Preparation and Support Effect of Graphdiyne Nanotubes with Abundant Cu Quantum Dots
Abstract
:1. Introduction
2. Results and Discussion
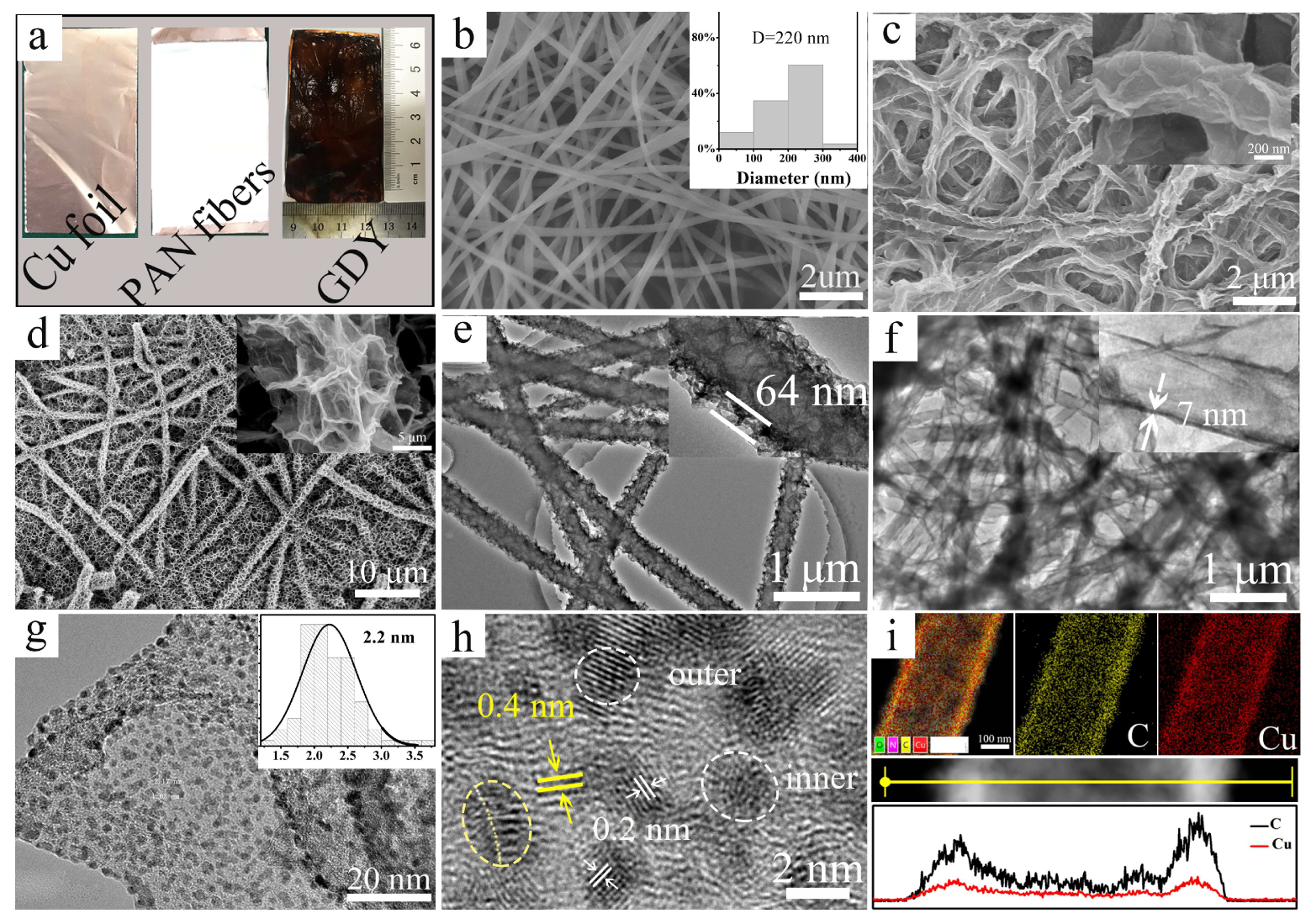
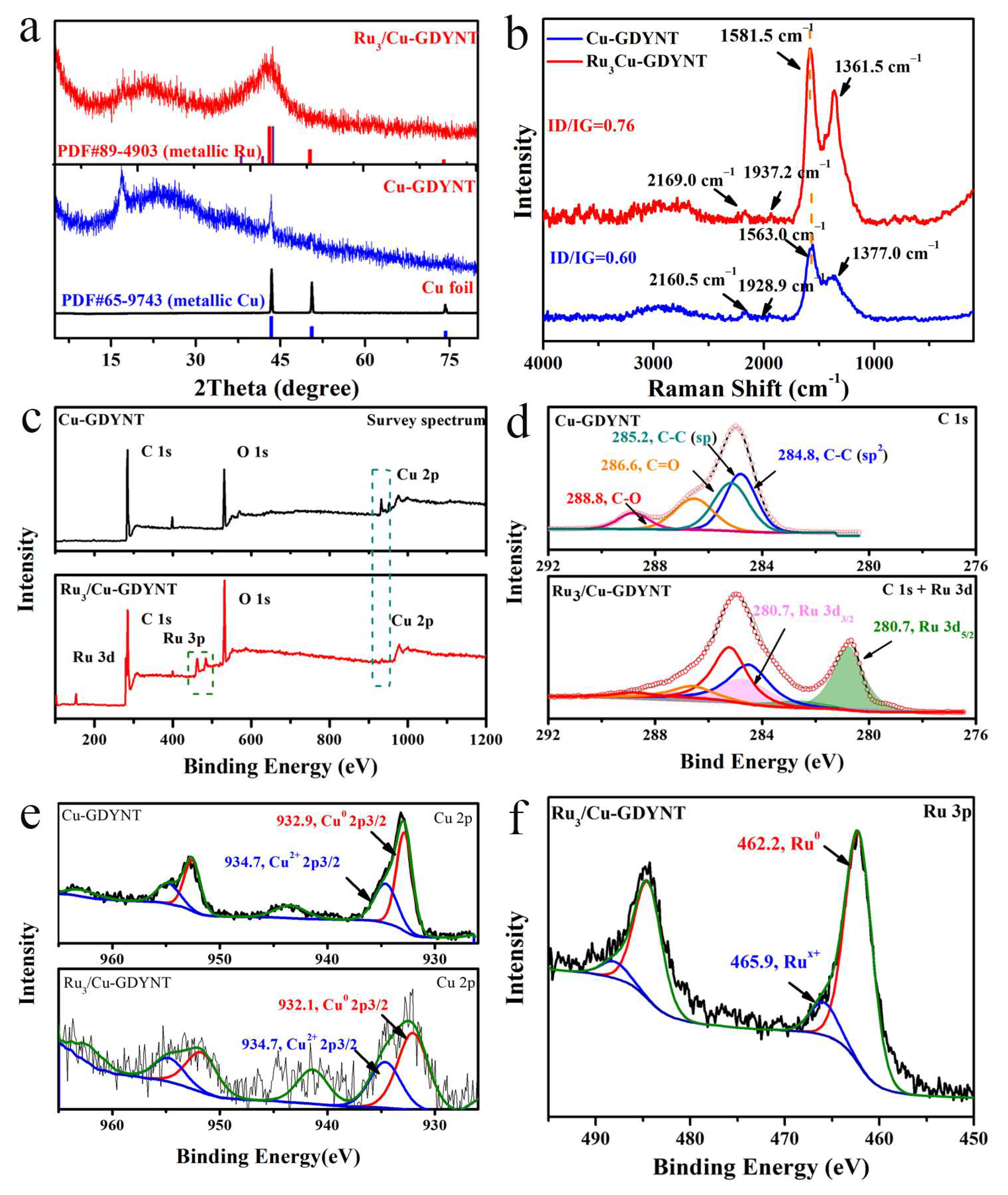
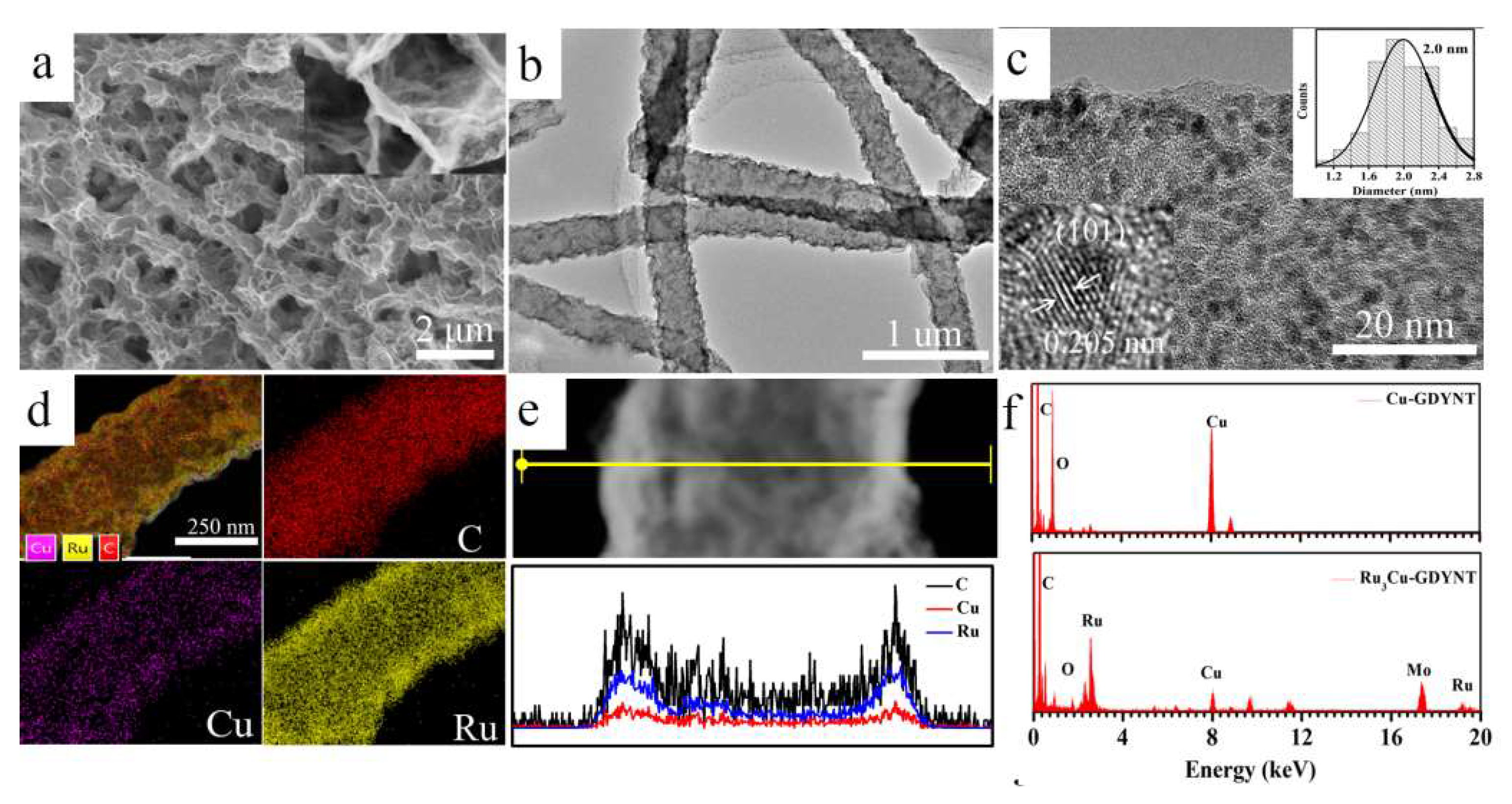
2.1. The Preparation and Characterization of Cu-GDYNT
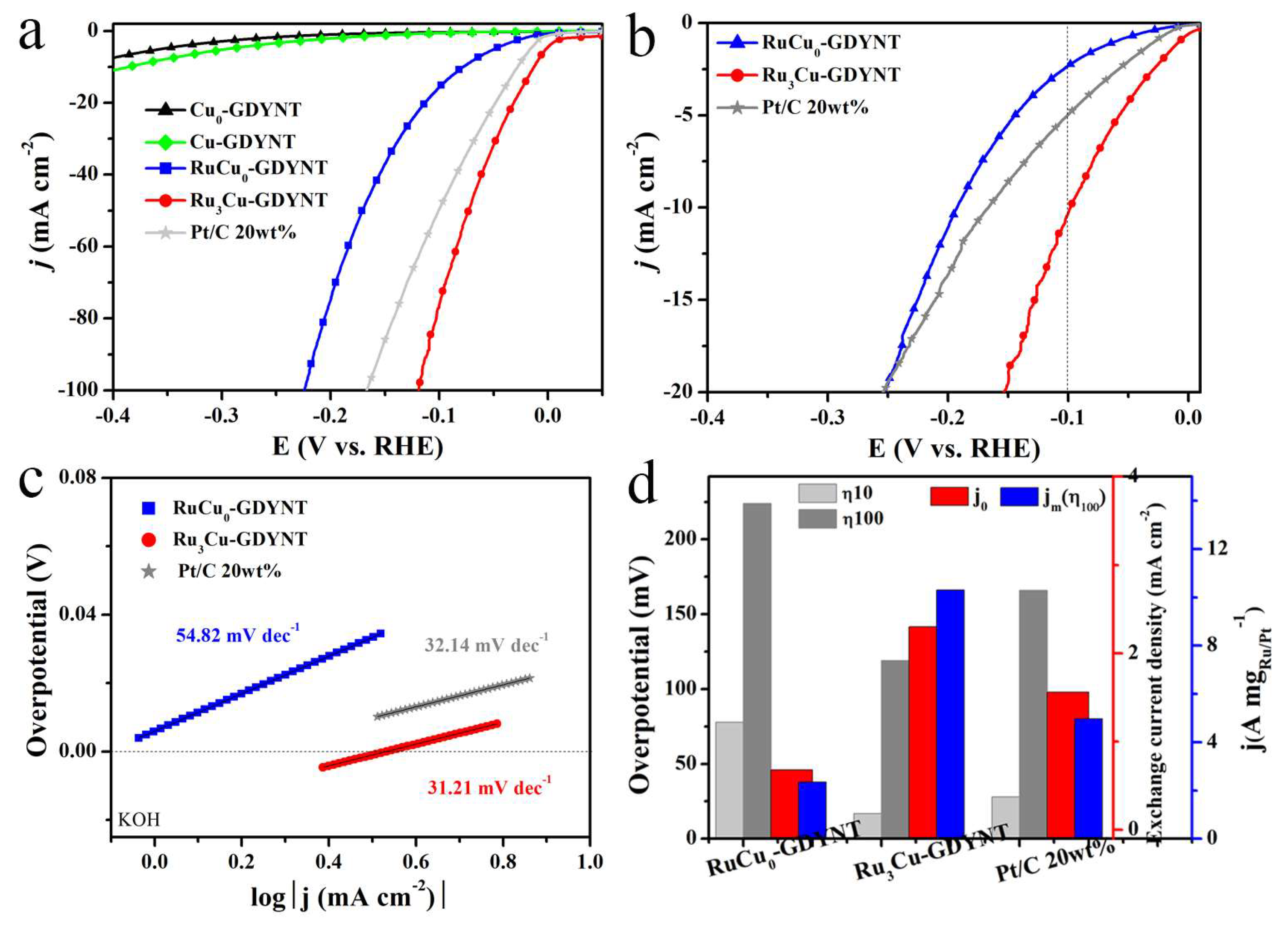
2.2. The Research on the Support Effect of Cu-GDYNT
2.3. The Verification of the Role of Cu
3. Materials and Method
3.1. Reagents and Materials
3.2. Synthesis of the Samples
3.3. Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Liu, H.; Wang, D.; Zhang, J. Graphdiyne: Synthesis, properties, and applications. Chem. Soc. Rev. 2019, 48, 908–936. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, Y.; Wang, N.; Xue, Y.; Zuo, Z.; Liu, H.; Li, Y. Progress in research into 2D graphdiyne-based materials. Chem. Rev. 2018, 118, 7744–7803. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-de-Arellano, J.M.; Canales, M.; Magaña, L.F. Carbon nanostructures doped with transition metals for pollutant gas adsorption systems. Molecules 2021, 26, 5346. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, Y.; Liu, H.; Guo, Y.; Li, Y.; Zhu, D. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256–3258. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, X.; Zhu, L.; Ghazzal, M.N.; Zhang, J.; Tung, C.-H.; Wu, L.-Z. Graphdiyne for crucial gas involved catalytic reactions in energy conversion applications. Energy Environ. Sci. 2020, 13, 1326–1346. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, H.; Yang, N.; Wang, D. Graphdiyne: Recent achievements in photo- and electrochemical conversion. Adv. Sci. 2018, 5, 1800959. [Google Scholar] [CrossRef]
- Zuo, Z.; Wang, D.; Zhang, J.; Lu, F.; Li, Y. Synthesis and applications of graphdiyne-based metal-free catalysts. Adv. Mater. 2019, 31, e1803762. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, X.; Zhang, Y.; Gao, H.; Wang, Y.; Shi, Q.; Rao, D.; Liu, Y.; Deng, K.; Lu, R. Graphdiyne as a high-efficiency membrane for separating oxygen from harmful gases: A first-principles study. ACS Appl. Mater. Interfaces 2016, 8, 28166–28170. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, X.; Yang, N. Graphdiyne electrochemistry: Progress and perspectives. Small 2022, 18, e2201135. [Google Scholar] [CrossRef]
- Wang, N.; He, J.; Wang, K.; Zhao, Y.; Jiu, T.; Huang, C.; Li, Y. Graphdiyne-based materials: Preparation and application for electrochemical energy storage. Adv. Mater. 2019, 31, e1803202. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Xie, Y.; Du, L.; Fan, Z.; Chen, X.; Fu, X.; Xie, W.; Wang, M.; Yuan, M. Stabilization of cobalt clusters with graphdiyne enabling efficient overall water splitting. Nano Energy 2020, 74, 104852. [Google Scholar] [CrossRef]
- Du, Y.; Xue, Y.; Zhang, C.; Liu, Y.; Fang, Y.; Xing, C.; He, F.; Li, Y. Photoinduced electrocatalysis on 3D flexible OsOx quantum dots. Adv. Energy Mater. 2021, 11, 2100234. [Google Scholar] [CrossRef]
- Ma, D.; Zeng, Z.; Liu, L.; Jia, Y. Theoretical screening of the transition metal heteronuclear dimer anchored graphdiyne for electrocatalytic nitrogen reduction. J. Energy Chem. 2021, 54, 501–509. [Google Scholar] [CrossRef]
- Xie, W.; Liu, K.; Shi, G.; Fu, X.; Chen, X.; Fan, Z.; Liu, M.; Yuan, M.; Wang, M. CoS2 nanowires supported graphdiyne for highly efficient hydrogen evolution reaction. J. Energy Chem. 2021, 60, 272–278. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, Y.; Pan, C.; Wang, C.; Hu, S.; Xiao, W.; Chi, X.; Fang, Y.; Yang, J.; Deng, H.; et al. Interfacial sp C-O-Mo hybridization originated high-current density hydrogen evolution. J. Am. Chem. Soc. 2021, 143, 8720–8730. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, X.; Lin, H.; Li, J.; Zhang, H.; Guo, J.; Jia, D.; Zhang, H. A novel carbon support: Few-layered graphdiyne-decorated carbon nanotubes capture metal clusters as effective metal-supported catalysts. Small 2021, 17, 2006442. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wu, X.; Jia, W.; Guo, J.; Zhang, H.; Liu, H.; Jia, D.; Tong, F. Graphdiyne-anchored ultrafine NiFe hydroxide nanodots electrocatalyst for water oxidation with high mass activity and superior durability. Carbon 2020, 169, 45–54. [Google Scholar] [CrossRef]
- Yu, H.; Xue, Y.; Li, Y. Graphdiyne and its assembly architectures: Synthesis, functionalization, and applications. Adv. Mater. 2019, 31, e1803101. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xue, Y.; Li, Y.; Yu, H.; Hui, L.; Liu, Y.; Xing, C.; Zhang, C.; Zhang, D.; Wang, Z.; et al. Graphdiyne interface engineering: Highly active and selective ammonia synthesis. Angew. Chem. Int. Ed. 2020, 59, 13021–13027. [Google Scholar] [CrossRef]
- Hui, L.; Xue, Y.; He, F.; Jia, D.; Li, Y. Efficient hydrogen generation on graphdiyne-based heterostructure. Nano Energy 2019, 55, 135–142. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Jiao, D.; Tian, Y.; Shang, Y.; Yin, L.; Cai, Q.; Zhao, J. Tuning precise numbers of supported nickel clusters on graphdiyne for efficient CO2 electroreduction toward various multi-carbon products. J. Energy Chem. 2022, 69, 456–465. [Google Scholar] [CrossRef]
- Li, B.; Lai, C.; Zhang, M.; Zeng, G.; Lu, S.; Huang, D.; Qin, L.; Liu, X.; Yi, H.; Xu, F.; et al. Graphdiyne: A rising star of electrocatalyst support for energy conversion. Adv. Energy Mater. 2020, 10, 2000177. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Qian, X.; Liu, H.; Lin, H.; Chen, N.; Li, Y. Construction of tubular molecule aggregations of graphdiyne for highly efficient field emission. J. Phys. Chem. C 2011, 115, 2611–2615. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Xie, Z.; Gao, X.; Zhou, J.; Xiong, Y.; Chen, C.; Zhang, J.; Liu, Z. Diatomite-templated synthesis of freestanding 3D graphdiyne for energy storage and catalysis application. Adv. Mater. 2018, 30, e1800548. [Google Scholar] [CrossRef]
- Zhao, F.; Li, X.; He, J.; Wang, K.; Huang, C. Preparation of hierarchical graphdiyne hollow nanospheres as anode for lithium-ion batteries. Chem. Eng. J. 2021, 413, 127486. [Google Scholar] [CrossRef]
- Shang, H.; Zuo, Z.; Li, L.; Wang, F.; Liu, H.; Li, Y.; Li, Y. Ultrathin graphdiyne nanosheets grown in situ on copper nanowires and their performance as lithium-ion battery Anodes. Angew. Chem. Int. Ed. 2018, 57, 774–778. [Google Scholar] [CrossRef]
- Zhan, S.; Chen, X.; Xu, B.; Wang, L.; Tong, L.; Yu, R.; Yang, N.; Wang, D. Hollow multishelled structured graphdiyne realized radioactive water safe-discharging. Nano Today 2022, 47, 101626. [Google Scholar] [CrossRef]
- Pan, H.; Jiang, Z.; Zuo, Z.; He, F.; Wang, F.; Li, L.; Chang, Q.; Guan, B.; Li, Y. Proton selective anode nanochannel for efficient methanol utilization. Nano Today 2021, 39, 101213. [Google Scholar] [CrossRef]
- Zhao, X.; Jia, W.; Wu, X.; Lv, Y.; Qiu, J.; Guo, J.; Wang, X.; Jia, D.; Yan, J.; Wu, D. Ultrafine MoO3 anchored in coal-based carbon nanofibers as anode for advanced lithium-ion batteries. Carbon 2020, 156, 445–452. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, X.; Liu, R.; Xie, Z.; Yang, J.; Zhang, S.; Zhang, G.; Liu, H.; Li, Y.; Zhang, J.; et al. Synthesis of graphdiyne nanowalls using acetylenic coupling reaction. J. Am. Chem. Soc. 2015, 137, 7596–7599. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; He, F.; Wang, F.; Li, L.; Li, Y. Spontaneously splitting copper nanowires into quantum dots on graphdiyne for suppressing lithium dendrites. Adv. Mater. 2020, 32, e2004379. [Google Scholar] [CrossRef]
- Yu, H.; Xue, Y.; Huang, B.; Hui, L.; Zhang, C.; Fang, Y.; Liu, Y.; Zhao, Y.; Li, Y.; Liu, H.; et al. Ultrathin nanosheet of graphdiyne-supported palladium atom catalyst for efficient hydrogen production. iScience 2019, 11, 31–41. [Google Scholar] [CrossRef]
- He, Q.; Zhou, Y.; Shou, H.; Wang, X.; Zhang, P.; Xu, W.; Qiao, S.; Wu, C.; Liu, H.; Liu, D.; et al. Synergic reaction kinetics over adjacent ruthenium sites for superb hydrogen generation in alkaline media. Adv. Mater. 2022, 34, e2110604. [Google Scholar] [CrossRef]
- Gao, Y.; Xue, Y.; Qi, L.; Xing, C.; Zheng, X.; He, F.; Li, Y. Rhodium nanocrystals on porous graphdiyne for electrocatalytic hydrogen evolution from saline water. Nat. Commun. 2022, 13, 5227. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Qi, L.; Xue, Y.; Li, Y. Highly selective and durable of monodispersed metal atoms in ammonia production. Nano Today 2022, 43, 101431. [Google Scholar] [CrossRef]
- Cao, D.; Wang, J.; Xu, H.; Cheng, D. Growth of highly active amorphous RuCu nanosheets on Cu nanotubes for the hydrogen evolution reaction in wide pH values. Small 2020, 16, e2000924. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, H.; Zhu, W.; Li, W.; Wang, C.; Lu, X. RuNi nanoparticles embedded in N-doped carbon nanofibers as a robust bifunctional catalyst for efficient overall water splitting. Adv. Sci. 2020, 7, 1901833. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, T.; Li, J.; Li, B.; Liu, Y.; Zhang, B.; Xiong, D.; Amorim, I.; Li, W.; Liu, L. Boosting the hydrogen evolution performance of ruthenium clusters through synergistic coupling with cobalt phosphide. Energy Environ. Sci. 2018, 11, 1819–1827. [Google Scholar] [CrossRef]
- Pu, Z.; Amiinu, I.S.; Kou, Z.; Li, W.; Mu, S. RuP2-based catalysts with platinum-like activity and higher durability for the hydrogen evolution reaction at all pH values. Angew. Chem. Int. Ed. 2017, 56, 11559–11564. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-supported nanoporous cobalt phosphide nanowire arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Su, P.; Pei, W.; Wang, X.; Ma, Y.; Jiang, Q.; Liang, J.; Zhou, S.; Zhao, J.; Liu, J.; Lu, G.Q.M. Exceptional electrochemical HER performance with enhanced electron transfer between Ru nanoparticles and single atoms dispersed on a carbon substrate. Angew. Chem. Int. Ed. 2021, 60, 16044–16050. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, Y.; Yi, Y.; Li, Y.; Liu, H.; Li, D.; Yang, W.; Li, Y. Self-catalyzed growth of Cu@graphdiyne core–shell nanowires array for high efficient hydrogen evolution cathode. Nano Energy 2016, 30, 858–866. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Murerials Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blӧchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 953–978. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Annu. Rev. Condens. Matter Phys. 1992, 46, 6671–6687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, P.; Li, M.; Li, Y.; Zhang, X.; Chen, J.; Fang, X.; Liu, Y.; Yuan, X.; Dai, X.; et al. Interfacial synergy between dispersed Ru sub-nanoclusters and porous NiFe layered double hydroxide on accelerated overall water splitting by intermediate modulation. Nanoscale 2020, 12, 9669–9679. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Dai, J.; Zhao, J.; Wu, Y.; Li, C.; Ji, L.; Zhang, X.; Yang, F. Modulation in ruthenium-cobalt electronic structure for highly efficient overall water splitting. ACS Appl. Energy Mater. 2020, 3, 1869–1874. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. High electrocatalytic hydrogen evolution activity of an anomalous ruthenium catalyst. J. Am. Chem. Soc. 2016, 138, 16174–16181. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Z.; Li, J.; Xiong, J.; Zhou, S.; Liang, J.; Cai, W.; Wang, C.; Yang, Z.; Cheng, H. Engineering of Ru/Ru2P interfaces superior to Pt active sites for catalysis of the alkaline hydrogen evolution reaction. J. Mater. Chem. A 2019, 7, 5621–5625. [Google Scholar] [CrossRef]
- Chi, J.Q.; Gao, W.K.; Lin, J.H.; Dong, B.; Yan, K.L.; Qin, J.F.; Liu, B.; Chai, Y.M.; Liu, C.G. Hydrogen evolution activity of ruthenium phosphides encapsulated in nitrogen- and phosphorous-codoped hollow carbon nanospheres. ChemSusChem 2018, 11, 743–752. [Google Scholar] [CrossRef]
- Zhang, L.; Jang, H.; Wang, Y.; Li, Z.; Zhang, W.; Kim, M.G.; Yang, D.; Liu, S.; Liu, X.; Cho, J. Exploring the dominant role of atomic-and nano-ruthenium as active sites for hydrogen evolution reaction in both acidic and alkaline media. Adv. Sci. 2021, 8, 2004516. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Yan, T.; Wang, Y.; Long, Y.; Fan, G. Carbon nanopore and anchoring site-assisted general construction of encapsulated metal (Rh, Ru, Ir) nanoclusters for highly efficient hydrogen evolution in pH-universal electrolytes and natural seawater. Green Chem. 2021, 23, 4551–4559. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Li, H.; Cai, P.; Li, Y.; Wen, Z. Ru-RuO2/CNT hybrids as high-activity pH-universal electrocatalysts for water splitting within 0.73 V in an asymmetric-electrolyte electrolyzer. Nano Energy 2019, 61, 576–583. [Google Scholar] [CrossRef]
- Li, F.; Han, G.F.; Noh, H.J.; Ahmad, I.; Jeon, I.Y.; Baek, J.B. Mechanochemically assisted synthesis of a Ru catalyst for hydrogen evolution with performance superior to Pt in both acidic and alkaline media. Adv. Mater. 2018, 30, 1803676. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Lee, J.; Seo, B.; Kim, B.; Baik, H.; Joo, S.H.; Lee, K. Cactus-like hollow Cu2-xS@Ru nanoplates as excellent and robust electrocatalysts for the alkaline hydrogen evolution reaction. Small 2017, 13, 1700052. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qi, L. Heterostructured inter-doped ruthenium-cobalt oxide hollow nanosheet arrays for highly efficient overall water splitting. Angew. Chem. Int. Ed. 2020, 59, 17219–17224. [Google Scholar] [CrossRef]
- Ding, J.; Shao, Q.; Feng, Y.; Huang, X. Ruthenium-nickel sandwiched nanoplates for efficient water splitting electrocatalysis. Nano Energy 2018, 47, 1–7. [Google Scholar] [CrossRef]
- Xu, Y.; Yin, S.; Li, C.; Deng, K.; Xue, H.; Li, X.; Wang, H.; Wang, L. Low-ruthenium-content NiRu nanoalloys encapsulated in nitrogen-doped carbon as highly efficient and pH-universal electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 1376–1381. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Wang, Y.; Zhang, Q.; Gu, L.; Zhao, S.; Xu, D.; Li, Y.; Bao, J.; Dai, Z. Ru modulation effects in the synthesis of unique rod-like Ni@Ni2P-Ru heterostructures and their remarkable electrocatalytic hydrogen evolution performance. J. Am. Chem. Soc. 2018, 140, 2731–2734. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Wang, W.; Li, Z.; Liang, F. Preparation and Support Effect of Graphdiyne Nanotubes with Abundant Cu Quantum Dots. Molecules 2024, 29, 1410. https://doi.org/10.3390/molecules29061410
Lv Y, Wang W, Li Z, Liang F. Preparation and Support Effect of Graphdiyne Nanotubes with Abundant Cu Quantum Dots. Molecules. 2024; 29(6):1410. https://doi.org/10.3390/molecules29061410
Chicago/Turabian StyleLv, Yan, Wenzhou Wang, Zhangwei Li, and Fucang Liang. 2024. "Preparation and Support Effect of Graphdiyne Nanotubes with Abundant Cu Quantum Dots" Molecules 29, no. 6: 1410. https://doi.org/10.3390/molecules29061410




