Tailoring the Formation of Functionalized Furans from Glucose in Water with Nature-Sourced Catalysts and In Situ NMR
Abstract
:1. Introduction
2. Results and Discussion
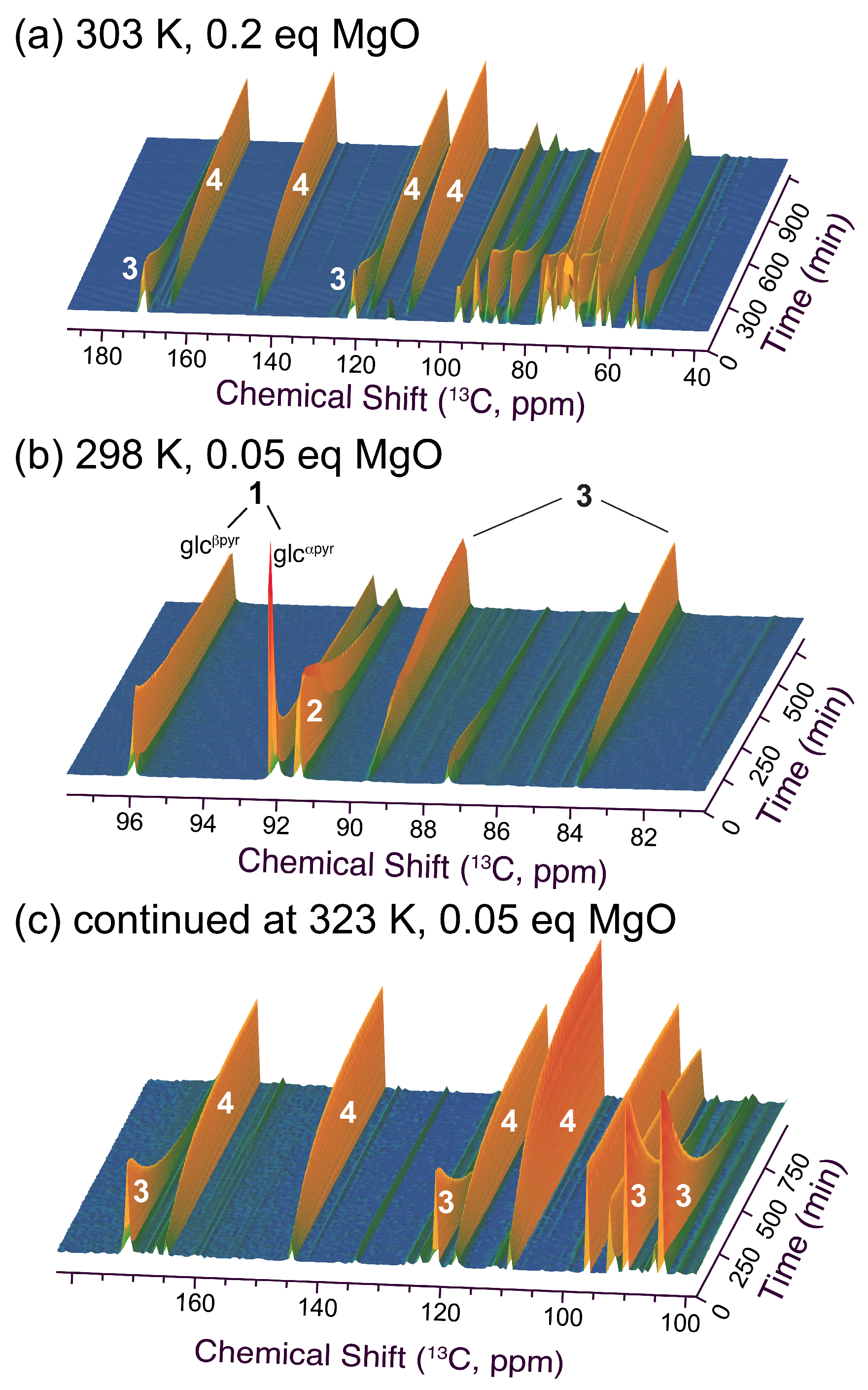
2.1. In Situ 13C NMR on MgO-Catalyzed Glucose Reaction with Malononitrile Clarifies Mechanism and Identifies the Kinetically Most Stable Intermediates
2.2. Filtration Test to Gain Insight on the Catalytically Active Species from MgO
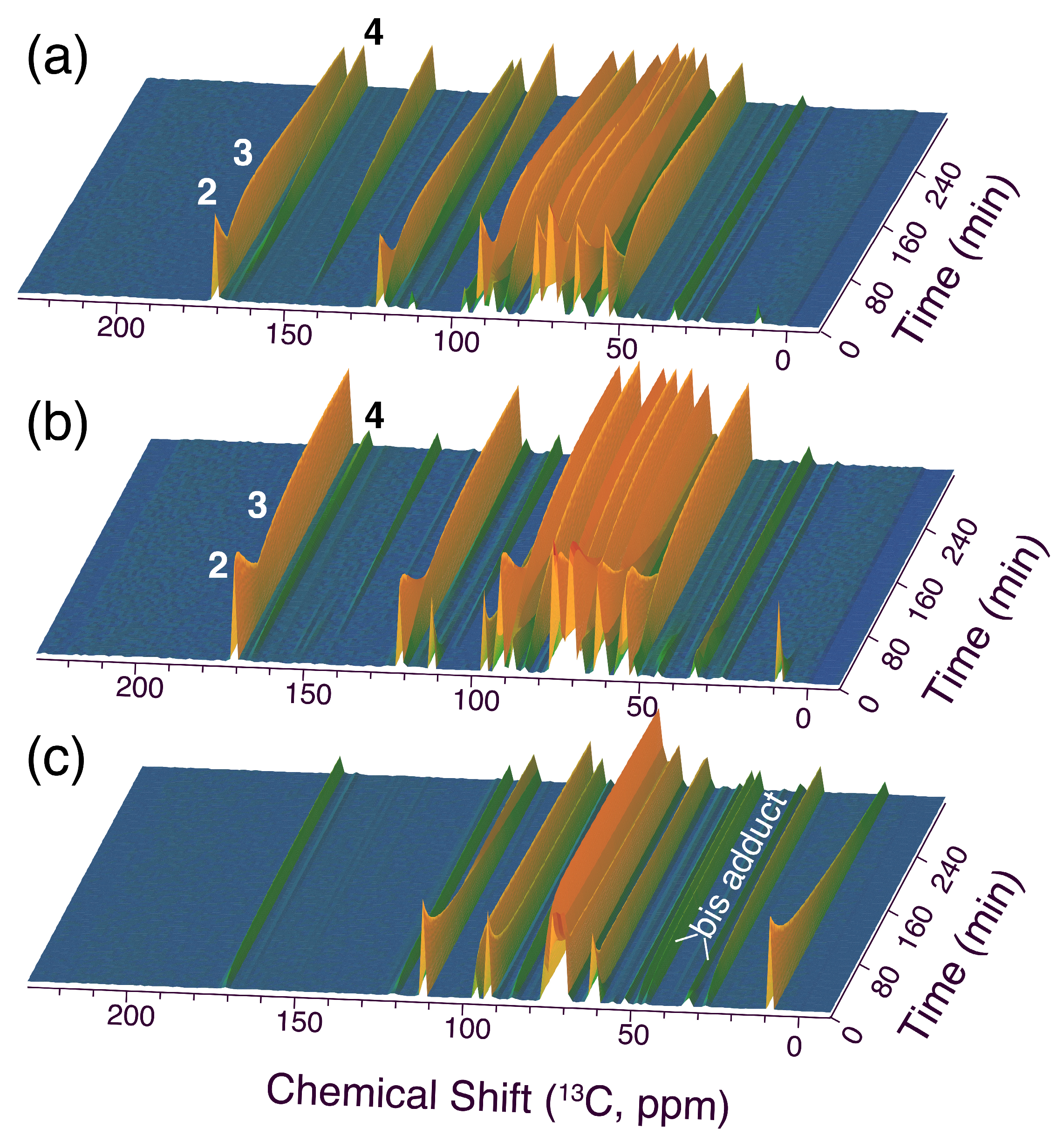
2.3. Viability of a Glucose-Derived Catalyst: N-Methyl-d-Glucamine
2.4. Catalysis by Chitosan
3. Materials and Methods
3.1. Chemicals
3.2. General Reaction Procedure
3.3. NMR Samples
3.4. NMR Spectroscopy
3.5. Filtration Test and pH Changes during the Reaction
3.6. Data Plotting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dusselier, M.; Van Wouwe, P.; de Clippel, F.; Dijkmans, J.; Gammon, D.W.; Sels, B.F. Mechanistic Insight into the Conversion of Tetrose Sugars to Novel α-Hydroxy Acid Platform Molecules. ChemCatChem 2013, 5, 569–575. [Google Scholar] [CrossRef]
- De Clippel, F.; Dusselier, M.; Van Rompaey, R.; Vanelderen, P.; Dijkmans, J.; Makshina, E.; Giebeler, L.; Oswald, S.; Baron, G.V.; Denayer, J.F.M.; et al. Fast and Selective Sugar Conversion to Alkyl Lactate and Lactic Acid with Bifunctional Carbon–Silica Catalysts. J. Am. Chem. Soc. 2012, 134, 10089–10101. [Google Scholar] [CrossRef]
- Dusselier, M.; Van Wouwe, P.; De Smet, S.; De Clercq, R.; Verbelen, L.; Van Puyvelde, P.; Du Prez, F.E.; Sels, B.F. Toward Functional Polyester Building Blocks from Renewable Glycolaldehyde with Sn Cascade Catalysis. ACS Catal. 2013, 3, 1786–1800. [Google Scholar] [CrossRef]
- Holm, M.S.; Saravanamurugan, S.; Taarning, E. Conversion of Sugars to Lactic Acid Derivatives Using Heterogeneous Zeotype Catalysts. Science 2010, 328, 602–605. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)Furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, F.W.; Peters, S. Carbohydrates as Green Raw Materials for the Chemical Industry. Comptes. Rendus. Chim. 2004, 7, 65–90. [Google Scholar] [CrossRef]
- Chatterjee, C.; Pong, F.; Sen, A. Chemical Conversion Pathways for Carbohydrates. Green Chem. 2015, 17, 40–71. [Google Scholar] [CrossRef]
- Binder, J.B.; Blank, J.J.; Cefali, A.V.; Raines, R.T. Synthesis of Furfural from Xylose and Xylan. ChemSusChem 2010, 3, 1268–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, Z.; Song, J.; Zhou, Y.; Han, B. Efficient Conversion of Glucose into 5-Hydroxymethylfurfural Catalyzed by a Common Lewis Acid SnCl4 in an Ionic Liquid. Green Chem. 2009, 11, 1746. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent Catalytic Routes for the Preparation and the Upgrading of Biomass Derived Furfural and 5-Hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Meier, S. Mechanism and Malleability of Glucose Dehydration to HMF: Entry Points and Water-Induced Diversions. Catal. Sci. Technol. 2020, 10, 1724–1730. [Google Scholar] [CrossRef]
- Drabo, P.; Fischer, M.; Toussaint, V.; Flecken, F.; Palkovits, R.; Delidovich, I. What Are the Catalytically Active Species for Aqueous-Phase Isomerization of D-Glucose into D-Fructose in the Presence of Alkaline Earth Metal (Hydr)Oxides? J. Catal. 2021, 402, 315–324. [Google Scholar] [CrossRef]
- Guo, Z.; Pedersen, C.M.; Chang, H.; Wang, Y.; Qiao, Y. Highly Efficient Production and Purification of Fructose via Glucose Isomerization by Calcium Chloride and Triethylamine. Green Chem. 2023, 25, 6297–6305. [Google Scholar] [CrossRef]
- Saravanamurugan, S.; Riisager, A.; Taarning, E.; Meier, S. Mechanism and Stereoselectivity of Zeolite-Catalysed Sugar Isomerisation in Alcohols. Chem. Commun. 2016, 52, 12773–12776. [Google Scholar] [CrossRef] [PubMed]
- Román-Leshkov, Y.; Moliner, M.; Labinger, J.A.; Davis, M.E. Mechanism of Glucose Isomerization Using a Solid Lewis Acid Catalyst in Water. Angew. Chem. 2010, 122, 9138–9141. [Google Scholar] [CrossRef]
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. The Mechanism of Glucose Isomerization to Fructose over Sn-BEA Zeolite: A Periodic Density Functional Theory Study. ChemSusChem 2013, 6, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Monrad, R.N.; Madsen, R. Modern Methods for Shortening and Extending the Carbon Chain in Carbohydrates at the Anomeric Center. Tetrahedron 2011, 67, 8825–8850. [Google Scholar] [CrossRef]
- Barsøe, L.R.; Saravanamurugan, S.; Taarning, E.; Espin, J.S.M.; Meier, S. Heterogeneous Base-Catalyzed Conversion of Glycolaldehyde to Aldotetroses: Mechanistic and Kinetic Insight. ChemCatChem 2021, 13, 5141–5147. [Google Scholar] [CrossRef]
- Szekrenyi, A.; Garrabou, X.; Parella, T.; Joglar, J.; Bujons, J.; Clapés, P. Asymmetric Assembly of Aldose Carbohydrates from Formaldehyde and Glycolaldehyde by Tandem Biocatalytic Aldol Reactions. Nat. Chem. 2015, 7, 724–729. [Google Scholar] [CrossRef]
- Van de Vyver, S.; Odermatt, C.; Romero, K.; Prasomsri, T.; Román-Leshkov, Y. Solid Lewis Acids Catalyze the Carbon–Carbon Coupling between Carbohydrates and Formaldehyde. ACS Catal. 2015, 5, 972–977. [Google Scholar] [CrossRef]
- Lambu, M.R.; Judeh, Z.M.A. Efficient, One-Step, Cascade Synthesis of Densely Functionalized Furans from Unprotected Carbohydrates in Basic Aqueous Media. Green Chem. 2019, 21, 821–829. [Google Scholar] [CrossRef]
- Scherrmann, M.-C. Knoevenagel Reaction of Unprotected Sugars. In Carbohydrates in Sustainable Development II; Rauter, A.P., Vogel, P., Queneau, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–18. ISBN 978-3-642-15161-3. [Google Scholar]
- Voigt, B.; Matviitsuk, A.; Mahrwald, R. Organocatalyzed Knoevenagel-Addition—Simple Access to Carbon Chain-Elongated Branched Carbohydrates. Tetrahedron 2013, 69, 4302–4310. [Google Scholar] [CrossRef]
- Van Beurden, K.; De Koning, S.; Molendijk, D.; Van Schijndel, J. The Knoevenagel Reaction: A Review of the Unfinished Treasure Map to Forming Carbon–Carbon Bonds. Green Chem. Lett. Rev. 2020, 13, 349–364. [Google Scholar] [CrossRef]
- Van Der Helm, M.P.; Klemm, B.; Eelkema, R. Organocatalysis in Aqueous Media. Nat. Rev. Chem. 2019, 3, 491–508. [Google Scholar] [CrossRef]
- Chavan, H.V.; Bandgar, B.P. Aqueous Extract of Acacia Concinna Pods: An Efficient Surfactant Type Catalyst for Synthesis of 3-Carboxycoumarins and Cinnamic Acids via Knoevenagel Condensation. ACS Sustain. Chem. Eng. 2013, 1, 929–936. [Google Scholar] [CrossRef]
- Mohr, J.T.; Krout, M.R.; Stoltz, B.M. Natural Products as Inspiration for the Development of Asymmetric Catalysis. Nature 2008, 455, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Ronaghi, N.; Jones, E.V.; Moon, H.J.; Park, S.J.; Jones, C.W.; France, S. Selective Conversion of Malononitrile and Unprotected Carbohydrates to Bicyclic Polyhydroxyalkyl Dihydrofurans Using Magnesium Oxide as a Recyclable Catalyst. ACS Sustain. Chem. Eng. 2022, 10, 5966–5975. [Google Scholar] [CrossRef]
- Elliot, S.G.; Taarning, E.; Madsen, R.; Meier, S. NMR Spectroscopic Isotope Tracking Reveals Cascade Steps in Carbohydrate Conversion by Tin-Beta. ChemCatChem 2018, 10, 1414–1419. [Google Scholar] [CrossRef]
- Dumez, J.-N. NMR Methods for the Analysis of Mixtures. Chem. Commun. 2022, 58, 13855–13872. [Google Scholar] [CrossRef]
- Jensen, P.R.; Meier, S. Catalytic Cycle of Carbohydrate Dehydration by Lewis Acids: Structures and Rates from Synergism of Conventional and DNP NMR. Chem. Commun. 2020, 56, 6245–6248. [Google Scholar] [CrossRef]
- Bertz, S.H.; Cope, S.; Murphy, M.; Ogle, C.A.; Taylor, B.J. Rapid Injection NMR in Mechanistic Organocopper Chemistry. Preparation of the Elusive Copper(III) Intermediate. J. Am. Chem. Soc. 2007, 129, 7208–7209. [Google Scholar] [CrossRef]
- Ben-Tal, Y.; Boaler, P.J.; Dale, H.J.A.; Dooley, R.E.; Fohn, N.A.; Gao, Y.; García-Domínguez, A.; Grant, K.M.; Hall, A.M.R.; Hayes, H.L.D.; et al. Mechanistic Analysis by NMR Spectroscopy: A Users Guide. Prog. Nucl. Magn. Reson. Spectrosc. 2022, 129, 28–106. [Google Scholar] [CrossRef] [PubMed]
- Blasco, T. Insights into Reaction Mechanisms in Heterogeneous Catalysis Revealed by in Situ NMR Spectroscopy. Chem. Soc. Rev. 2010, 39, 4685. [Google Scholar] [CrossRef]
- Kovacs, H.; Moskau, D.; Spraul, M. Cryogenically Cooled Probes—A Leap in NMR Technology. Prog. Nucl. Magn. Reson. Spectrosc. 2005, 46, 131–155. [Google Scholar] [CrossRef]
- Warthegau, S.S.; Karlsson, M.; Madsen, R.; Jensen, P.R.; Meier, S. Kinetic and Mechanistic Study of Aldose Conversion to Functionalized Furans in Aqueous Solutions. Catalysts 2024, 14, 199. [Google Scholar] [CrossRef]
- Mohy Eldin, M.S.; Soliman, E.A.; Hashem, A.I.; Tamer, T.M. Antimicrobial Activity of Novel Aminated Chitosan Derivatives for Biomedical Applications. Adv. Polym. Technol. 2012, 31, 414–428. [Google Scholar] [CrossRef]
- Malerz, S.; Mudryk, K.; Tomaník, L.; Stemer, D.; Hergenhahn, U.; Buttersack, T.; Trinter, F.; Seidel, R.; Quevedo, W.; Goy, C.; et al. Following in Emil Fischer’s Footsteps: A Site-Selective Probe of Glucose Acid–Base Chemistry. J. Phys. Chem. A 2021, 125, 6881–6892. [Google Scholar] [CrossRef]
- Feng, S.; Bagia, C.; Mpourmpakis, G. Determination of Proton Affinities and Acidity Constants of Sugars. J. Phys. Chem. A 2013, 117, 5211–5219. [Google Scholar] [CrossRef] [PubMed]
- Delidovich, I. Toward Understanding Base-Catalyzed Isomerization of Saccharides. ACS Catal. 2023, 13, 2250–2267. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warthegau, S.S.; Meier, S. Tailoring the Formation of Functionalized Furans from Glucose in Water with Nature-Sourced Catalysts and In Situ NMR. Molecules 2024, 29, 1368. https://doi.org/10.3390/molecules29061368
Warthegau SS, Meier S. Tailoring the Formation of Functionalized Furans from Glucose in Water with Nature-Sourced Catalysts and In Situ NMR. Molecules. 2024; 29(6):1368. https://doi.org/10.3390/molecules29061368
Chicago/Turabian StyleWarthegau, Stefan S., and Sebastian Meier. 2024. "Tailoring the Formation of Functionalized Furans from Glucose in Water with Nature-Sourced Catalysts and In Situ NMR" Molecules 29, no. 6: 1368. https://doi.org/10.3390/molecules29061368





