. [Google Scholar]
- Strosberg, J.R.; Mizuno, N.; Doi, T.; Grande, E.; Delord, J.-P.; Shapira-Frommer, R.; Bergsland, E.K.; Shah, M.H.; Fakih, M.; Takahashi, S.; et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study. Clin. Cancer Res. 2020, 26, 2124–2130. [Google Scholar] [CrossRef]
- Liu, M.; Guo, F. Recent updates on cancer immunotherapy. Precis. Clin. Med. 2018, 1, 65–74. [Google Scholar] [CrossRef]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Kwok, G.; Yau, T.C.C.; Chiu, J.W.; Tse, E.; Kwong, Y.-L. Pembrolizumab (Keytruda). Hum. Vaccines Immunother. 2016, 12, 2777–2789. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.C.; Strosberg, J.; Fazio, N.; Pavel, M.E.; Bergsland, E.; Ruszniewski, P.; Halperin, D.M.; Li, D.; Tafuto, S.; Raj, N.; et al. Spartalizumab in metastatic, well/poorly differentiated neuroendocrine neoplasms. Endocr. Relat. Cancer 2021, 28, 161–172. [Google Scholar] [CrossRef]
- Kuhns, M.S.; Davis, M.M.; Garcia, K.C. Deconstructing the Form and Function of the TCR/CD3 Complex. Immunity 2006, 24, 133–139. [Google Scholar] [CrossRef] [Green Version]


| Biomarker | Carcinoid Location | Assess/Correlations | Sensitivity and Specificity |
|---|---|---|---|
| Chromogranin A(CgA) | All locations | Confirm diagnosis, assess treatment progress, tumor burden, correlated to tumor load, background levels variable in different populations [32,33,34,35] | 43–100% sensitivity 10–96% specificity [36] |
| Serotonin (5-HT) | Foregut, Midgut | Blood serum analysis, carcinoid syndrome [32,37] | 35% sensitivity up to ≈100% specificity [36] |
| 5-HIAA | Midgut | Urinary or serum analysis, carcinoid syndrome, used for screening and diagnosis [32,36] | 35% sensitivity Up to ≈100% specificity [36] |
| Pancreastatin | Pancreas, Midgut | Tumor activity [32,36,38] | 64% sensitivity 58–100% specificity [36] |
| Neurokinin A (NKA), Substance P | Midgut | Prognostic value, correlated with poor outcome [36] | 88% sensitivity No data for specificity [36] |
| Neuron-specific enolase (NSE) | All locations | Elevated levels suggest poor differentiation [32,36] | 33% sensitivity Up to 100% specificity [36] |
| Progastrin- releasing peptide (proGRP) | Lung | Expression associated with survival, >90 ng/L negatively correlated with outcome [36] | 99% sensitivity 43% specificity [36] |
| Pancreatic Polypeptide (PP) | Pancreas, Midgut, Colon | No known clinical utility [36] | 50–80% sensitivity No data for specificity [36] |
| N-terminal pro-brain natriuretic peptide (NT-proBNP) | Midgut | Prognostic value, correlates with survival in carcinoid heart disease [36] | 87% sensitivity 80% specificity [36] |
| Connective Tissue Growth Factor (CTGF) | Midgut | Elevations predict reduced right ventricular function in carcinoid heart disease [36] | 88% sensitivity 69% specificity [36] |
| Paraneoplastic Ma antigen 2 (PNMA2) | Small intestines, Lung | Assess recurrence risk [39] | 46–50% sensitivity SI-NETs 35% sensitivity lung 98% overall specificity [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vellani, S.D.; Nigro, A.; Varatharajan, S.; Dworkin, L.D.; Creeden, J.F. Emerging Immunotherapeutic and Diagnostic Modalities in Carcinoid Tumors. Molecules 2023, 28, 2047. https://doi.org/10.3390/molecules28052047
Vellani SD, Nigro A, Varatharajan S, Dworkin LD, Creeden JF. Emerging Immunotherapeutic and Diagnostic Modalities in Carcinoid Tumors. Molecules. 2023; 28(5):2047. https://doi.org/10.3390/molecules28052047
Chicago/Turabian StyleVellani, Shahnaz D., Anthony Nigro, Shangari Varatharajan, Lance D. Dworkin, and Justin Fortune Creeden. 2023. "Emerging Immunotherapeutic and Diagnostic Modalities in Carcinoid Tumors" Molecules 28, no. 5: 2047. https://doi.org/10.3390/molecules28052047






