Donor-π-Acceptor-Type Fluorinated Tolane Containing a Semifluoroalkoxy Chain as a Condensed-Phase Luminophore
Abstract
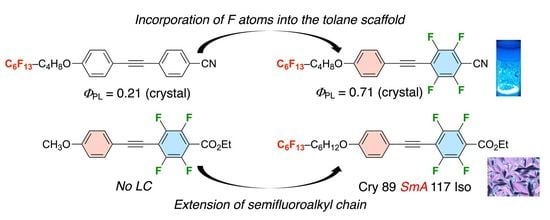
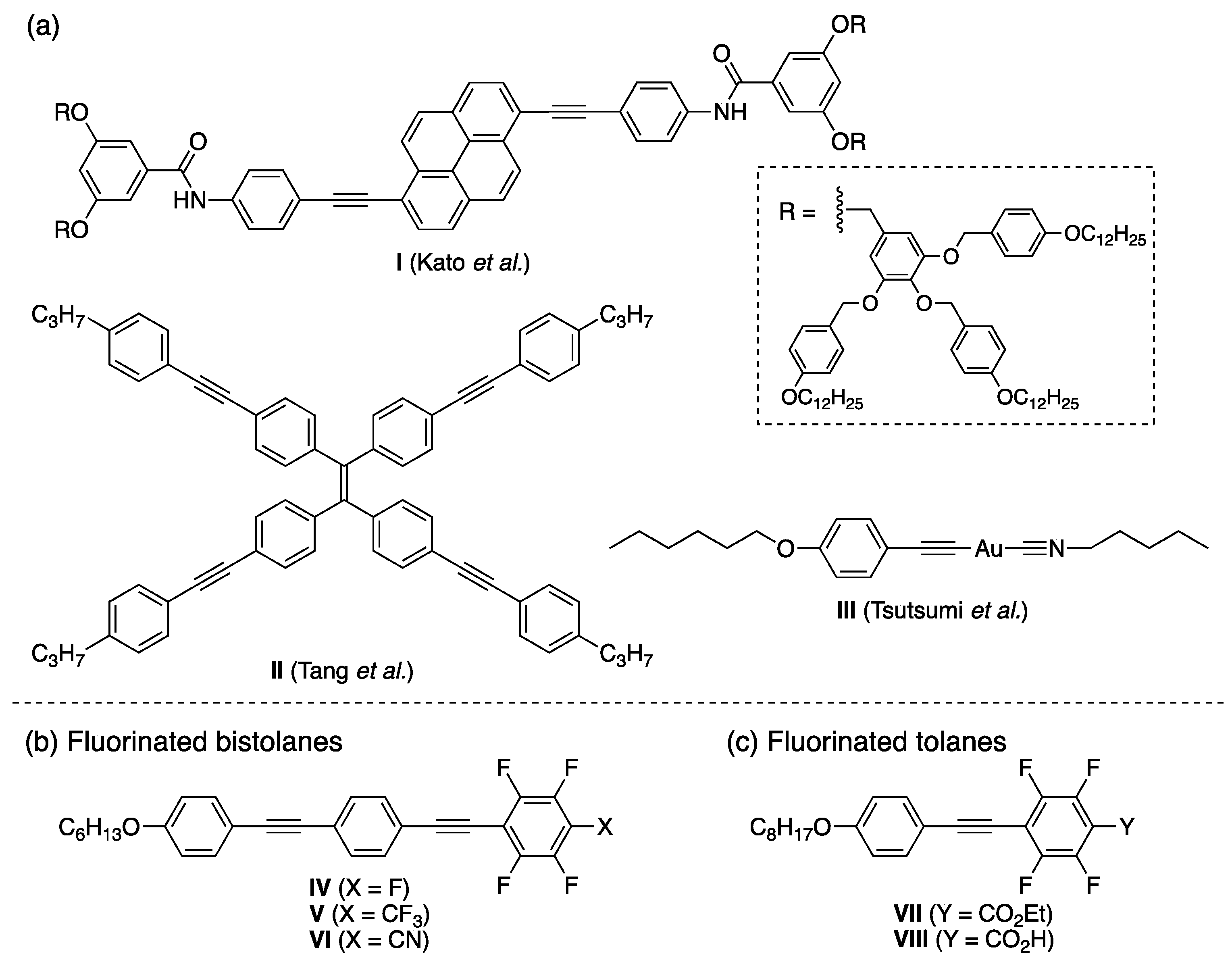
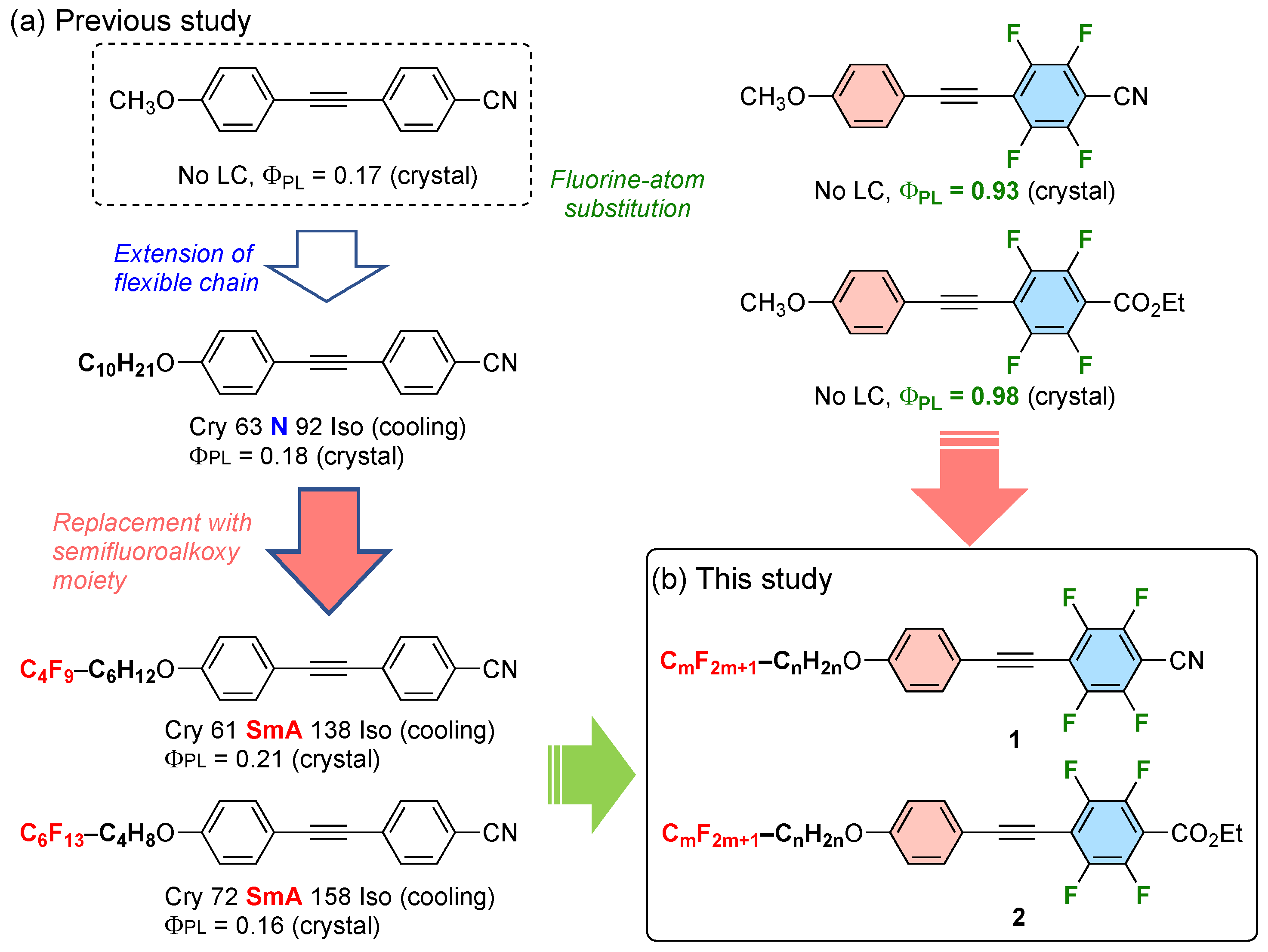
:1. Introduction
2. Results and Discussion
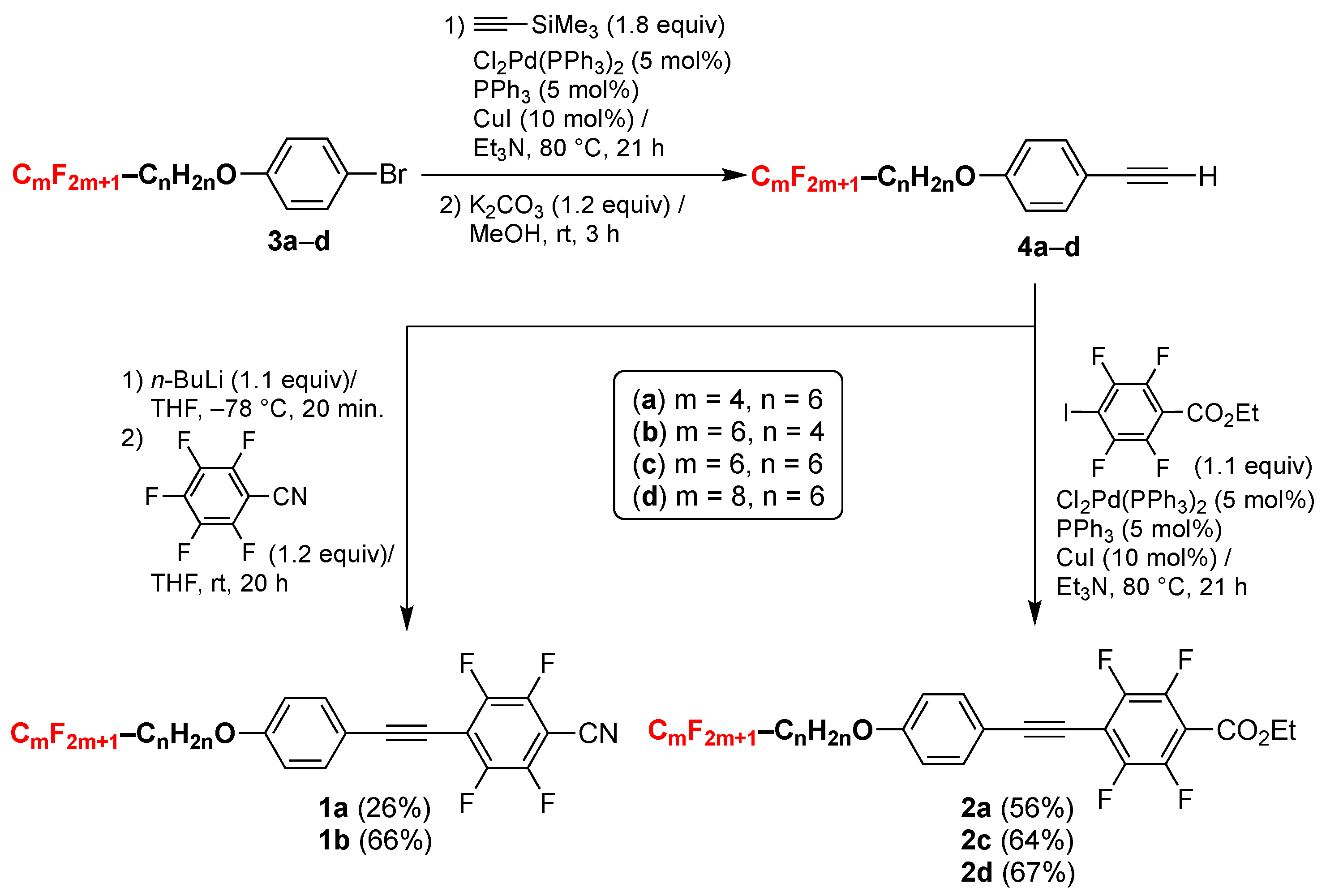
2.1. Synthesis
2.2. Photophysical Behavior
2.3. Phase Transition Behavior
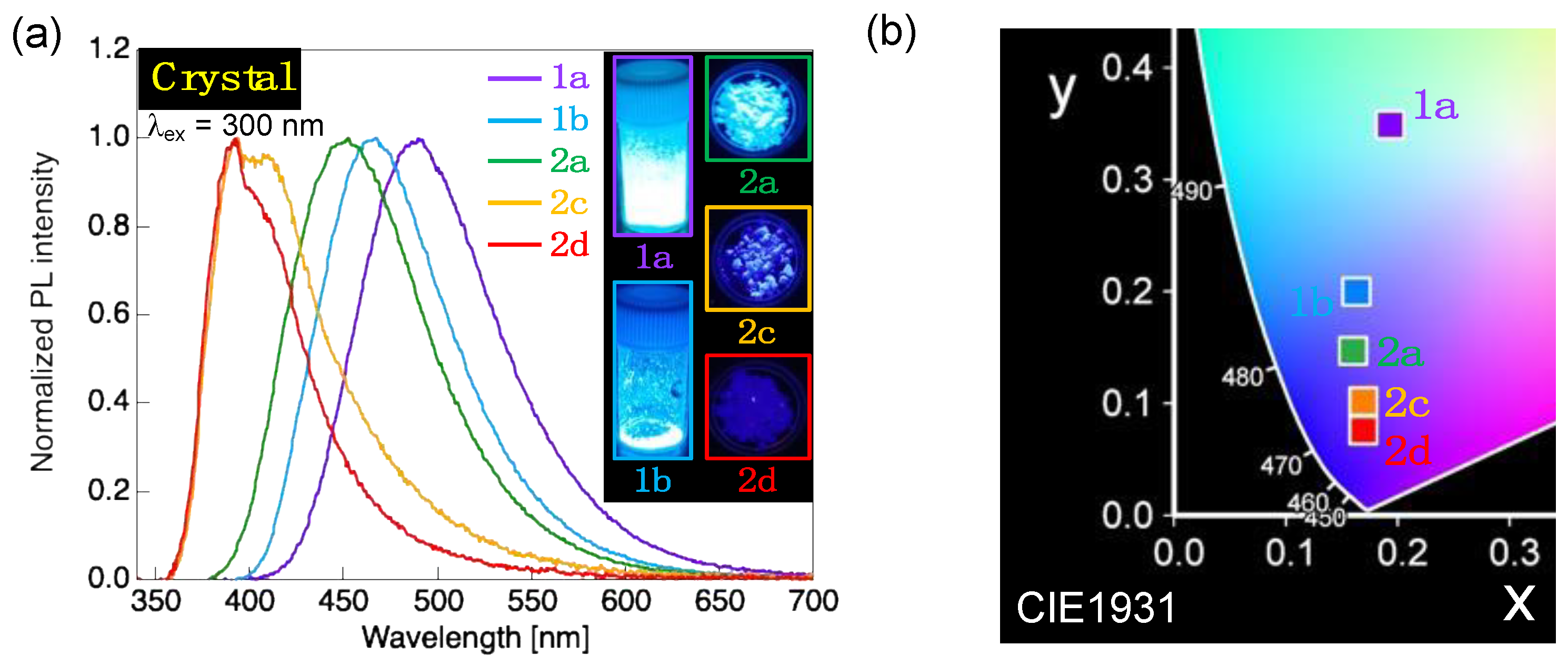
2.4. PL Behavior in the SmA Phase
3. Materials and Methods
3.1. General
3.2. Synthesis Procedure of 2,3,5,6-Tetrafluoro-4-[2-{4-(7,7,8,8,9,9,10,10,10-nonafluorodecyloxy)phenyl}ethyn-1-yl]benzonitrile (1a)
3.2.1. 2,3,5,6-Tetrafluoro-4-[2-{4-(7,7,8,8,9,9,10,10,10-nonafluorodecyloxy)phenyl}ethyn-1-yl]benzonitrile (1a)
3.2.2. 2,3,5,6-Tetrafluoro-4-[2-{4-(5,5,6,6,7,7,8,8,9,9,10,10,10-tridecafluorodecyloxy)phenyl}ethyn-1-yl]benzonitrile (1b)
3.3. Synthesis Procedure of Ethyl 2,3,5,6-Tetrafluoro-4-[2-{4-(7,7,8,8,9,9,10,10,10-nonafluorodecyloxy)phenyl}Ethyn-1-yl]benzoate (2a)
3.3.1. Ethyl 2,3,5,6-Tetrafluoro-4-[2-{4-(7,7,8,8,9,9,10,10,10-nonafluorodecyloxy)phenyl}ethyn-1-yl]benzoate (2a)
3.3.2. Ethyl 2,3,5,6-Tetrafluoro-4-[2-{4-(7,7,8,8,9,9,10,10,11,11,12,12,12-trideccafluorododecyloxy)phenyl}ethyn-1-yl]benzoate (2c)
3.3.3. Ethyl 2,3,5,6-Tetrafluoro-4-[2-{4-(7,7,8,8,9,9,10,10,11,11,12,12,13,13,14,14,14-heptadeccafluorotetradecyloxy)phenyl}ethyn-1-yl]benzoate (2d)
3.4. Theoretical Assessment
3.5. Photophysical Behavior
3.6. Phase Transition Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, Q.; Li, Z. Molecular packing: Another key point for the performance of organic and polymeric optoelectronic materials. Acc. Chem. Res. 2020, 53, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Sagara, Y.; Yamane, S.; Mitani, M.; Weder, C.; Kato, T. Mechanoresponsive luminescent molecular assemblies: An emerging class of materials. Adv. Mater. 2016, 28, 1073–1095. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Wu, F.; Liu, Z.; Fan, Z.; Chen, L.; Chen, Y. Recent developments of n-type organic thermoelectric materials: Influence of structure modification on molecule arrangement and solution processing. ChemSusChem. 2022, 15, e202102420. [Google Scholar] [CrossRef]
- Shao, D.; Wang, X. Development of single-molecule magnets. Chin. J. Chem. 2020, 38, 1005–1018. [Google Scholar] [CrossRef]
- Yu, H.; Song, X.; Xie, N.; Wang, J.; Li, C.; Wang, Y. Reversible crystal-to-crystal phase transitions with high-contrast luminescent alterations for a thermally activated delayed fluorescence emitter. Adv. Funct. Mater. 2021, 31, 2007511. [Google Scholar] [CrossRef]
- Seki, T.; Takamatsu, Y.; Ito, H. A screening approach for the discovery of mechanochromic gold(I) isocyanide complexes with crystal-to-crystal phase transitions. J. Am. Chem. Soc. 2016, 138, 6252–6260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oladepo, S.A. Development and application of liquid crystals as stimuli-responsive sensors. Molecules 2022, 27, 1453. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, K.; Li, H.; Zhang, T.; Liu, D.; Han, Y. Liquid crystal ordering on conjugated polymers film morphology for high performance. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1572–1591. [Google Scholar] [CrossRef] [Green Version]
- Ailincai, D.; Pamfil, D.; Marin, L. Multiple bio-responsive polymer dispersed liquid crystal composites for sensing applications. J. Mol. Liq. 2018, 272, 572–582. [Google Scholar] [CrossRef]
- Du, X.; Liu, Y.; Wang, F.; Zhao, D.; Gleeson, H.F.; Luo, D. A fluorescence sensor for Pb2+ detection based on liquid crystals and aggregation-induced emission luminogens. ACS Appl. Mater. Interfaces 2021, 13, 22361–22367. [Google Scholar] [CrossRef]
- Sagara, Y.; Kato, T. Stimuli-responsive luminescent liquid crystals: Change of photoluminescent colors triggered by a shear-induced phase transition. Angew. Chem. Int. Ed. 2008, 120, 5253–5256. [Google Scholar] [CrossRef]
- Zhao, D.; Fan, F.; Cheng, J.; Zhang, Y.; Wong, K.S.; Chigrinov, V.G.; Kwok, H.S.; Guo, L.; Tang, B.Z. Light-emitting liquid crystal displays based on an aggregation-induced emission luminogen. Adv. Opt. Mater. 2015, 3, 199–202. [Google Scholar] [CrossRef]
- Fujisawa, K.; Kawakami, N.; Onishi, Y.; Izumi, Y.; Tamai, S.; Sugimoto, N.; Tsutsumi, O. Photoluminescent properties of liquid crystalline gold(I) isocyanide complexes with a rod-like molecular structure. J. Mater. Chem. C 2013, 1, 5359–5366. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Agou, T.; Kubota, T.; Konno, T. Luminescence tuning of fluorinated bistolanes via electronic or aggregated-structure control. Appl. Sci. 2019, 9, 1905. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Miyano, K.; Konno, T.; Agou, T.; Kubota, T.; Hosokai, T. Fluorine-containing bistolanes as light-emitting liquid crystalline molecules. Org. Biomol. Chem. 2017, 15, 5949–5958. [Google Scholar] [CrossRef]
- Yamada, S.; Kataoka, M.; Yoshida, K.; Nagata, M.; Agou, T.; Fukumoto, H.; Konno, T. Photophysical and thermophysical behavior of D-π-A-type fluorinated diphenylacetylenes bearing an alkoxy and an ethoxycarbonyl group at both longitudinal molecular terminals. J. Fluor. Chem. 2022, 261–262, 110032. [Google Scholar] [CrossRef]
- Yamada, S.; Kataoka, M.; Yoshida, K.; Nagata, M.; Agou, T.; Fukumoto, H.; Konno, T. Development of hydrogen-bonded dimer-type photoluminescent liquid crystals of fluorinated tolanecarboxylic acid. Crystals 2023, 13, 25. [Google Scholar] [CrossRef]
- Ciastek, S.; Szymańska, K.; Kaszyński, P.; Jasiński, M.; Pociecha, D. Smectic behaviour of methyl 4-alkoxybenzoates with a partially fluorinated alkyl chain. Liq. Cryst. 2018, 45, 11–21. [Google Scholar] [CrossRef]
- Russell, T.P.; Rabolt, J.F.; Twieg, R.J.; Siemens, R.L.; Farmer, B.L. Structural characterization of semifluorinated n-alkanes. 2. Solid-solid transition behavior. Macromolecules 1986, 19, 1135–1143. [Google Scholar] [CrossRef]
- Yamada, S.; Yoshida, K.; Sakurai, T.; Hara, M.; Konno, T. Effect of fluorine atoms in flexible chains on the phase transitions and photophysical behavior of D-π-A-type 4-alkoxy-4′-cyanodiphenylacetylene. Mol. Syst. Des. Eng. 2022, 7, 720–724. [Google Scholar] [CrossRef]
- Zgierski, M.Z.; Lim, E.C. Nature of the ‘dark’ state in diphenylacetylene and related molecules: State switch from the linear ππ* state to the bent πσ*state. Chem. Phys. Lett. 2004, 387, 352–355. [Google Scholar] [CrossRef]
- Saltiel, J.; Kumar, V.K.R. Photophysics of diphenylacetylene: Light from the ‘dark state’. J. Phys. Chem. A 2012, 116, 10548–10558. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Nath, S.; Hajra, A.; Sinha, S. Fluorescence self-quenching of tetraphenylporphirin in liquid medium. J. Lumin. 2013, 141, 87–92. [Google Scholar] [CrossRef]
- Deptuch, A.; Lalik, S.; Jasiurkowska-Delaporte, M.; Juszyńska-Gałazka, E.; Drzewicz, A.; Urbańska, M.; Marzec, M. Comparative study of electrooptic, dielectric, and structural properties of two glassforming antiferroelectric mixtures with a high tilt angle. Phys. Rev. E 2022, 105, 024705. [Google Scholar] [CrossRef]
- Lalik, S.; Deptuch, A.; Jaworska-Gołab, T.; Fryń, P.; Dardas, D.; Stefańczyk, O.; Urbańska, M.; Marzec, M. Modification of AFLC physical properties by doping with BaTiO3 particles. J. Phys. Chem. B 2020, 124, 6055–6073. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hohenstein, E.G.; Chill, S.T.; Sherrill, C.D. Assessment of the performance of the M05-2X and M06-2X exchange-correlation functionals for noncovalent interactions in biomolecules. J. Chem. Theor. Comput. 2008, 4, 1996–2000. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jensen, J.H. Improving the efficiency and convergence of geometry optimization with the polarizable continuum model: New energy gradients and molecular surface tessellation. J. Comput. Chem. 2004, 25, 1449–1462. [Google Scholar] [CrossRef] [PubMed]



| CH2Cl2 Solution | Crystal | |||||
|---|---|---|---|---|---|---|
| Molecule | λabs [nm] 1 (ε [103, L mol–1 cm–1]) | λPL [nm] 2 (ΦPL) 3 | CIE Coordinate (x, y) | λPL [nm] (ΦPL) 3 | CIE Coordinate (x, y) | |
| 1a | 260 (25.0), 272 (20.0), 341 (36.0) | 437 (0.20) | (0.155, 0.090) | 490 (0.61) 4 | (0.193, 0.349) | |
| 1b | 261 (19.0), 274 (15.9), 351 (34.1) | 435 (0.18) | (0.155, 0.089) | 467 (0.71) 4 | (0.163, 0.200) | |
| 2a | 259 (9.94), 269 (10.8), 324 (30.8) | 428 (0.42) | (0.164, 0.115) | 453 (0.48) 4 | (0.160, 0.147) | |
| 2c | 258 (11.6), 268 (12.0), 325 (31.1) | 429 (0.42) | (0.163, 0.114) | 394, 409 (0.13) 5 | (0.169, 0.102) | |
| 2d | 259 (8.68), 269 (9.61), 325 (28.7) | 430 (0.39) | (0.164, 0.116) | 392, 408sh (0.14) 5 | (0.169, 0.076) | |
| Molecule | Phase Transition Sequence and Temperature [°C] (Enthalpy [kJ mol−1]) | |
|---|---|---|
| 1a | Heating | Cry 109 (28.6) Iso |
| Cooling | Cry 96 (−28.1) Iso | |
| 1b | Heating | Cry 113 (21.6) Iso |
| Cooling | Cry 106 (−20.7) Iso | |
| 2a | Heating | Cry1 77 (19.3) Cry2 80 (22.4) SmA 94 (6.7) Iso |
| Cooling | Cry1 63 (−21.4) SmA 94 (−6.7) Iso | |
| 2c | Heating | Cry 89 (29.8) SmA 117 (8.2) Iso |
| Cooling | Cry 67 (−27.7) SmA 117 (−8.3) Iso | |
| 2d | Heating | Cry1 45 (5.3) Cry2 101 (38.0) SmA 135 (10.1) Iso |
| Cooling | Cry1 39 (−5.4) Cry2 84 (−35.1) SmA 134 (−10.1) Iso 1 | |
| Molecule | Phase | λPL [nm] | IsmA/Icry |
|---|---|---|---|
| 2a | Cry (25 °C) | 448 | 0.22 |
| SmA (90 °C) | 429 | ||
| 2c | Cry (25 °C) | 393 | 0.32 |
| SmA (100 °C) | 444 | ||
| 2d | Cry (25 °C) | 390 | 0.20 |
| SmA (120 °C) | 433 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Yoshida, K.; Kataoka, M.; Hara, M.; Konno, T. Donor-π-Acceptor-Type Fluorinated Tolane Containing a Semifluoroalkoxy Chain as a Condensed-Phase Luminophore. Molecules 2023, 28, 2764. https://doi.org/10.3390/molecules28062764
Yamada S, Yoshida K, Kataoka M, Hara M, Konno T. Donor-π-Acceptor-Type Fluorinated Tolane Containing a Semifluoroalkoxy Chain as a Condensed-Phase Luminophore. Molecules. 2023; 28(6):2764. https://doi.org/10.3390/molecules28062764
Chicago/Turabian StyleYamada, Shigeyuki, Keigo Yoshida, Mitsuki Kataoka, Mitsuo Hara, and Tsutomu Konno. 2023. "Donor-π-Acceptor-Type Fluorinated Tolane Containing a Semifluoroalkoxy Chain as a Condensed-Phase Luminophore" Molecules 28, no. 6: 2764. https://doi.org/10.3390/molecules28062764