Multifaceted Characterization for the Hepatic Clearance of Graphene Oxide and Size-Related Hepatic Toxicity
Abstract
:1. Introduction
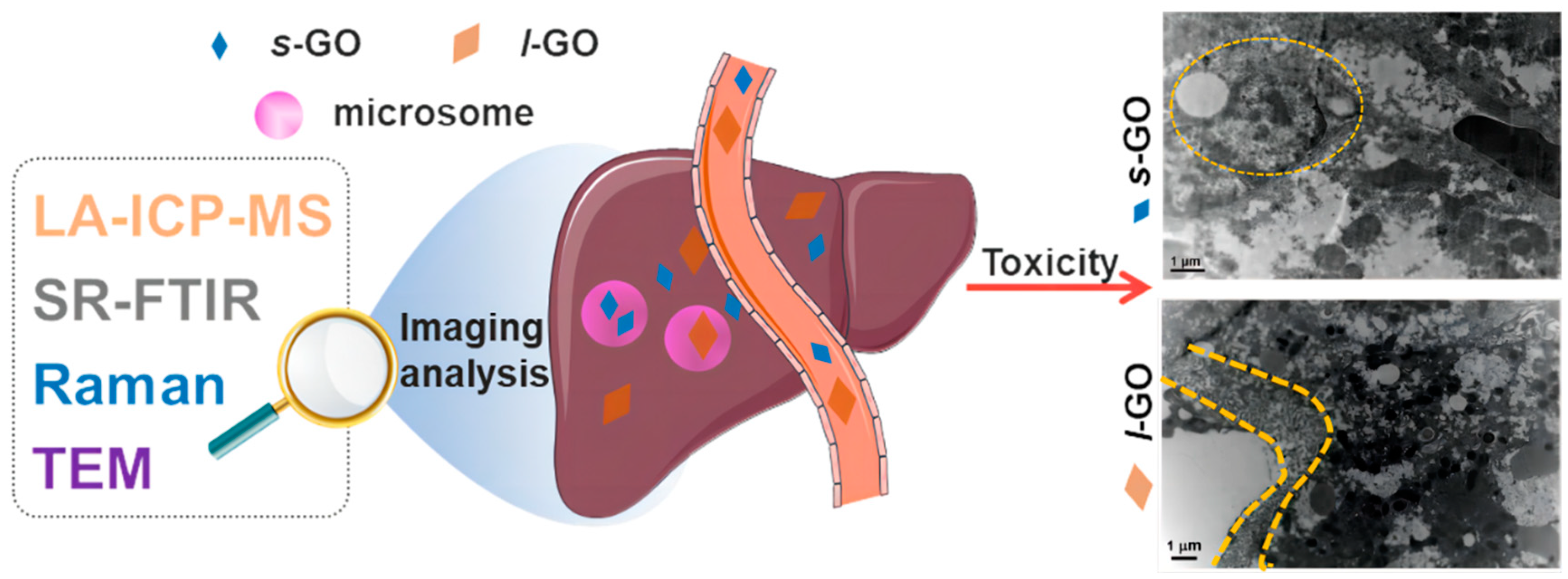
2. Results and Discussion
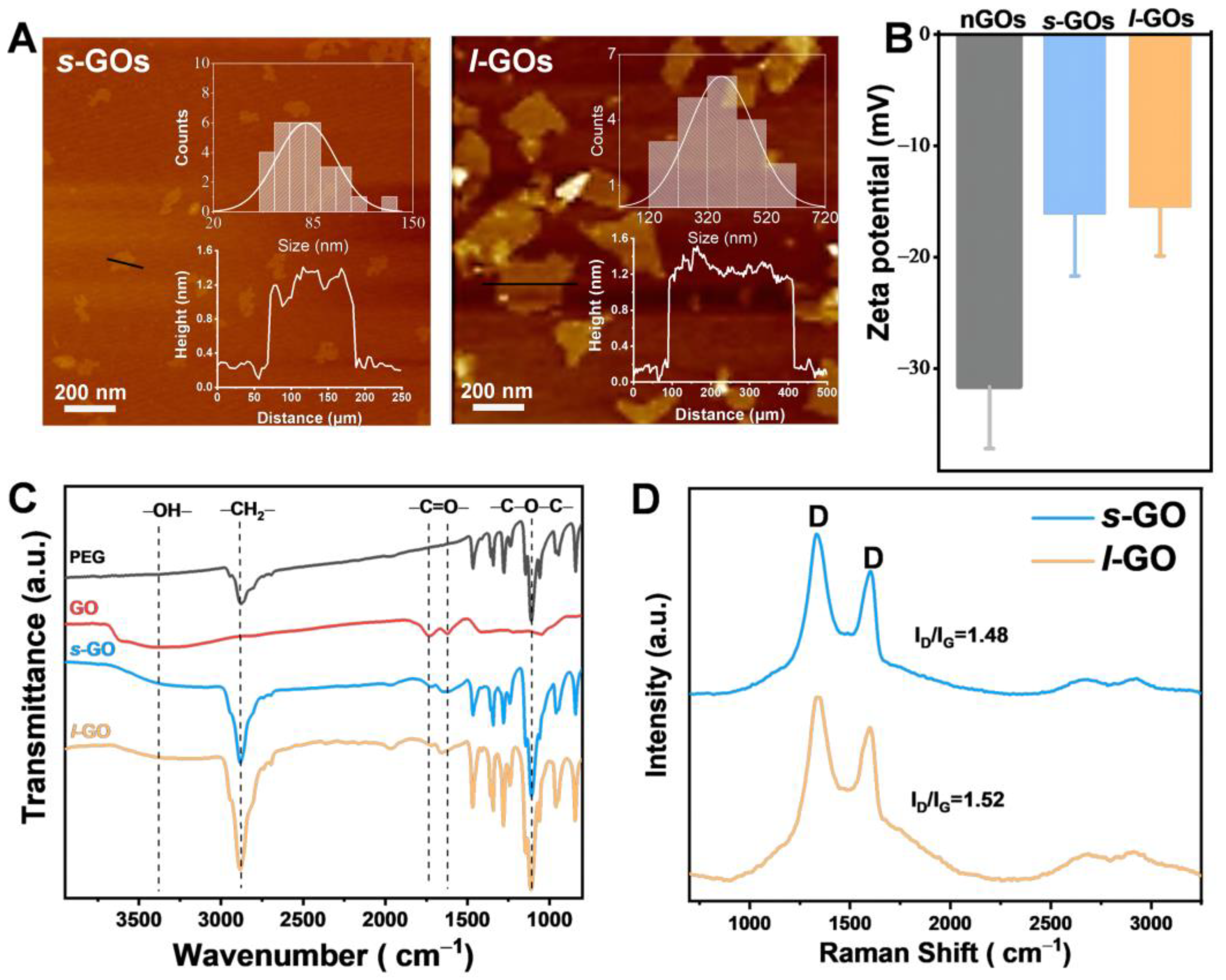
2.1. Physicochemical Properties of Graphene Oxide Nanosheets (GOs)
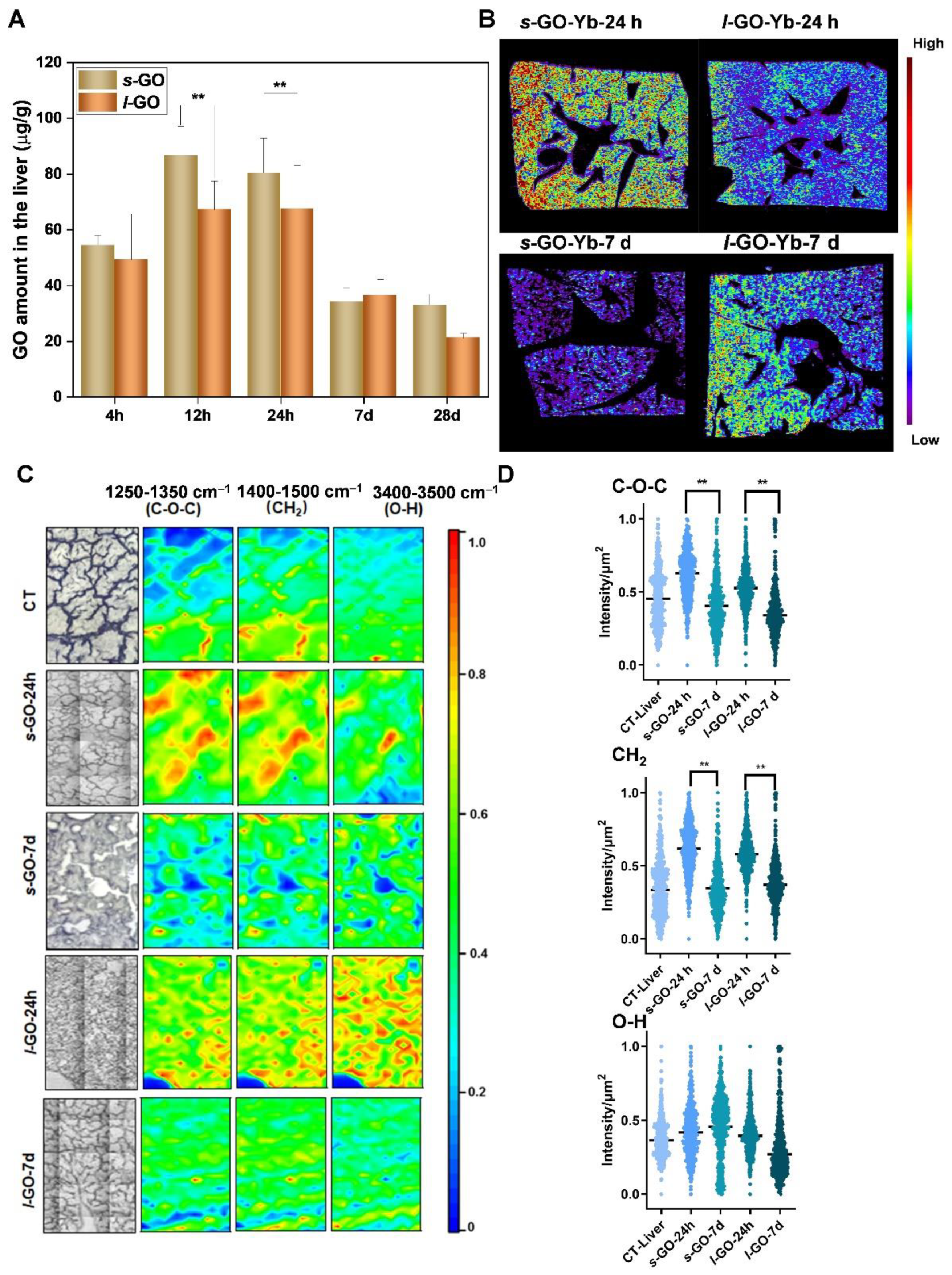
2.2. Size-Dependent Hepatic Clearance Patterns of GO
2.3. Hepatobiliary Excretion of s-GO and l-GO
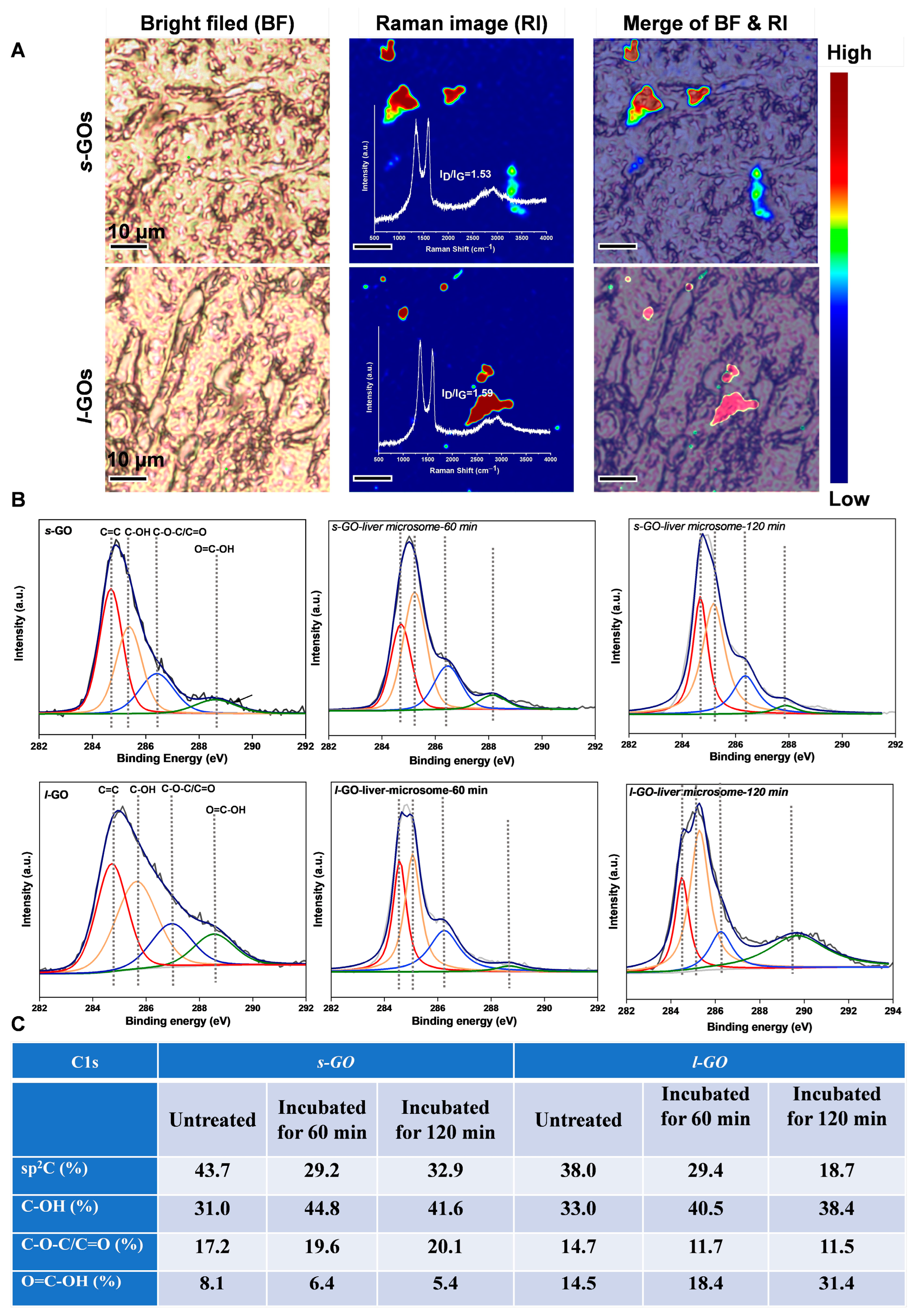
2.4. Size-Dependent Hepatic Toxic Effects of GO
3. Experimental Methods
3.1. Chemicals and Materials
3.2. Preparation and Physicochemical Characterization of PEGylated s-GOs and l-GOs
3.3. Animal Experiment
3.4. Rare Earth Element Yb Label, ICP-MS Analysis, and LA-ICP-MS Elemental Imaging Analysis
3.5. Confocal Raman Spectroscopy Imaging
3.6. Synchrotron Radiation-Based FT-IR Imaging
3.7. TEM Imaging
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, A.; Meng, K.; Liu, Y.; Pan, Y.; Qu, W.; Chen, D.; Xie, S. Absorption, distribution, metabolism, and excretion of nanocarriers in vivo and their influences. Adv. Colloid Interface Sci. 2020, 284, 102261. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.; Xia, T. Understanding nanomaterial-liver interactions to facilitate the development of safer nanoapplications. Adv. Mater. 2022, 34, 2106456. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.C.; Liu, L.; Pang, K.S.; Chan, W.C.W. Pharmacokinetics of nanoscale quantum dots: In vivo distribution, sequestration, and clearance in the rat. Adv. Funct. Mater. 2006, 16, 1299–1305. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Poon, W.; Tavares, A.J.; McGilvray, I.D.; Chan, W.C.W. Nanoparticle-liver interactions: Cellular uptake and hepatobiliary elimination. J. Control. Release 2016, 240, 332–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Rivers-Auty, J.; Crica, L.E.; Barr, K.; Rosano, V.; Arranz, A.E.; Loret, T.; Spiller, D.; Bussy, C.; Kostarelos, K.; et al. Dynamic interactions and intracellular fate of label-free, thin graphene oxide sheets within mammalian cells: Role of lateral sheet size. Nanoscale Adv. 2021, 3, 4166–4185. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Chen, Y.; Zareian, M.; Pandit, S.; Mijakovic, I. Cellular and subcellular interactions of graphene-based materials with cancerous and non-cancerous cells. Adv. Drug Deliv. Rev. 2022, 189, 114467. [Google Scholar] [CrossRef]
- Sun, T.; Kang, Y.; Liu, J.; Zhang, Y.; Ou, L.; Liu, X.; Lai, R.; Shao, L. Nanomaterials and hepatic disease: Toxicokinetics, disease types, intrinsic mechanisms, liver susceptibility, and influencing factors. J. Nanobiotechnol. 2021, 19, 108. [Google Scholar] [CrossRef]
- Tsoi, K.M.; MacParland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of hard-nanomaterial clearance by the liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef]
- Yu, H.; Wang, B.; Zhou, S.; Zhu, M.; Chen, W.; Chen, H.; Li, X.; Liang, S.; Wang, M.; Zheng, L.; et al. Polyvinylpyrrolidone functionalization induces deformable structure of graphene oxide nanosheets for lung-targeting delivery. Nano Today 2021, 38, 101151. [Google Scholar] [CrossRef]
- Abbina, S.; Takeuchi, L.E.; Anilkumar, P.; Yu, K.; Rogalski, J.C.; Shenoi, R.A.; Constantinescu, I.; Kizhakkedathu, J.N. Blood circulation of soft nanomaterials is governed by dynamic remodeling of protein opsonins at nano-biointerface. Nat. Commun. 2020, 11, 3048. [Google Scholar] [CrossRef]
- Cui, G.; Wu, J.; Lin, J.; Liu, W.; Chen, P.; Yu, M.; Zhou, D.; Yao, G. Graphene-based nanomaterials for breast cancer treatment: Promising therapeutic strategies. J. Nanobiotechnol. 2021, 19, 211. [Google Scholar] [CrossRef]
- Itoo, A.M.; Vemula, S.L.; Gupta, M.T.; Giram, M.V.; Kumar, S.A.; Ghosh, B.; Biswas, S. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J. Control. Release 2022, 350, 26–59. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, M.J.; Kwak, S.Y.; Jeong, J.; Choi, D.; Choi, S.W.; Ryu, J.; Kang, K.S. Bioengineered liver crosslinked with nano-graphene oxide enables efficient liver regeneration via MMP suppression and immunomodulation. Nat. Commun. 2023, 14, 801. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.P.; Gliga, A.R.; Lazzaretto, B.; Brandner, B.; Fielden, M.; Vogt, C.; Newman, L.; Rodrigues, A.F.; Shao, W.; Fournier, P.M.; et al. Graphene oxide is degraded by neutrophils and the degradation products are non-genotoxic. Nanoscale 2018, 10, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Russier, J.; Squillaci, M.A.; Treossi, E.; Menard-Moyon, C.; Del Rio-Castillo, A.E.; Vazquez, E.; Samori, P.; Palermo, V.; Bianco, A. Dispersibility-dependent biodegradation of graphene oxide by myeloperoxidase. Small 2015, 11, 3985–3994. [Google Scholar] [CrossRef] [PubMed]
- Kurapati, R.; Mukherjee, S.P.; Martin, C.; Bepete, G.; Vazquez, E.; Penicaud, A.; Fadeel, B.; Bianco, A. Degradation of single-layer and few-layer graphene by neutrophil myeloperoxidase. Angew. Chem. Int. Ed. 2018, 57, 11722–11727. [Google Scholar] [CrossRef]
- Liang, S.; Wang, B.; Li, X.; Chu, R.; Yu, H.; Zhou, S.; Wang, M.; Chen, H.; Zheng, L.; Chai, Z.; et al. In vivo pharmacokinetics, transfer and clearance study of graphene oxide by La/Ce dual elemental labelling method. NanoImpact 2020, 17, 100213. [Google Scholar] [CrossRef]
- Quagliarini, E.; Pozzi, D.; Cardarelli, F.; Caracciolo, G. The influence of protein corona on Graphene Oxide: Implications for biomedical theranostics. J. Nanobiotechnol. 2023, 21, 267. [Google Scholar] [CrossRef] [PubMed]
- Charmi, J.; Nosrati, H.; Mostafavi Amjad, J.; Mohammadkhani, R.; Danafar, H. Polyethylene glycol (PEG) decorated graphene oxide nanosheets for controlled release curcumin delivery. Heliyon 2019, 5, e01466. [Google Scholar] [CrossRef]
- Chen, W.; Wang, B.; Liang, S.; Wang, M.; Zheng, L.; Xu, S.; Wang, J.; Fang, H.; Yang, P.; Feng, W. Renal clearance of graphene oxide: Glomerular filtration or tubular secretion and selective kidney injury association with its lateral dimension. J. Nanobiotechnol. 2023, 21, 51. [Google Scholar] [CrossRef]
- Ray, K. Liver: Clearance of nanomaterials in the liver. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 560. [Google Scholar] [CrossRef]
- Wang, X.; Chang, C.H.; Jiang, J.; Liu, X.; Li, J.; Liu, Q.; Liao, Y.P.; Li, L.; Nel, A.E.; Xia, T. Mechanistic differences in cell death responses to metal-based engineered nanomaterials in Kupffer cells and hepatocytes. Small 2020, 16, 2000528. [Google Scholar] [CrossRef]
- Jasim, D.A.; Newman, L.; Rodrigues, A.F.; Vacchi, I.A.; Lucherelli, M.A.; Lozano, N.; Menard-Moyon, C.; Bianco, A.; Kostarelos, K. The impact of graphene oxide sheet lateral dimensions on their pharmacokinetic and tissue distribution profiles in mice. J. Control. Release 2021, 338, 330–340. [Google Scholar] [CrossRef]
- Jasim, D.A.; Murphy, S.; Newman, L.; Mironov, A.; Prestat, E.; McCaffrey, J.; Menard-Moyon, C.; Rodrigues, A.F.; Bianco, A.; Haigh, S.; et al. The effects of extensive glomerular filtration of thin graphene oxide sheets on kidney physiology. ACS Nano 2016, 10, 10753–10767. [Google Scholar] [CrossRef]
- Poon, W.; Zhang, Y.N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.Y.; Wilhelm, S.; Chan, W.C.W. Elimination pathways of nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef]
- Wang, X.; He, B.; Shi, J.; Li, Q.; Zhu, H.J. Comparative proteomics analysis of human liver microsomes and S9 fractions. Drug Metab. Dispos. 2020, 48, 31–40. [Google Scholar] [CrossRef]
- Kurapati, R.; Martìn, C.; Palermo, V.; Nishina, Y.; Bianco, A. Biodegradation of graphene materials catalyzed by human eosinophil peroxidase. Faraday Discuss. 2021, 227, 189–203. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Molecular mechanisms of the microsomal mixed function oxidases and biological and pathological implications. Redox Biol. 2015, 4, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Romaldini, A.; Spano, R.; Catalano, F.; Villa, F.; Poggi, A.; Sabella, S. Sub-Lethal Concentrations of Graphene Oxide Trigger Acute-Phase Response and Impairment of Phase-I Xenobiotic Metabolism in Upcyte Hepatocytes. Front. Bioeng. Biotechnol. 2022, 10, 867728. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, R.; Wang, X.; Liu, Q.; Chen, Y.; Valle, R.P.; Zuo, Y.; Xia, T.; Liu, S. Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals. ACS Nano 2015, 9, 10498–10515. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.F.; Newman, L.; Jasim, D.; Mukherjee, S.P.; Wang, J.; Vacchi, I.A.; Menard-Moyon, C.; Bianco, A.; Fadeel, B.; Kostarelos, K.; et al. Size-dependent pulmonary impact of thin graphene oxide sheets in mice: Toward safe-by-design. Adv. Sci. 2020, 7, 1903200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Mei, K.C.; Chang, C.H.; Jiang, J.; Liu, X.; Liu, Q.; Guiney, L.M.; Hersam, M.C.; Liao, Y.P.; et al. Lateral size of graphene oxide determines differential cellular uptake and cell death pathways in Kupffer cells, LSECs, and hepatocytes. Nano Today 2021, 37, 101061. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Wei, W.; Yue, Z.; Wang, B.; Luo, N.; Gao, Y.; Ma, D.; Ma, G.; Su, Z. The role of the lateral dimension of graphene oxide in the regulation of cellular responses. Biomaterials 2012, 33, 4013–4021. [Google Scholar] [CrossRef]
- Ding, X.; Pang, Y.; Liu, Q.; Zhang, H.; Wu, J.; Lei, J.; Zhang, T. GO-PEG Represses the Progression of Liver Inflammation via Regulating the M1/M2 Polarization of Kupffer Cells. Small 2024, 2306483. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Chen, W.; Liang, S.; Fang, H.; Zhang, M.; Wang, M.; Zheng, L.; Wang, B.; Bi, Y.; Feng, W. Multifaceted Characterization for the Hepatic Clearance of Graphene Oxide and Size-Related Hepatic Toxicity. Molecules 2024, 29, 1335. https://doi.org/10.3390/molecules29061335
Su Z, Chen W, Liang S, Fang H, Zhang M, Wang M, Zheng L, Wang B, Bi Y, Feng W. Multifaceted Characterization for the Hepatic Clearance of Graphene Oxide and Size-Related Hepatic Toxicity. Molecules. 2024; 29(6):1335. https://doi.org/10.3390/molecules29061335
Chicago/Turabian StyleSu, Zongyi, Wei Chen, Shanshan Liang, Hao Fang, Minglu Zhang, Meng Wang, Lingna Zheng, Bing Wang, Yi Bi, and Weiyue Feng. 2024. "Multifaceted Characterization for the Hepatic Clearance of Graphene Oxide and Size-Related Hepatic Toxicity" Molecules 29, no. 6: 1335. https://doi.org/10.3390/molecules29061335





