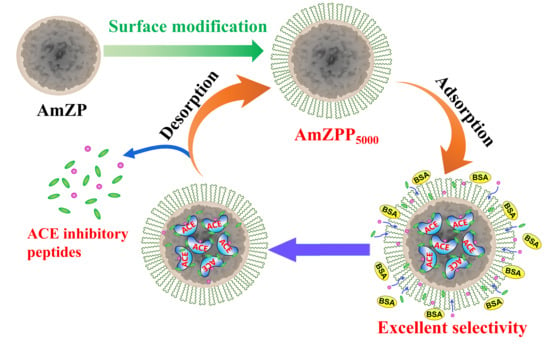
Anchoring of Polymer Loops on Enzyme-Immobilized Mesoporous ZIF-8 Enhances the Recognition Selectivity of Angiotensin-Converting Enzyme Inhibitory Peptides
Abstract
:1. Introduction
2. Results and Discussion
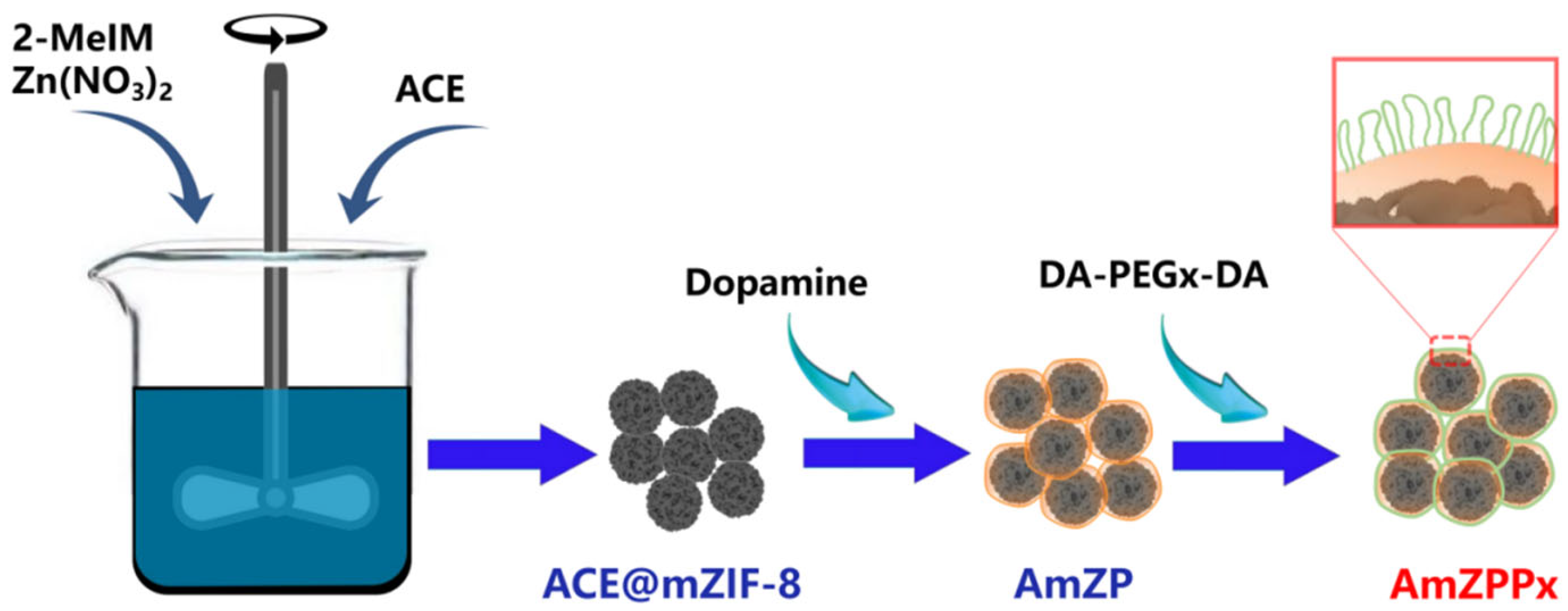
2.1. Synthesis of AmZPPx
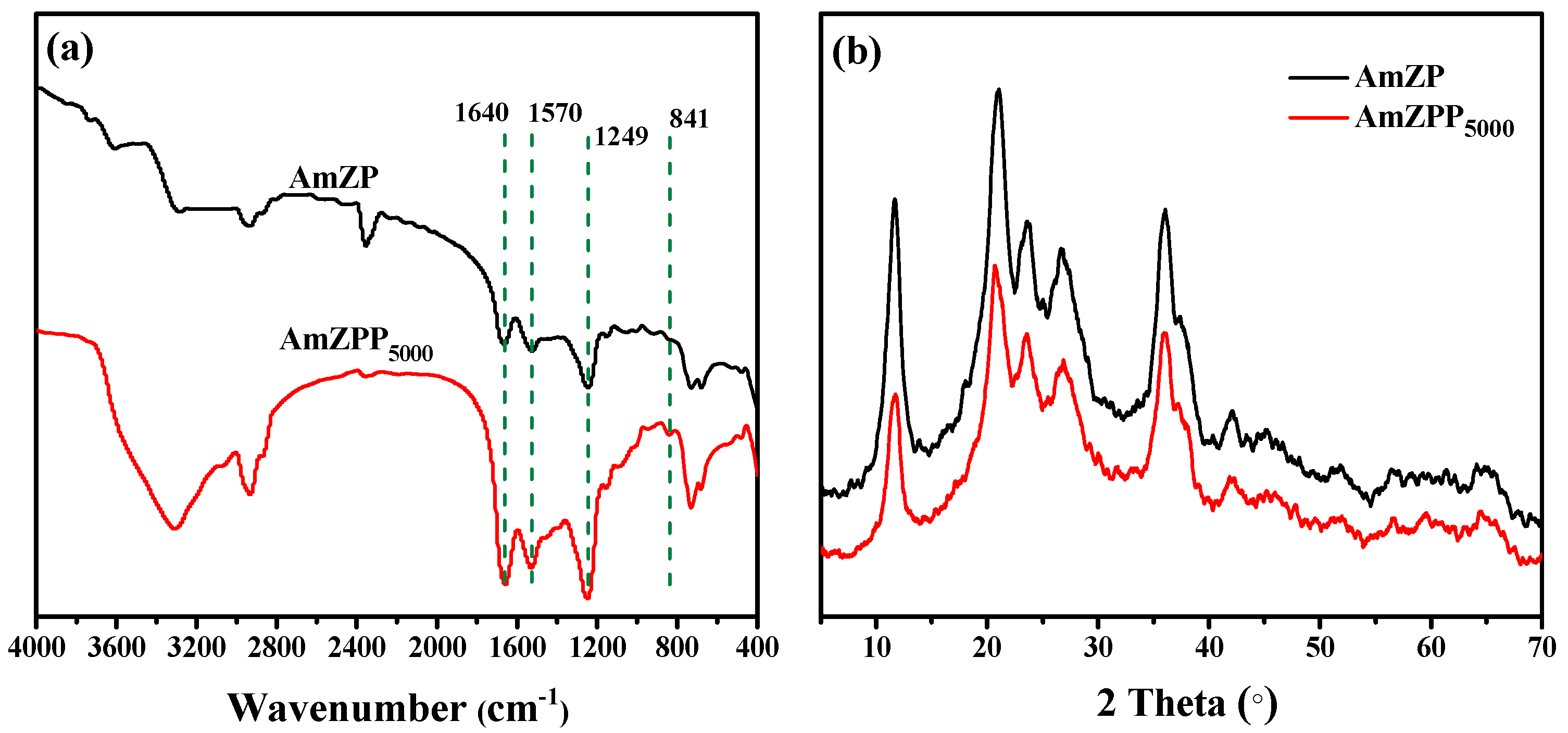
2.2. Characterization
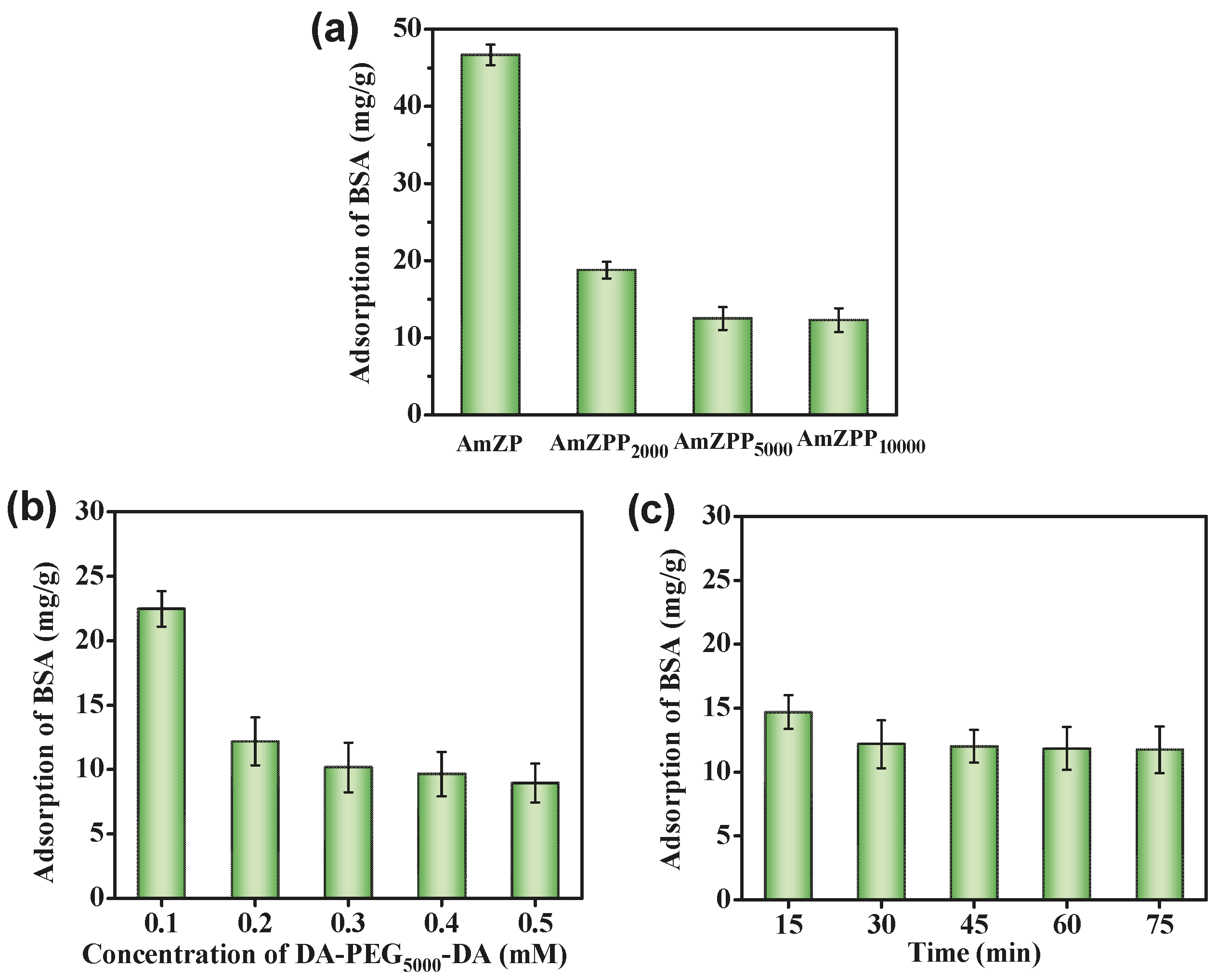
2.3. Specific Adsorption of AmZPP5000
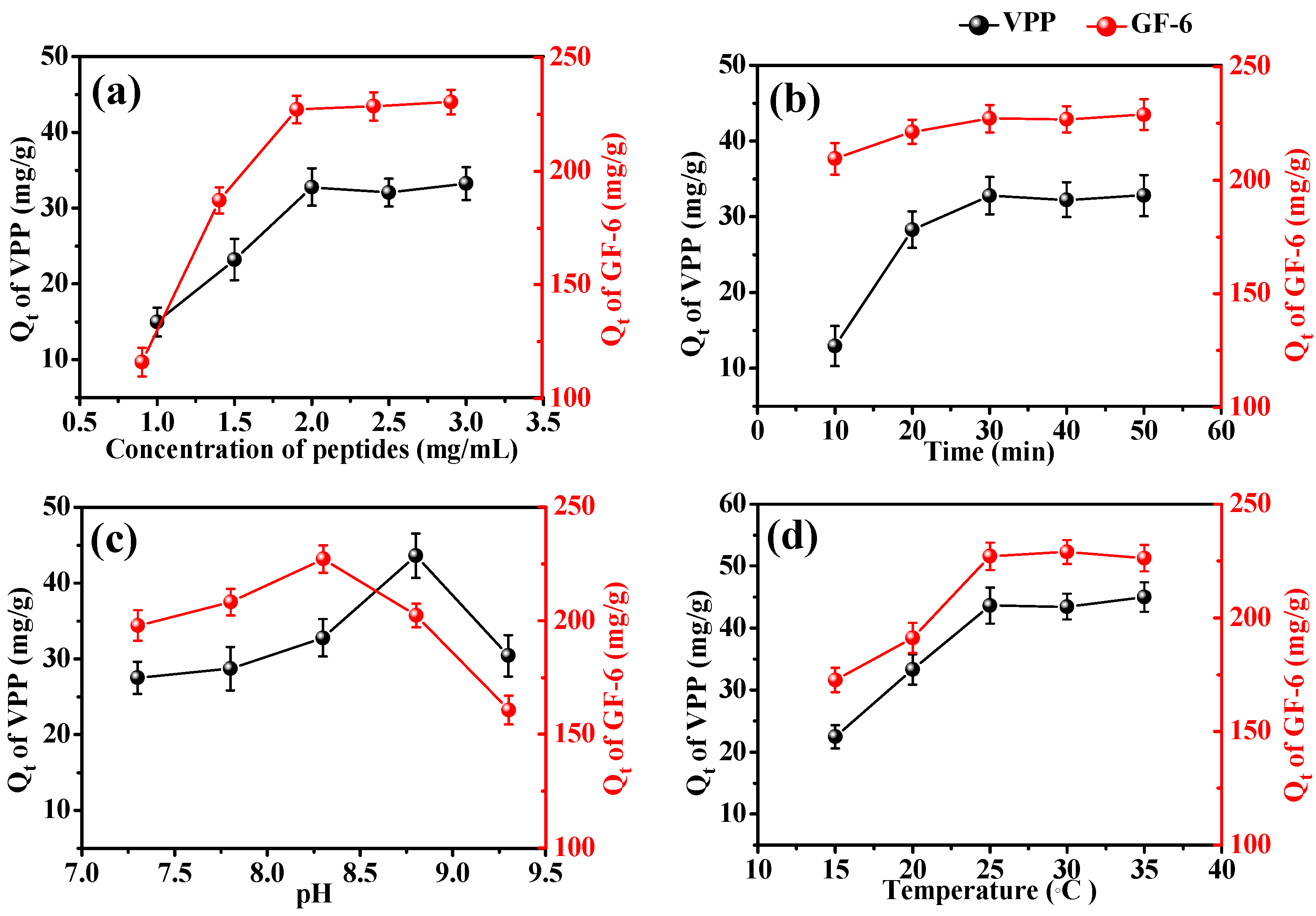
2.4. Adsorption Kinetics of AmZPP5000 for VPP and GF-6
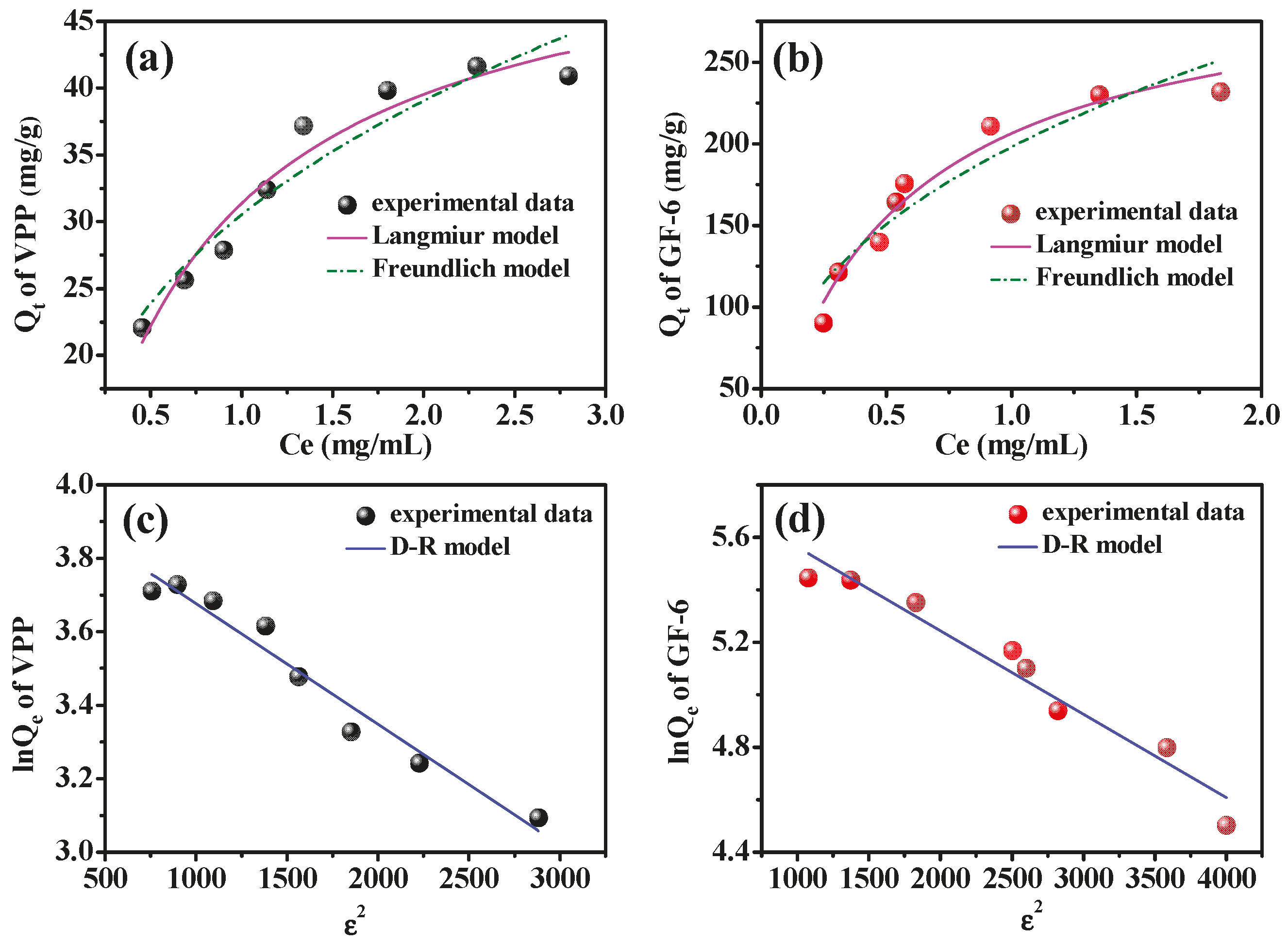
2.5. Adsorption Isotherm of AmZPP5000 for VPP and GF-6
2.6. Desorption and Reusability of AmZPP5000
2.7. Recognition Selectivity of AmZPP5000
2.8. Application of AmZPP5000 in Hydrolysate
3. Materials and Methods
3.1. Materials
3.2. Preparation of AmZP
3.3. Surface Modification with DA-PEGx-DA
3.4. Characterization
3.5. ACE Activity and Inhibitory Activity Assay
3.6. Concentration of VPP and GF-6 Analysis
3.7. Specific/Nonspecific Adsorption Tests
3.8. Studies of Stability
3.9. Application of AmZPP5000 in Hydrolysate Samples
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xie, D.; Shen, Y.; Su, E.; Du, L.; Xie, J.; Wei, D. The effects of angiotensin I-converting enzyme inhibitory peptide vginyw and the hydrolysate of α-lactalbumin on blood pressure, oxidative stress and gut microbiota of spontaneously hypertensive rats. Food Funct. 2022, 13, 2743–2755. [Google Scholar] [CrossRef] [PubMed]
- Bayannavar, P.K.; Kamble, R.R.; Joshi, S.D. Design and synthesis of angiotensin converting enzyme(ace)inhibitors: Analysis of the role of tetrazole ring appended to biphenyl moiety. Chem. Sel. 2022, 7, e202103336. [Google Scholar] [CrossRef]
- Wu, N.; Zhao, Y.; Wang, Y.; Shuang, Q. Effects of ultra-high pressure treatment on angiotensin-converting enzyme (ace) inhibitory activity, antioxidant activity, and physicochemical properties of milk fermented with lactobacillus delbrueckii qs306. J. Dairy Sci. 2022, 105, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Pan, F.; Ai, X.; Tang, X.; Cai, S.; Soliman, M.M.; Yanli, N. Novel angiotensin-converting enzyme (ace) inhibitory mechanism of peptides from macadamia integrifolia antimicrobial protein 2 (miamp2). J. Food Biochem. 2022, 46, e14168. [Google Scholar] [CrossRef]
- Dong, J.; Wang, S.; Yin, X.; Fang, M.; Gong, Z.; Wu, Y. Angiotensin i converting enzyme(ace)inhibitory activity and antihypertensive effects of rice peptides. Food Sci. Hum. Well. 2022, 11, 1539–1543. [Google Scholar] [CrossRef]
- Noguchi, Y.; Murayama, A.; Esaki, H.; Sugioka, M.; Koyama, A.; Tachi, T.; Teramachi, H. Angioedema caused by drugs that prevent the degradation of vasoactive peptides: A pharmacovigilance database study. J. Clin. Med. 2021, 10, 5507. [Google Scholar]
- Kaya, A.; Tatlisu, M.A.; Kaya, T.K.; Yildirimturk, O.; Gungor, B.; Karatas, B. Sublingual vs. oral captopril in hypertensive crisis. J. Emerg. Med. 2016, 50, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.B.; Reyes, J.V.M.; Baig, M. Lisinopril-induced Acute Necrotizing Pancreatitis. Cureus 2021, 13, e14642. [Google Scholar] [CrossRef] [PubMed]
- Solanki, D.; Sakure, A.; Prakash, S. Characterization of angiotensin I-converting enzyme (ACE) inhibitory peptides produced in fermented camel milk (Indian breed) by Lactobacillus acidophilus NCDC-15. J. Food Sci. Technol. 2022, 59, 3567–3577. [Google Scholar]
- Martinez-Sanchez, S.M.; Gabaldon-Hernandez, A.; Montoro-Garcia, S. Unravelling the molecular mechanisms associated with the role of food-derived bioactive peptides in promoting cardiovascular health. J. Funct. Foods. 2020, 64, 103645. [Google Scholar] [CrossRef]
- Shanmugam, V.P.; Kapila, S.; Kemgang, T.S.; Reddi, S.; Kapila, R.; Muthukumar, S.; Rajesh, D. Isolation and Characterization of Angiotensin Converting Enzyme Inhibitory Peptide from Buffalo Casein. Int. J. Pept. Res. Ther. 2021, 27, 1481–1491. [Google Scholar] [CrossRef]
- Li, X.; Su, Z.; Luo, Y.; Chen, X.; Luo, J.; Pinelo, M. Modelling of oligodextran production via an immobilized enzyme membrane reactor: Bioreaction-separation coupling mechanism. Sep. Purif. Technol. 2021, 282, 120024. [Google Scholar] [CrossRef]
- Mujtaba, N.; Jahan, N.; Sultana, B.; Zia, M.A. Isolation and characterization of antihypertensive peptides from soy bean protein. Braz. J. Pharm. Sci. 2021, 57, e19061. [Google Scholar] [CrossRef]
- Chuang, H.; Huang, C.; Hung, T.; Huang, L.; Lin, N. Development of biotinylated and magnetic bead-immobilized enzymes for efficient glyco-engineering and isolation of antibodies. Bioorg. Chem. 2021, 112, 104863. [Google Scholar] [CrossRef]
- Wan, G.; Ma, X.; Jin, L.; Chen, J. α-glucosidase immobilization on magnetic core-shell metal-organic frameworks for inhibitor screening from traditional chinese medicines. Colloid. Surf. B 2021, 205, 111847. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Jia, B.; Zhou, J.; Liu, J.; Wang, J.; Ma, D.; Li, P.; Chen, J. A method using angiotensin converting enzyme immobilized on magnetic beads for inhibitor screening. J. Pharmaceut. Biomed. 2019, 164, 223–230. [Google Scholar] [CrossRef]
- Wei, N.; Zhao, J.; Wu, G.; Cao, W.; Luo, P.; Zhang, Z.; Chen, G.; Wen, L. Rapid Screening and Identification of Antitumor Ingredients from the Mangrove Endophytic Fungus Using an Enzyme-Immobilized Magnetic Nanoparticulate System. Molecules 2021, 26, 2255. [Google Scholar] [CrossRef]
- Wu, X.; Qiu, B.; Chen, Y.; Shi, Y.; Zhu, J.; Liu, X.; Zhao, D. Online coupling Fe3O4@ZIF-67@alpha-glucosidase biomicroreactor with high performance liquid chromatography for rapid screening of alpha-glucosidase inhibitors in tea and their inhibitory activity research. J. Chromatogr. B. 2020, 1159, 122398. [Google Scholar] [CrossRef]
- Megías, C.; Pedroche, J.; Yust, M.; Alaiz, M.; Girón-Calle, J.; Millán, F.; Vioque, J. Immobilization of angiotensin-converting enzyme on glyoxyl-agarose. J. Agric. Food Chem. 2006, 54, 4641–4645. [Google Scholar] [CrossRef]
- Hong, Y.; Li, T.; Chen, B.; Yao, S. Immobilization of angiotensin converting-enzyme on chitosan microspheres. Chem. J. Chin. Univ. 2009, 30, 328–331. [Google Scholar]
- Hui, L.; Yin, Q.; Jin, Q.; Xinyu, L. A novel strategy for screening angiotensin-converting enzyme inhibitors from natural products based on enzyme-immobilized ligand fishing combined with active-site blocking and directional enrichment. J. Chromatogr. B. 2022, 1195, 123203. [Google Scholar]
- Meizhong, T.; Jingwu, K. Enzyme inhibitor screening by capillary electrophoresis with an on-column immobilized enzyme microreactor created by an ionic binding technique. Anal. Chem. 2006, 78, 2514–2520. [Google Scholar]
- Feng, X.; Liao, D.; Sun, L.; Wu, S.; Lan, P.; Wang, Z.; Li, C.; Zhou, Q.; Lu, Y.; Lan, X. Affinity Purification of Angiotensin Converting Enzyme Inhibitory Peptides from Wakame (Undaria Pinnatifida) Using Immobilized ACE on Magnetic Metal Organic Frameworks. Mar. Drugs. 2021, 19, 177. [Google Scholar] [CrossRef] [PubMed]
- Altinkaynak, C.; Tavlasoglu, S.; Ÿzdemir, N.; Ocsoy, I. A new generation approach in enzyme immobilization: Organic-inorganic hybrid nanoflowers with enhanced catalytic activity and stability. Enzyme Microb. Tech. 2016, 93–94, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Wang, X.; Zhou, H. Enzyme-mof (metal-organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef]
- Fried, D.; Brieler, F.; Frba, M. Designing inorganic porous materials for enzyme adsorption and applications in biocatalysis. ChemCatChem 2013, 5, 862–884. [Google Scholar] [CrossRef]
- Liang, W.; Xu, H.; Carraro, F.; Maddigan, N.K.; Li, Q.; Bell, S.G.; Huang, D.; Tarzia, A.; Solomon, M.B.; Amenitsch, H. Enhanced activity of enzymes encapsulated in hydrophilic metal-organic frameworks. J. Am. Chem. Soc. 2019, 141, 2348–2355. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, J.; Ren, H.; Ji, C.; Tao, Y.; Wu, Y.; Zhang, Y.; Cheng, L. Fine-tuning the micro-environment to optimize the catalytic activity of enzymes immobilized in multivariate metal-organic frameworks. J. Am. Chem. Soc. 2021, 143, 15378–15390. [Google Scholar] [CrossRef]
- Chen, Y.; Jimenez-Angeles, F.; Qiao, B.; Krzyaniak, M.D.; Farha, O.K. Insights into the enhanced catalytic activity of cytochrome c when encapsulated in a metal-organic framework. J. Am. Chem. Soc. 2020, 142, 18579–18582. [Google Scholar] [CrossRef]
- Peng, S.; Liu, J.; Qin, Y.; Wang, H.; Yu, X. Metal-organic framework encapsulating hemoglobin as a high-stable and long-circulating oxygen carriers to treat hemorrhagic shock. ACS Appl. Mater. Interfaces 2019, 11, 35604–35612. [Google Scholar] [CrossRef]
- Qian, L.; Liu, W.; Liu, H.; Nica, V.; Zhang, S.; Zhou, Q.; Song, W.; Zhang, Q. Fabrication of raspberry-like cytochrome c surface-imprinted nanoparticles based on mof composites for high-performance protein separation. ACS Appl. Mater. Interfaces 2021, 13, 31010–31020. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, X.; Xie, M.; Wu, H.; Yoshioka, T.; Saeki, D.; Matsuyama, H. Antifouling thin-film composite membranes with multi-defense properties by controllably constructing amphiphilic diblock copolymer brush layer. J. Membr. Sci. 2020, 614, 118515. [Google Scholar] [CrossRef]
- Niu, J.; Wang, H.; Chen, J.; Chen, X.; Han, X.; Liu, H. Bio-inspired zwitterionic copolymers for antifouling surface and oil-water separation. Colloid. Surf. A 2021, 626, 127016. [Google Scholar] [CrossRef]
- He, Y.; Wan, X.; Lin, W.; Li, J.; Li, Z.; Luo, F.; Li, J.; Tan, H.; Fu, Q. The synergistic effect of hierarchical structure and alkyl chain length on the antifouling and bactericidal properties of cationic/zwitterionic block polymer brushes. Biomater. Sci. 2020, 8, 6890–6902. [Google Scholar] [CrossRef]
- Kanamaru, T.; Sakurai, K.; Envelope, S.F. Impact of polyethylene glycol (peg) conformations on the in vivo fate and drug release behavior of pegylated core-cross-linked polymeric nanoparticles. Biomacromolecules 2022, 23, 3909–3918. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Jiang, S.; Simon, J.; Passlick, D.; Frey, M.L.; Wagner, M.; Mailander, V.; Crespy, D.; Landfester, K. Brush conformation of polyethylene glycol determines the stealth effect of nanocarriers in the low protein adsorption regime. Nano Lett. 2021, 21, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Kim, K.; Kim, S.; Park, H.; Shin, H.; Joo, J. Tailored polyethylene glycol grafting on porous nanoparticles for enhanced targeting and intracellular sirna delivery. Nanoscale 2022, 14, 14482–14490. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Banquy, X.; Heo, J.H.; Lim, C.N.; Lynd, N.A.; Lundberg, P.; Oh, D.X.; Lee, H.K.; Hong, Y.K.; Hwang, D.S.; et al. Mussel-inspired anchoring of polymer loops that provide superior surface lubrication and antifouling properties. Acs Nano. 2016, 10, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wang, H.; Zhang, Z.; Zhang, Z.; Chen, J.; Chen, J. Conformation-dominated surface antifouling and aqueous lubrication. Colloid. Surf. B 2022, 214, 112452. [Google Scholar] [CrossRef]
- Xiong, C.; Xiong, W.; Mu, Y.; Pei, D.; Wan, X. Mussel-inspired polymeric coatings with the antifouling efficacy controlled by topologies. J. Mater. Chem. B 2022, 10, 9295–92304. [Google Scholar] [CrossRef]
- Shin, E.; Lim, C.; Kang, U.J.; Kim, M.; Park, J.; Kim, D.; Choi, W.; Hong, J.; Baig, C.; Lee, D.W. Mussel-inspired copolyether loop with superior antifouling behavior. Macromolecules 2020, 53, 3551–3562. [Google Scholar] [CrossRef]
- Pina, A.S.; Roque, A.C.A. Studies on the molecular recognition between bioactive peptides and angiotensin-converting enzyme. J. Mol. Recognit. 2010, 22, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Sakamoto, Y.; Toh, M.; Ohara, N.; Hatanaka, Y.; Naka, A.; Kishimoto, Y.; Kondo, K.; Iida, K. Milk-derived peptide val-pro-pro (VPP) inhibits obesity-induced adipose inflammation via an angiotensin-converting enzyme (ACE) dependent cascade. Mol. Nutr. Food Res. 2015, 59, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Liao, D.; Wu, S.; Wang, F.; Sun, J.; Tong, Z. Rapid purification and characterization of angiotensin converting enzyme inhibitory peptides from lizard fish protein hydrolysates with magnetic affinity separation. Food Chem. 2015, 182, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Sun, L.; Muhammad, Y.; Wang, Z.; Liu, H.; Sun, J.; Zhou, L.; Feng, X.; Liao, D.; Wang, S. Studies on the interaction between angiotensin-converting enzyme (ace) and ace inhibitory peptide from saurida elongata. J. Agric. Food Chem. 2018, 66, 13414–13422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, J.; Zhang, Y.; Wu, Y.; Chen, Y.; Messersmith, P.B.; Jiang, Z. Polymer@mofs capsules prepared through controlled interfacial mineralization for switching on/off enzymatic reactions. Appl. Mater. Today. 2018, 13, 320–328. [Google Scholar] [CrossRef]
- Rashid, S.; Ward-Bond, J.; Krupin, O.; Berini, P. Non-specific adsorption of protein to microfluidic materials. Colloid. Surf. B 2021, 208, 112138. [Google Scholar] [CrossRef]
- Hu, Y.L.; Dai, L.; Liu, D.; Du, W. Hydrophobic pore space constituted in macroporous ZIF-8 for lipase immobilization greatly improving lipase catalytic performance in biodiesel preparation. Biotechnol. Biofuels. 2020, 13, 86. [Google Scholar] [CrossRef]
- Wu, X.; Yue, H.; Zhang, Y.; Gao, X.; Li, X.; Wang, L.; Cao, Y.; Hou, M.; An, H.; Zhang, L.; et al. Packaging and delivering enzymes by amorphous metal-organic frameworks. Nat. Commun. 2019, 10, 5165. [Google Scholar] [CrossRef] [Green Version]
- Dou, D.; Wei, D.; Guan, X.; Liang, Z.; Lan, L.; Lan, X.; Liu, P.; Mo, H.; Lan, P. Adsorption of copper (II) and cadmium (II) ions by in situ doped nano-calcium carbonate high-intensity chitin hydrogels. J. Hazard. Mater. 2021, 423, 127137. [Google Scholar] [CrossRef]
- Georgianos, P.; Pournara, A.D.; Andreou, E.K.; Armatas, G.S.; Manos, M.J. Composite Materials Based on a Zr4+ MOF and Aluminosilicates for the Simultaneous Removal of Cationic and Anionic Dyes from Aqueous Media. Molecules 2023, 28, 815. [Google Scholar] [CrossRef] [PubMed]
- Simonin, J.P.; Bouté, J. Intraparticle diffusion-adsorption model to describe liquid/solid adsorption kinetics. Rev. Mex. Ing. Quim. 2016, 15, 161–173. [Google Scholar]
- Tourani, S.; Behvandi, A. Synthesis of MIL-101(Cr)/Sulfasalazine (Cr-TA@SSZ) hybrid and its use as a novel adsorbent for adsorptive removal of organic pollutants from wastewaters. J. Porous Mat. 2022, 29, 1441–1462. [Google Scholar] [CrossRef]
- Gao, Y.; Doherty, C.M.; Mulet, X. A Systematic Study of the Stability of Enzyme/Zeolitic Imidazolate FrameworkComposites in Various Biologically Relevant Solutions. Chem. Sel. 2020, 5, 13766–13774. [Google Scholar]
- Shujin, P.; Xuan, Z.; Yang, C.; NAseer, S.; Zhang, X.; Ouyang, J.; Li, D. The effects of NaCl on enzyme encapsulation by zeolitic imidazolate frameworks-8. Enzyme Microb. Tech. 2019, 122, 1–6. [Google Scholar]
- Wang, S.; Chen, K.; Li, L.; Guo, X. Binding between proteins and cationic spherical polyelectrolyte brushes: Effect of pH, ionic strength, and stoichiometry. Biomacromolecules 2013, 14, 818–827. [Google Scholar] [CrossRef]


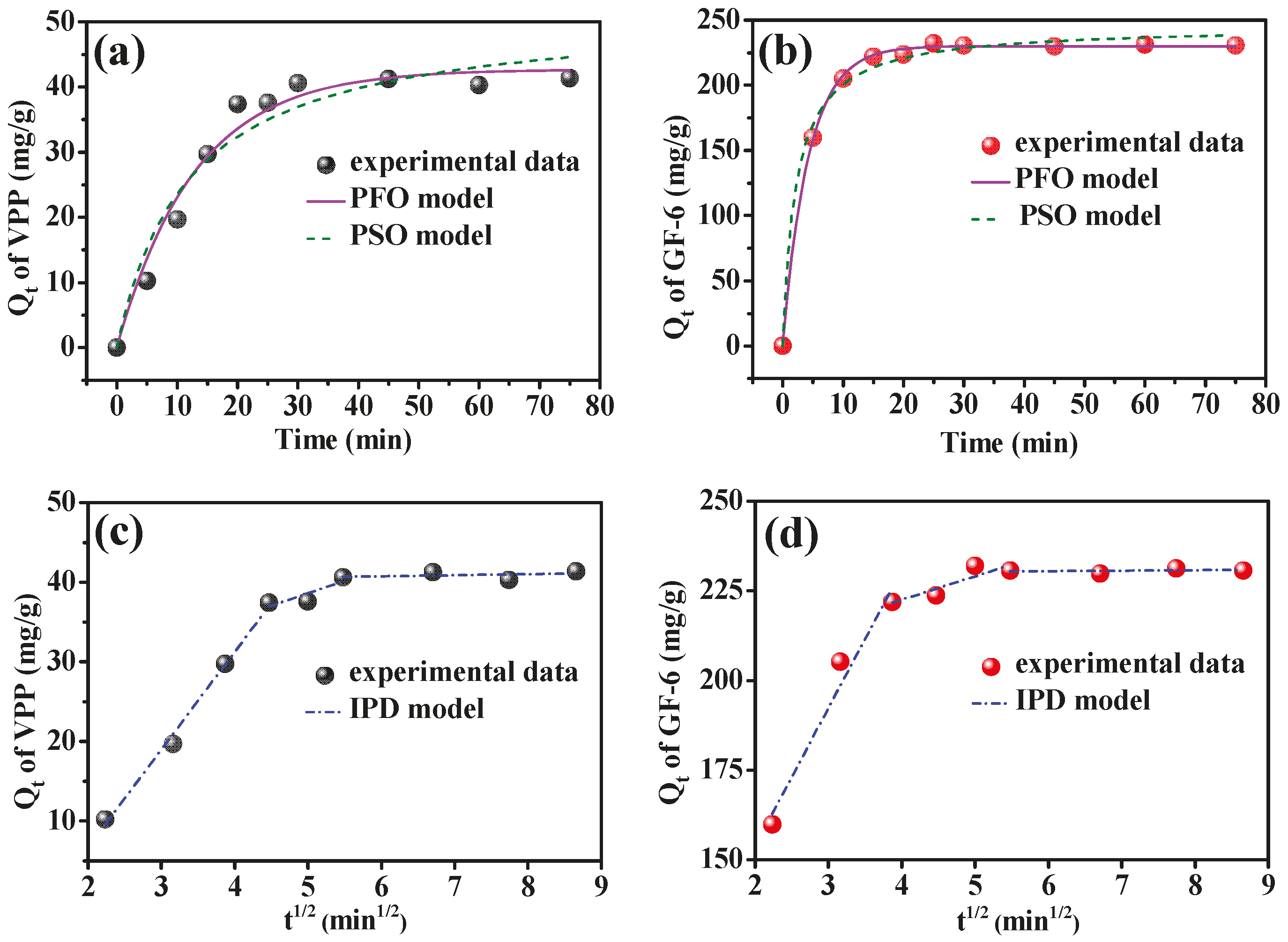



| PFO Model | PSO Model | ||||||
|---|---|---|---|---|---|---|---|
| Qe,exp (mg/g) | Qe,cal (mg/g) | k1 | R2 | Qe,cal (mg/g) | k2 | R2 | |
| VPP | 40.88 | 42.68 | 0.0774 | 0.9757 | 51.61 | 0.00163 | 0.9472 |
| GF-6 | 230.37 | 229.98 | 0.2319 | 0.9992 | 245.29 | 0.00182 | 0.9926 |
| First Step | Second Step | Third Step | |||||
|---|---|---|---|---|---|---|---|
| Kid (mg/(g∙min1/2) | C | R2 | Kid (mg/(g∙min1/2) | C | R2 | ||
| VPP | 12.26 | −17.84 | 0.9924 | 3.134 | 22.91 | 0.8777 | Balance stage |
| GF-6 | 38.49 | 76.70 | 0.9326 | 6.511 | 196.45 | 0.7069 | |
| Langmuir Model | Freundlich Model | D–R Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| kL (mL/mg) | Qm (mg/g) | R2 | kF | n | R2 | Qm (mg/g) | E (KJ/mol) | R2 | |
| VPP | 1.4211 | 53.44 | 0.9501 | 30.53 | 2.816 | 0.9107 | 54.86 | 0.552 | 0.9556 |
| GF-6 | 2.0131 | 308.78 | 0.9589 | 198.02 | 2.544 | 0.8860 | 357.70 | 0.561 | 0.9441 |
| Protein release (%) | |||
| Agents | ACE@mZIF-8 | AmZP | AmZPP5000 |
| 2 mg/mL VPP | 7.32 | 1.37 | 1.24 |
| 2 mg/mL GF-6 | 6.21 | 1.08 | 1.13 |
| Enzyme activity release (%) | |||
| 2 mol/L NaCl | 3.84 | 0.21 | 0.20 |
| 0.5 mg/mL BSA | 1.52 | 0.00 | 0.00 |
| Sample | IC50 (mg/mL) |
|---|---|
| Hydrolysates | 0.623 |
| Desorbate of AmZP | 0.095 |
| Desorbate of AmZPP5000 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhou, Q.; Liu, S.; Liao, D.; Liu, P.; Lan, X. Anchoring of Polymer Loops on Enzyme-Immobilized Mesoporous ZIF-8 Enhances the Recognition Selectivity of Angiotensin-Converting Enzyme Inhibitory Peptides. Molecules 2023, 28, 3117. https://doi.org/10.3390/molecules28073117
Wang Z, Zhou Q, Liu S, Liao D, Liu P, Lan X. Anchoring of Polymer Loops on Enzyme-Immobilized Mesoporous ZIF-8 Enhances the Recognition Selectivity of Angiotensin-Converting Enzyme Inhibitory Peptides. Molecules. 2023; 28(7):3117. https://doi.org/10.3390/molecules28073117
Chicago/Turabian StyleWang, Zefen, Qian Zhou, Siyuan Liu, Dankui Liao, Pengru Liu, and Xiongdiao Lan. 2023. "Anchoring of Polymer Loops on Enzyme-Immobilized Mesoporous ZIF-8 Enhances the Recognition Selectivity of Angiotensin-Converting Enzyme Inhibitory Peptides" Molecules 28, no. 7: 3117. https://doi.org/10.3390/molecules28073117