An Optimized Method for Evaluating the Preparation of High-Quality Fuel from Various Types of Biomass through Torrefaction
Abstract
:1. Introduction
2. Results and Discussion
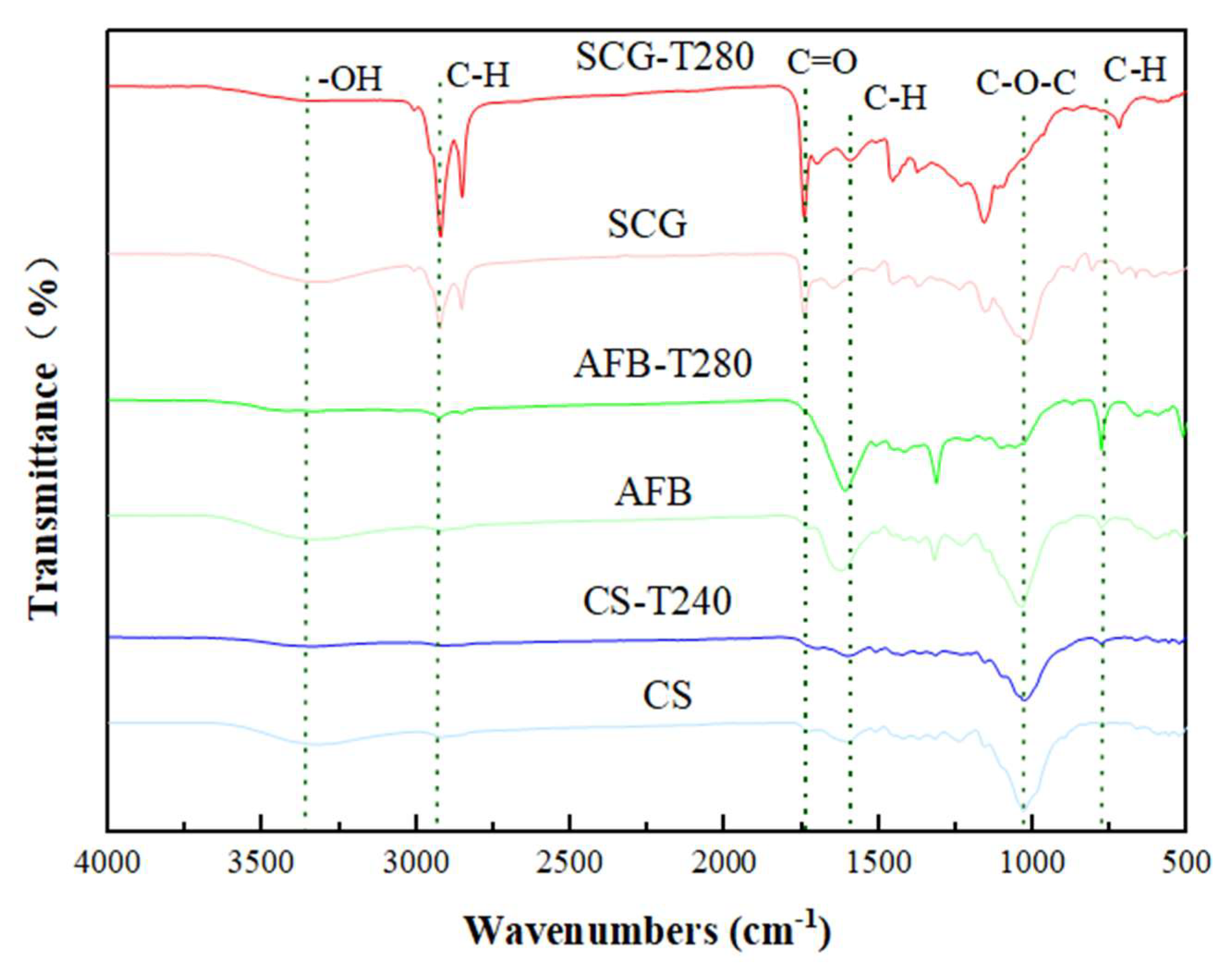
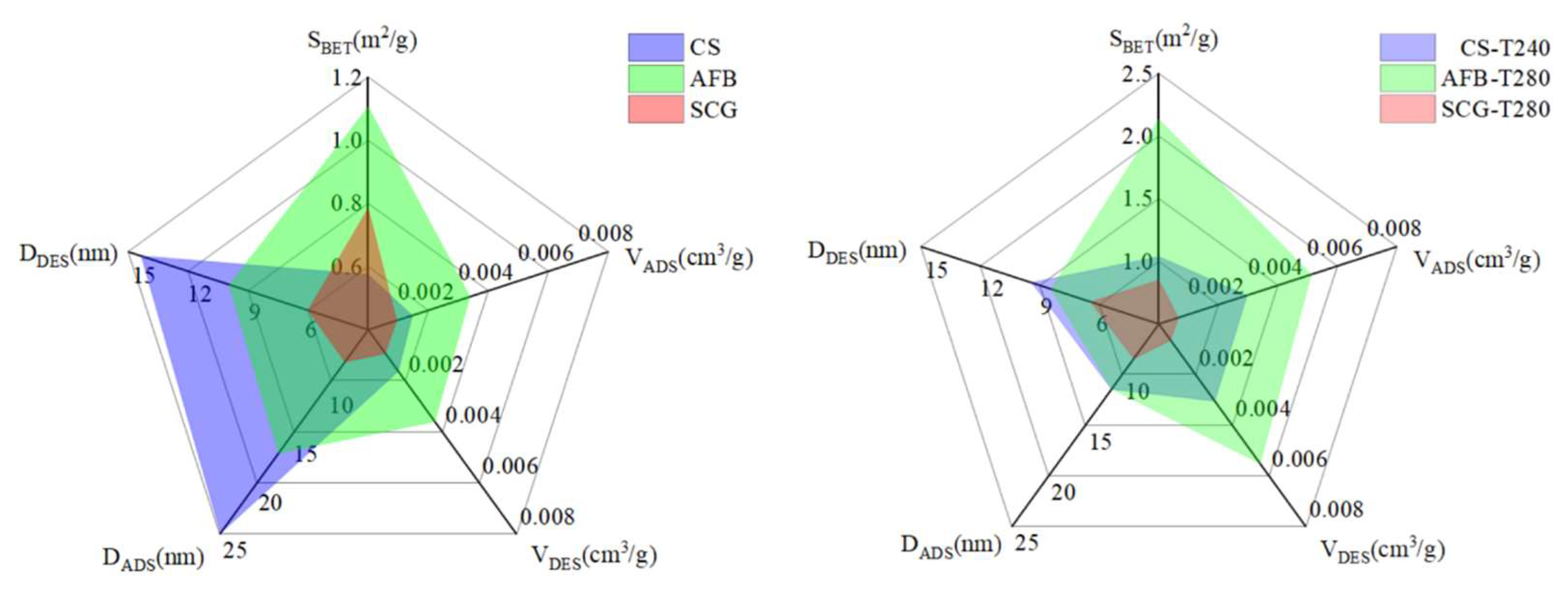
2.1. Physicochemical Properties of Biochar Samples
2.2. Normalized Assessment of Various Raw Material Torrefied Biochar
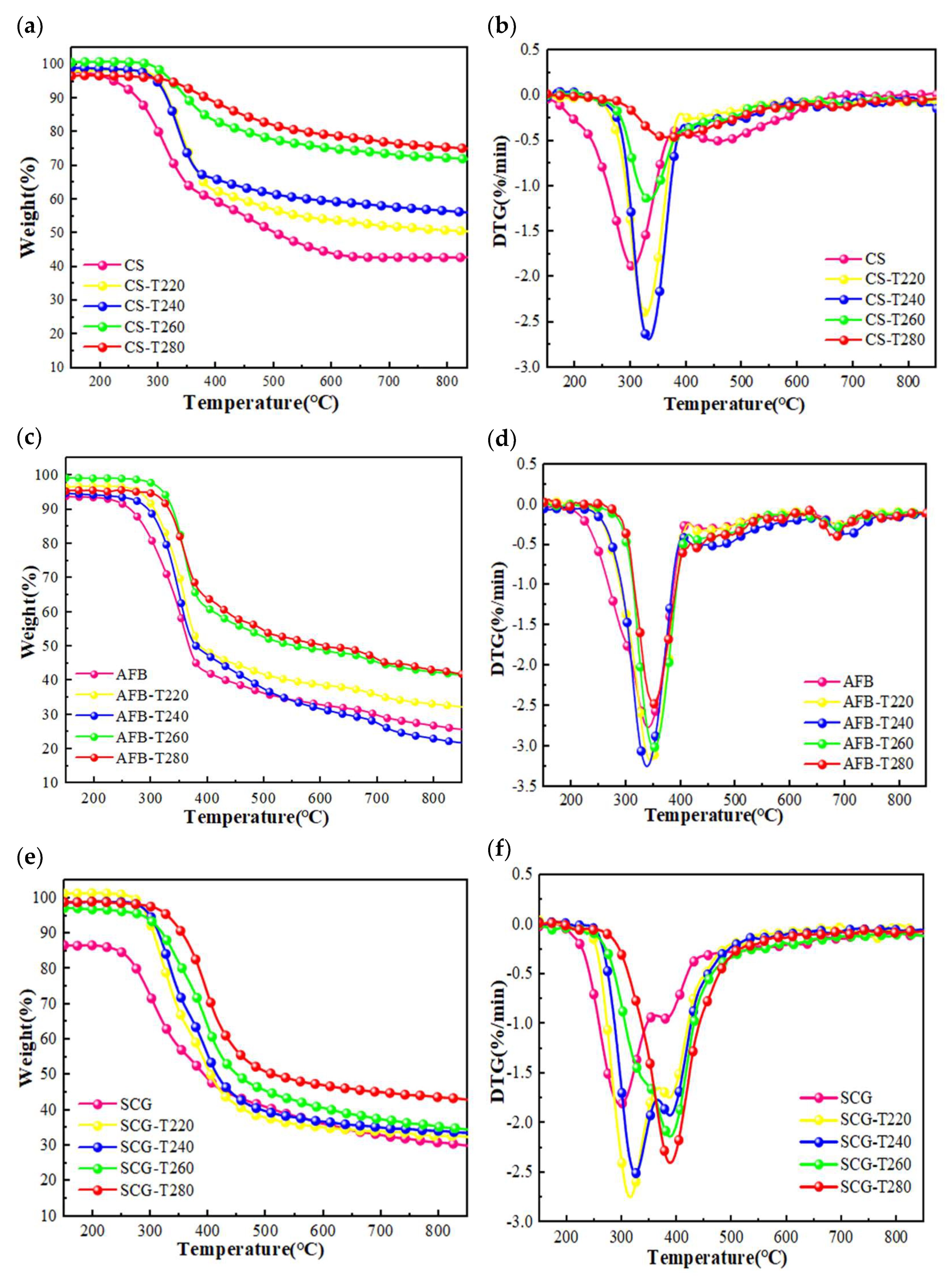
2.2.1. Analysis of Pyrolysis Behavior Characteristics
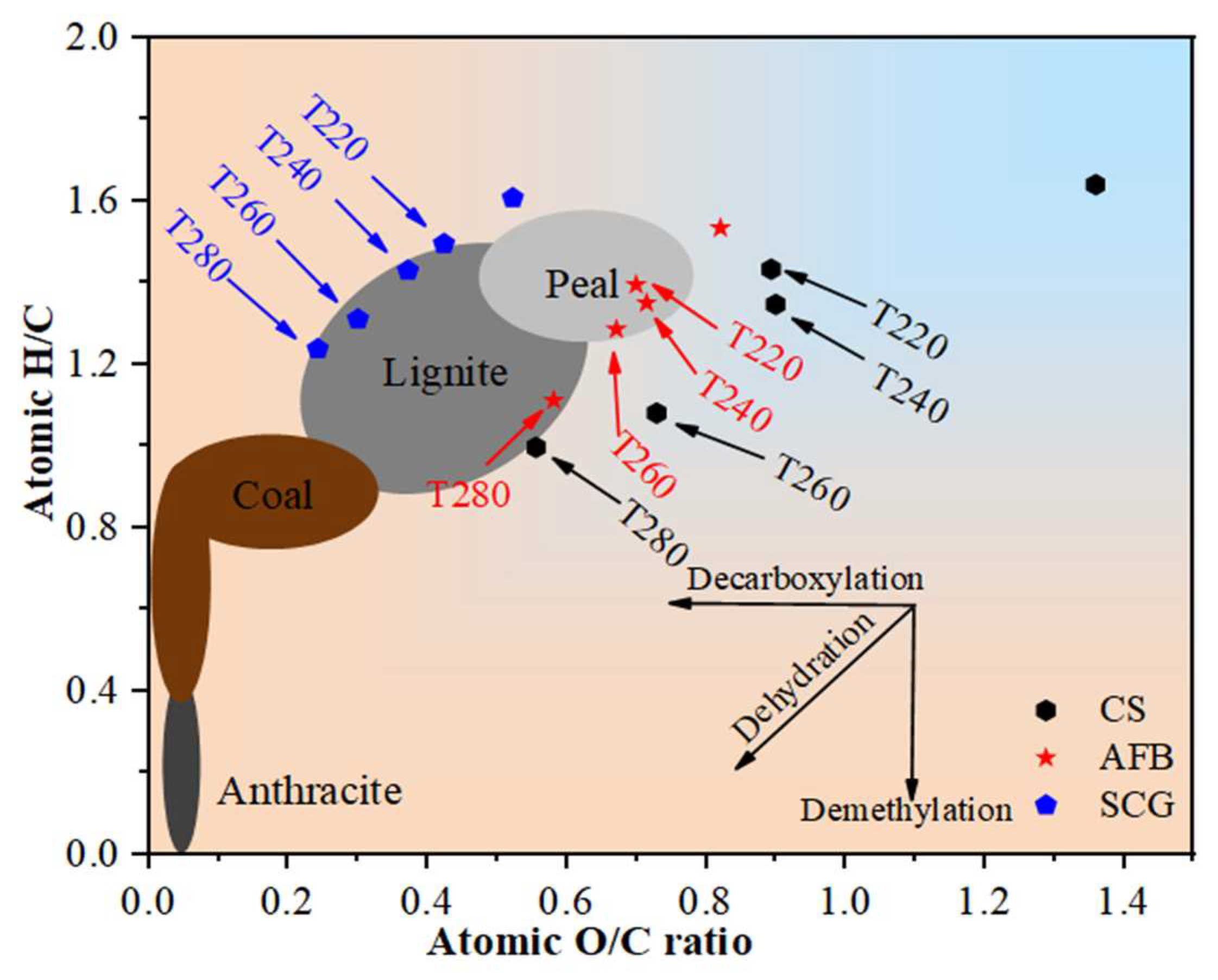
2.2.2. Assessment of Displacement Level
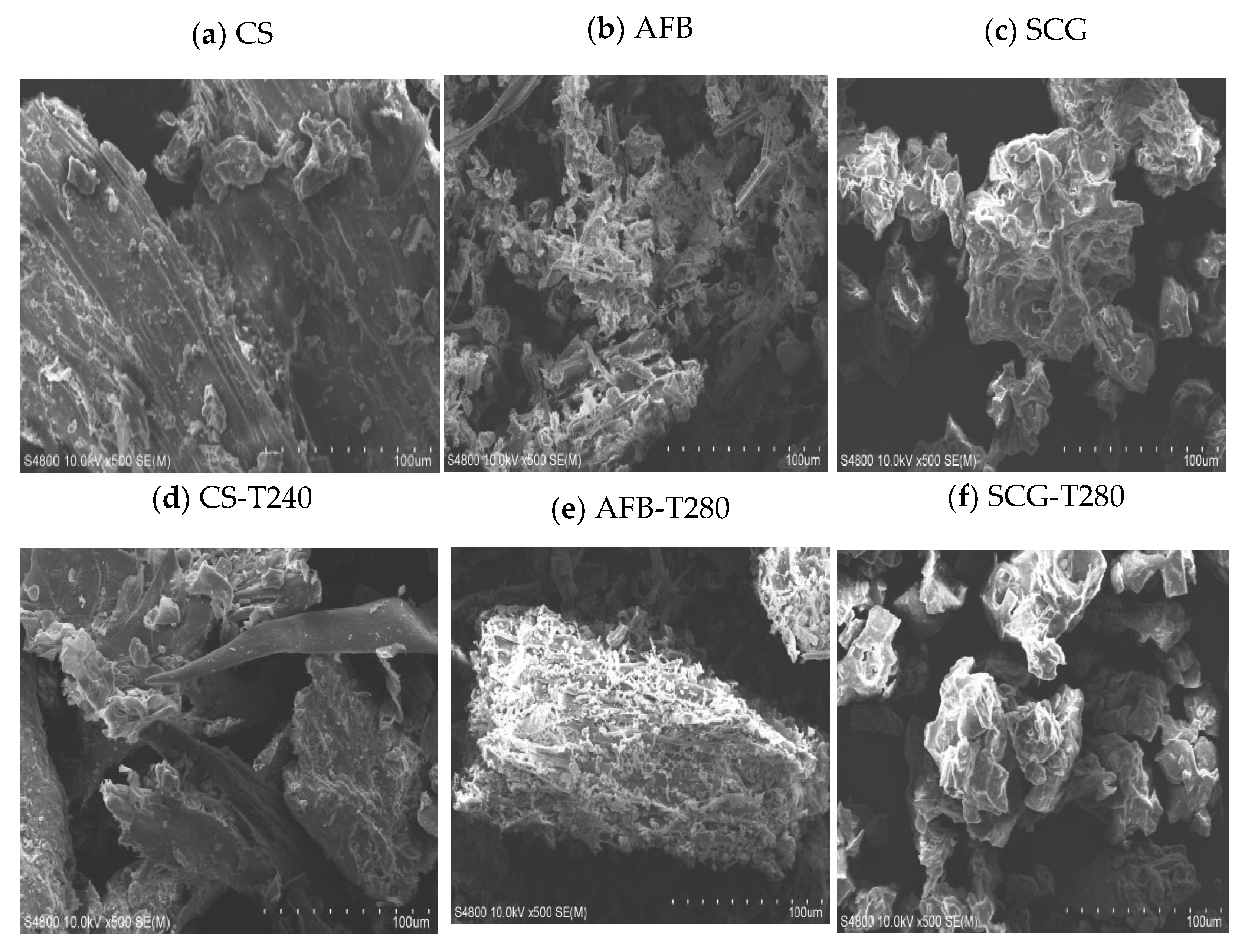
2.3. Microstructural Analysis of Biomass Raw Material and Optimal Torrefied Biochar
2.4. Energy Consumption Calculation
3. Materials and Methods
3.1. Materials Selection
3.2. Torrefaction Experiments
3.3. Analytical Method
3.3.1. Basic Characteristics of Biomass Feedstock and Torrefied Biochar
3.3.2. Normalized Assessment of Various Biomass Torrefied Biochars
3.3.3. Microscopic Characterization of Biomass Feedstock and Optimal Torrefied Biochar
3.3.4. Energy Consumption of Biomass Feedstock and Optimal Torrefied Biochar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ehlig, C. A Geologic carbon dioxide sequestration methods, opportunities, and impacts. Curr. Opin. Chem. Eng. 2023, 42, 1009557. [Google Scholar]
- Liu, T.; Conkey, B.; Huffman, T.; Smith, S.; Gregor, B.; Yemshanov, D.; Kulshreshtha, S. Potential and impacts of renewable energy production from agricultural biomass in Canada. Appl. Energy 2014, 30, 222–229. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, B.; Wu, S.; Gou, W.; Zhang, J.; Wu, Z.; Wang, Z.; Peng, H.; Lim, J. Effect of torrefaction pretreatment on the fast pyrolysis behavior of biomass: Product distribution and kinetic analysis on spruce-pin-fir sawdust. J. Anal. Appl. Pyrol. 2021, 158, 105259. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Ma, J.; Chu, Y.; Kong, W.; Yuan, P.; Sun, R.; Shen, B. Analysis of functionality distribution and microstructural characteristics of upgraded rice husk after undergoing non-oxidative and oxidative torrefaction. Fuel 2022, 310, 122477. [Google Scholar] [CrossRef]
- Basu, P.; Sadhukhan, A.; Gupta, P.; Rao, S.; Dhungana, A.; Acharya, B. An experimental and theoretical investigation on torrefaction of a large wet wood particle. Bioresour. Technol. 2014, 159, 1215–1222. [Google Scholar] [CrossRef]
- Peng, J.; Bi, X.; Velebil, J.; Sokhansanj, S. Torrefaction and densification of different species of softwood residues. Fuel 2013, 111, 411–421. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, S.; Zhao, Y.; Wang, Z.; Ma, J.; Xu, L.; Yang, J.; Shen, B. Investigation on the fuel quality and hydrophobicity of upgraded rice husk derived from various inert and oxidative torrefaction conditions. Renew. Energy 2022, 189, 1234–1248. [Google Scholar] [CrossRef]
- Simonic, M.; Goricanec, D.; Urbancl, D. Impact of torrefaction on biomass properties depending on temperature and operation time. Sci. Total Environ. 2020, 740, 1440086. [Google Scholar] [CrossRef] [PubMed]
- Toscano, G.; Pizz, A.; Pedretti, E.; Rossin, G.; Ciceri, G.; Martignon, G.; Duca, D. Torrefaction of tomato industry residues. Fuel 2015, 143, 89–97. [Google Scholar] [CrossRef]
- Kanwal, S.; Chaudhry, N.; Munir, S.; Sana, H. Effect of torrefaction conditions on the physicochemical characterization of agricultural waste. Waste Manag. 2019, 88, 280–290. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q.; Zhu, X.; Liu, Q. Upgrading of Rice Husk by Torrefaction and its Influence on the Fuel Properties. Bioresources 2014, 9, 5893–5905. [Google Scholar] [CrossRef]
- Ivanovski, M.; Goricanec, D.; Krope, J.; Urbancl, D. Torrefaction pretreatment of lignocellulosic biomass for sustainable solid biofuel production. Energy 2022, 240, 122483. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Zhang, L.; Wang, Z.; Zhao, Z.; Zhu, W.; Ma, J.; Shen, B. Effects of Torrefaction Pretreatment on the Structural Features and Combustion Characteristics of Biomass-Based Fue. Molecules 2023, 28, 4732. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahma, A.; Mohammed, S.; WanAhmad, W.; Vijean, V.; Santiagoo, R.; Ibrahim, N.; Udin, N. Influence of Torrefaction on Sewage Sludg. Earth Environ. Sci. 2023, 1135, 012036. [Google Scholar]
- Nepal, R.; Kim, H.; Poudel, J.; Oh, S. A study on torrefaction of spent coffee ground to improve its fuel properties. Fuel 2022, 318, 123643. [Google Scholar] [CrossRef]
- Mukhtar, H.; Feroze, N.; Munir, H.; Javed, F.; Kazm, M. Torrefaction process optimization of agriwaste for energy densification. Energy Source 2020, 42, 2526–2544. [Google Scholar] [CrossRef]
- Garba, M.; Gambo, S.; Musa, U.; Tauheed, K.; Alhassan, M.; Adeniyi, O. Impact of torrefaction on fuel property of tropical biomass feedstocks. Biofuels 2018, 9, 369–377. [Google Scholar] [CrossRef]
- Aniza, R.; Chen, W.; Yang, F.; Pugazhendh, A.; Singh, Y. Integrating Taguchi method and artificial neural network for predicting and maximizing biofuel production via torrefaction and pyrolysis. Bioresour. Technol. 2022, 343, 126140. [Google Scholar] [CrossRef]
- Jaquelinede, O.; Thaiane, A.; Isabelade, O.; Jaciane, L.; Rosely, A.; Juanmanuel, L.; Humberto, J.; Enrique, R.; Reginade, F. Mechanistic insights and kinetics of torrefaction of pine wood biomasses using solid-state NMR. J. Anal. Appl. Pyrol. 2023, 172, 106019. [Google Scholar]
- Singh, R.; Sarkar, A.; Chakraborty, J. Effect of torrefaction on the physicochemical properties of eucalyptus derived biofuels: Estimation of kinetic parameters and optimizing torrefaction using response surface methodology (RSM). Energy 2020, 198, 117369. [Google Scholar] [CrossRef]
- Guo, S.; Guo, T.; Che, D.; Liu, H.; Sun, B. Response surface analysis of energy balance and optimum condition for torrefaction of corn straw. Korean J. Chem. Eng. 2022, 39, 1287–1298. [Google Scholar] [CrossRef]
- Guo, S.; Liu, L.; Zhao, D.; Zhao, C.; Li, X.; Li, G. Optimization of Briquette Fuels by Co-Torrefaction of Residual Biomass and Plastic Waste Using Response Surface Methodology. Molecules 2023, 28, 2568. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Nobre, C.; Correia, R.; Gouveia, L.; Gonçalves, M. Optimization of Biochar Production by Co-Torrefaction of Microalgae and Lignocellulosic Biomass Using Response Surface Methodology. Energies 2021, 14, 7330. [Google Scholar] [CrossRef]
- Jaime, M.-P.; Jodar, J.; Miguel, R.; Montserrat, Z.; Tourigny, G. Determination of the Optimal Operative Conditions for the Torrefaction of Olive Waste Biomass. Sustainability 2020, 12, 6411. [Google Scholar] [CrossRef]
- Lester, E.; Gong, M.; Thompson, A. A method for source apportionment in biomass/coal blends using thermogravimetric analysis. J. Anal. Appl. Pyrol. 2007, 80, 111–117. [Google Scholar] [CrossRef]
- Kévin, C.; Mounir, C.; Stéphane, D.; Anélie, P.; Mathieu, P.; Philippe, G. Utilization of thermodesorption coupled to GC–MS to study stability of different wood species to thermodegradation. J. Anal. Appl. Pyrol. 2011, 92, 376–383. [Google Scholar]
- Smith, J.; Safferman, S.; Saffron, C. Development and application of a decision support tool for biomass co-firing in existing coal-fired power plants. J. Clean. Prod. 2019, 236, 117375. [Google Scholar] [CrossRef]
- Arriola, E.; Chen, W.; Chih, Y.; DeLuna, M.; Show, P. Impact of post-torrefaction process on biochar formation from wood pellets and self-heating phenomena for production safety. Energy 2019, 186, 115795. [Google Scholar] [CrossRef]
- Kang, K.; Nanda, S.; Sun, G.; Qiu, L.; Gu, Y.; Zhang, T.; Zhu, M.; Sun, R. Microwave-assisted hydrothermal carbonization of corn stalk for solid biofuel production: Optimization of process parameters and characterization of hydrochar. Energy 2019, 186, 115795. [Google Scholar] [CrossRef]
- DayaRam, N.; Prabir, B.; Bishnu, A. A Comprehensive Review on Biomass Torrefaction. Int. J. Renew Energy Biofuel 2014, 2014, 506376. [Google Scholar]
- Granados, D.; Ruiz, R.; Vega, L.; Chejne, F. Study of reactivity reduction in sugarcane bagasse as consequence of a torrefaction process. Energy 2017, 139, 818–827. [Google Scholar] [CrossRef]
- Sakulkit, P.; Palamanit, A.; Dejchanchaiwong, R.; Reubroycharoen, P. Characteristics of pyrolysis products from pyrolysis and co-pyrolysis of rubber wood and oil palm trunk biomass for biofuel and value-added applications. J. Environ. Chem. Eng. 2020, 8, 104561. [Google Scholar] [CrossRef]
- Jamari, S.; Howse, J. The effect of the hydrothermal carbonization process on palm oil empty fruit bunch. Biomass Bioenergy 2012, 47, 82–90. [Google Scholar] [CrossRef]
- Lee, X.; Lee, L.; Gan, S.; Thangalazhy, S.; Hoon, K. Biochar potential evaluation of palm oil wastes through slow pyrolysis: Thermochemical characterization and pyrolytic kinetic studies. Bioresour. Technol. 2017, 236, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Summers, S.; Turn, S.; Kusch, W. upgraded pongamia pod via torrefaction for the production of bioenergy. Fuel 2021, 291, 120260. [Google Scholar] [CrossRef]
- Peng, D.; Ouyang, F.; Deng, M.; Nie, L.; Kong, S. A Comprehensive Characterization of Different Fractions of Corn Stover and Their Relationships to Multipollutant Sorption Characteristics. Adsorpt. Sci. Technol. 2021, 2021, 9988938. [Google Scholar] [CrossRef]
- Zhang, C.; Ho, S.H.; Chen, W.; Xie, Y.; Liu, Z.; Chang, J. Torrefaction performance and energy usage of biomass wastes and their correlations with torrefaction severity index. Appl. Energy 2018, 220, 598–604. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, J.; Zhang, Q. Effects of Torrefaction on the Pyrolysis Behavior and Bio-Oil Properties of Rice Husk by Using TG-FTIR and Py-GC/MS. Energy Fuel 2014, 28, 5857–5863. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, J.; Cen, K.; Chen, F.; Chen, D.; Li, Y. Investigation of the relevance between thermal degradation behavior and physicochemical property of cellulose under different torrefaction severities. Biomass Bioenergy 2021, 148, 106061. [Google Scholar] [CrossRef]
- Janewit, W.; Bundit, F.; Nakorn, W. Effects of temperature and holding time during torrefaction on the pyrolysis behaviors of woody biomass. J. Anal. Appl. Pyrol. 2011, 92, 99–105. [Google Scholar]
- Guo, S.; Deng, X.; Liu, L.; Ge, L.; Lisak, G. Comprehensive analysis of combustion behavior, kinetics, and gas emissions of fungus bran biofuel through torrefaction pretreatment and polypropylene additio. Fuel 2024, 364, 131014. [Google Scholar] [CrossRef]
- Kutlu, O.; Kocar, G. Upgrading lignocellulosic waste to fuel by torrefaction: Characterisation and process optimization by response surface methodology. Int. J. Energy Res. 2018, 15, 4746–4760. [Google Scholar] [CrossRef]
- Tsai, W.; Jiang, T.; Tang, M.; Chang, C.; Kuo, T. Enhancement of thermochemical properties on rice husk under a wide range of torrefaction conditions. Biomass. Convers. Bior. 2021. [Google Scholar] [CrossRef]
- Thaim, T.; Abdulrasid, R.; Wan, I.; Wanismail, W. Torrefaction of oil palm fronds (OPF) as a potential feedstock for energy production processes. J. Environ. Eng. Landsc. Manag. 2019, 27, 64–71. [Google Scholar] [CrossRef]
- Acquah, G.; Via, B.; Fasina, O.; Eckhardt, L. Rapid Quantitative Analysis of Forest Biomass Using Fourier Transform Infrared Spectroscopy and Partial Least Squares Regression. J. Anal. Methods Chem. 2016, 2016, 1839598. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Feng, H.; Wang, Y.; Wang, F.; Wang, H.; Liu, H.; Liu, H. Influence mechanism of K on cellulose pyrolysis by stepwise isothermal method in-situ DRIFTS method. Fuel 2024, 360, 130601. [Google Scholar] [CrossRef]
- Enrico, B.; Federica, B.; Leonardo, T. Devolatilization of biomass fuels and biomass components studied by TG/FTIR technique. Ind. Eng. Chem. Res. 2006, 45, 4486–4493. [Google Scholar]
- Zhao, Y.; Feng, D.; Zhang, Y.; Huang, Y.; Sun, S. Effect of pyrolysis temperature on char structure and chemical speciation of alkali and alkaline earth metallic species in biochar. Fuel Process. Technol. 2016, 141, 54–60. [Google Scholar] [CrossRef]
- Krysanova, K.; Krylova, A.; Kulikova, M.; Kulikov, A.; Rusakova, O. Biochar characteristics produced via hydrothermal carbonization and torrefaction of peat and sawdus. Fuel 2022, 328, 125220. [Google Scholar] [CrossRef]
- Jiang, H.; Ye, Y.; Lu, P.; Zhao, M.; Xu, G.; Chen, D.; Song, T. Effects of torrefaction conditions on the hygroscopicity of biochars. J. Energy Inst. 2021, 96, 260–268. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.; Zhao, Y.; Xie, X.; Yang, S.; Hua, D.; Wang, C.; Li, T. Effect of Torrefaction on the Physiochemical Characteristics and Pyrolysis of the Corn Stalk. Polymers 2023, 15, 4069. [Google Scholar] [CrossRef] [PubMed]
- Zia, F.; Zia, K.; Zuber, M.; Kamal, S.; Aslam, N. Starch based polyurethanes: A critical review updating recent literature. Carbohydr. Polym. 2015, 134, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.; Liu, S.; Hsieh, C. Preparation and fuel properties of biochars from the pyrolysis of exhausted coffee residue. J. Anal. Appl. Pyrol. 2011, 93, 63–67. [Google Scholar] [CrossRef]
- Lee, K.; Cheng, C.; Lee, D.; Chen, W.; Vo, D.N.; Ding, L.; Lam, S. Spent coffee grounds biochar from torrefaction as a potential adsorbent for spilled diesel oil recovery and as an alternative fuel. Energy 2022, 239, 122467. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Qiu, T.; Liu, C.; Cui, L.; Liu, H.; Kashif, M.; Zhang, Y. Comparison of corn straw biochars from electrical pyrolysis and microwave pyrolysis. Energy Source 2023, 45, 636–649. [Google Scholar] [CrossRef]
- Tumpa, R.; Ramin, A.; Ajay, K.; Meda, V. Physicochemical and Fuel Characteristics of Torrefied Agricultural Residues for Sustainable Fuel Production. Enerny Fuel 2020, 34, 14169–14181. [Google Scholar]
- Fatih, G.; LuisMiguel, R.; Orla, W.; Kostas, E.; Abby, S.; Edward, L. Hydrothermal conversion of different lignocellulosic biomass feedstocks—Effect of the process conditions on hydrochar structures. Fuel 2021, 302, 112116. [Google Scholar]





| Sample | Mass Yield (%) | Energy Yield (%) | HHV (MJ/kg) | Energy Density |
|---|---|---|---|---|
| CS | - | - | 15.8 | - |
| CS-T220 | 81.07 | 86.21 | 16.8 | 1.06 |
| CS-T240 | 73.86 | 79.51 | 17 | 1.07 |
| CS-T260 | 61.74 | 71.47 | 18.29 | 1.16 |
| CS-T280 | 53.14 | 63.92 | 18.5 | 1.2 |
| AFB | - | - | 17.1 | - |
| AFB-T220 | 87.76 | 96.15 | 18.74 | 1.09 |
| AFB-T240 | 83.19 | 93.1 | 19.14 | 1.12 |
| AFB-T260 | 72.99 | 83.95 | 19.67 | 1.15 |
| AFB-T280 | 63.62 | 78.87 | 21.2 | 1.24 |
| SCG | - | - | 23.03 | - |
| SCG-T220 | 85.88 | 92.92 | 24.92 | 1.08 |
| SCG-T240 | 76.36 | 84.84 | 25.59 | 1.11 |
| SCG-T260 | 64.63 | 76.36 | 27.22 | 1.18 |
| SCG-T280 | 58.14 | 71.97 | 28.51 | 1.24 |
| Biomass | Optimal Methods | Optimal Torrefaction Conditions | EY (%) | HHV (MJ/kg) | H/O, O/C | Author |
|---|---|---|---|---|---|---|
| Cotton stalks | Response surface methodology | 305 °C, 32 min | 64.00 | 19.70 | 1.00, 0.17 | Kutlu et al. [42] |
| Olive waste | Numerical model | 275 °C, 30 min | 72.01 | 20.43 | / | Jaime et al. [24] |
| Rick husk | / | 360 °C, no retention time | 56.31 | 19.71 | 0.83, 0.34 | Majam et al. [43] |
| Oil palm frond | / | 300 °C, 30 min | 65.80 | 20.32 | 0.67, 0.11 | Tasi et al. [44] |
| CS, AFB, and SCGs | Displacement level | 240, 280, 280 °C and 30 min | 73.86, 63.62, and 58.14 | 17.00, 21.1, and 28.51 | 1.23–1.43, 0.24–0.89 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Deng, X.; Zhao, D.; Zhu, S.; Qu, H.; Li, X.; Zhao, Y. An Optimized Method for Evaluating the Preparation of High-Quality Fuel from Various Types of Biomass through Torrefaction. Molecules 2024, 29, 1889. https://doi.org/10.3390/molecules29081889
Guo S, Deng X, Zhao D, Zhu S, Qu H, Li X, Zhao Y. An Optimized Method for Evaluating the Preparation of High-Quality Fuel from Various Types of Biomass through Torrefaction. Molecules. 2024; 29(8):1889. https://doi.org/10.3390/molecules29081889
Chicago/Turabian StyleGuo, Shuai, Xiaoyan Deng, Deng Zhao, Shujun Zhu, Hongwei Qu, Xingcan Li, and Yan Zhao. 2024. "An Optimized Method for Evaluating the Preparation of High-Quality Fuel from Various Types of Biomass through Torrefaction" Molecules 29, no. 8: 1889. https://doi.org/10.3390/molecules29081889





