Reactive Force Field Molecular Dynamics Investigation of NH3 Generation Mechanism during Protein Pyrolysis Process
Abstract
:1. Introduction
2. Results and Discussion
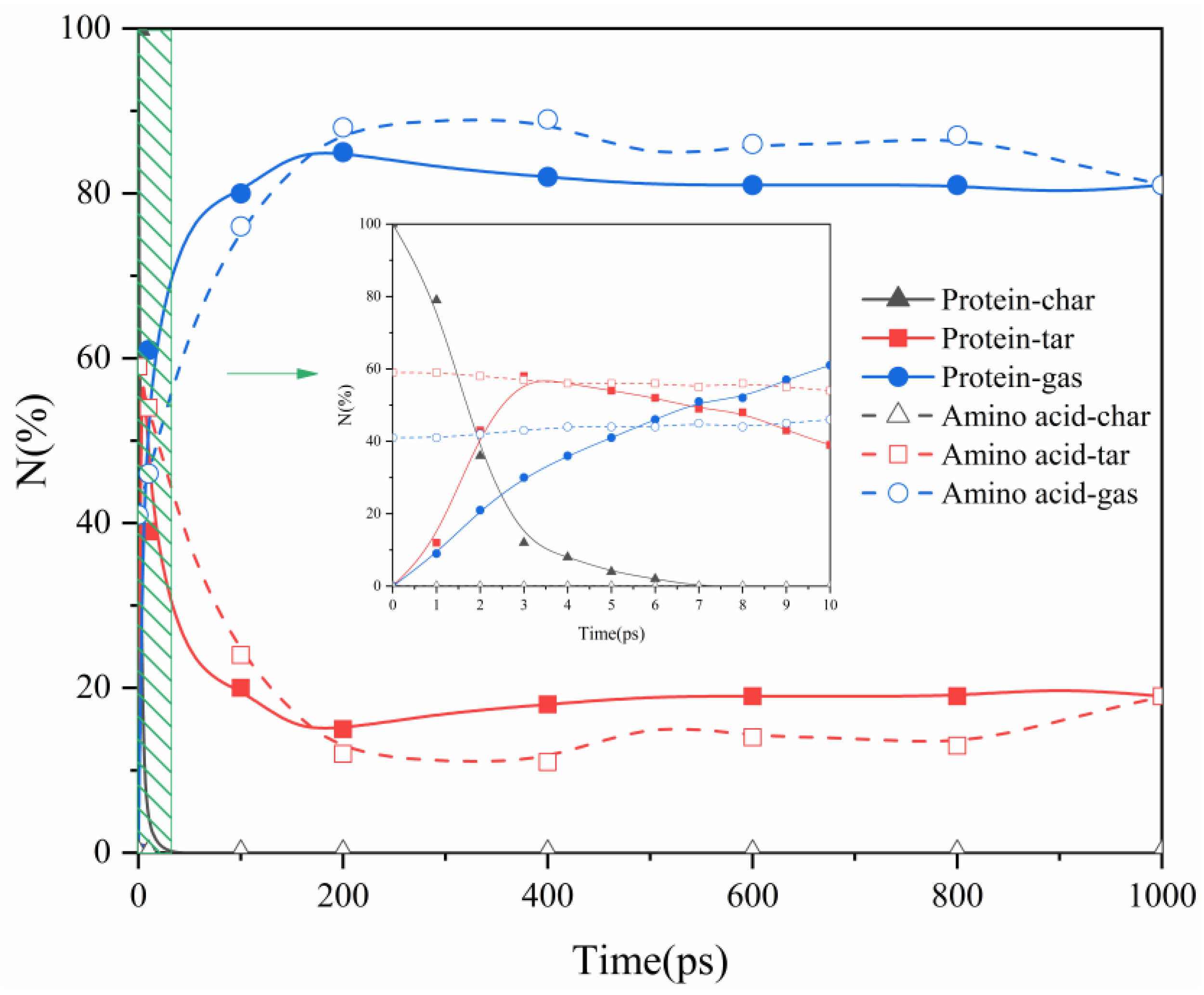
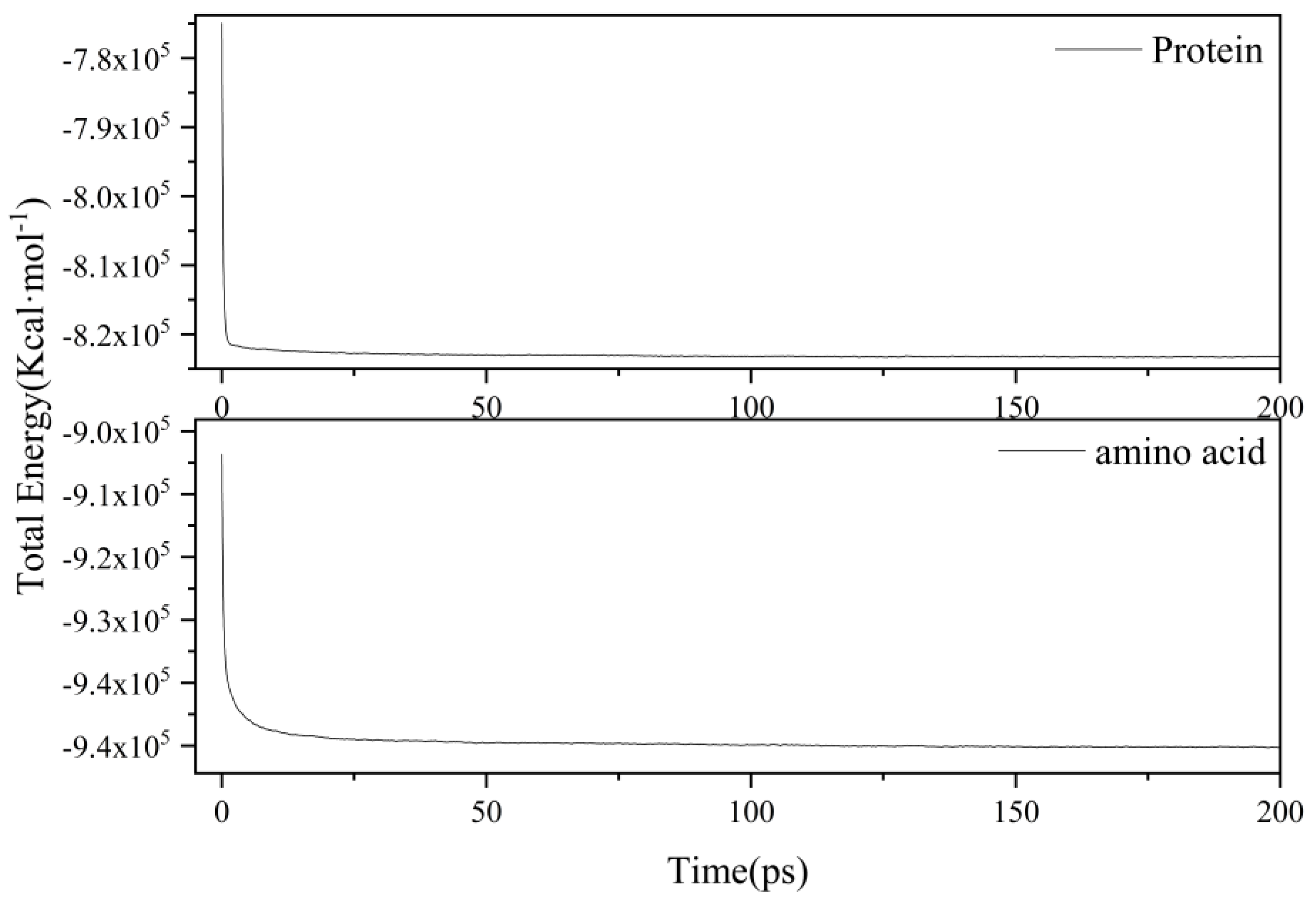
2.1. Proteins and Amino Acids Are Separately Thermally Decomposed
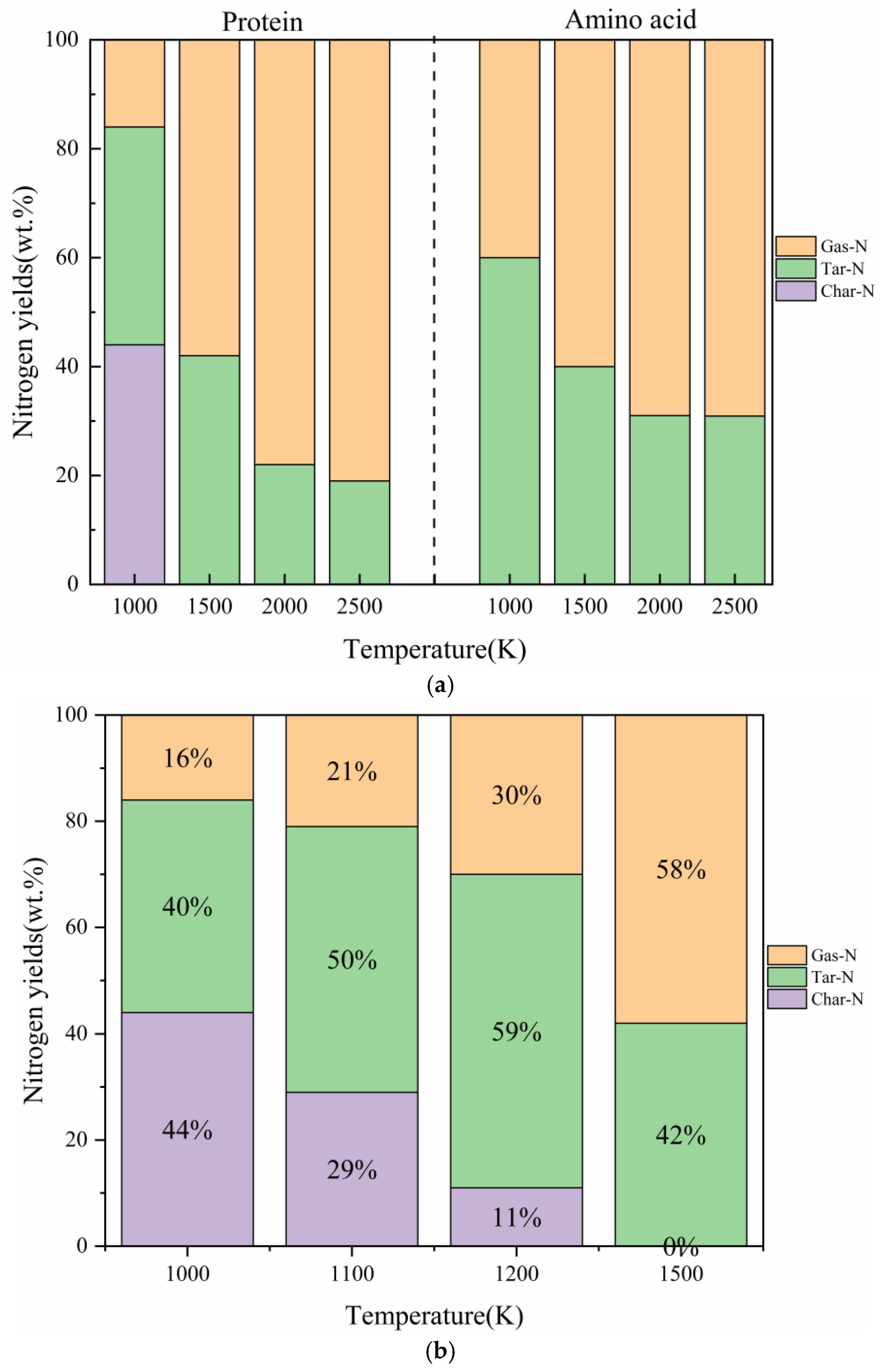
2.2. The Influence of Pyrolysis Temperature
2.2.1. The Comparative Impact of Pyrolysis Temperature on the Distribution of Three-Phase Products
2.2.2. The Impact of Pyrolysis Temperature on the Generation of NH3
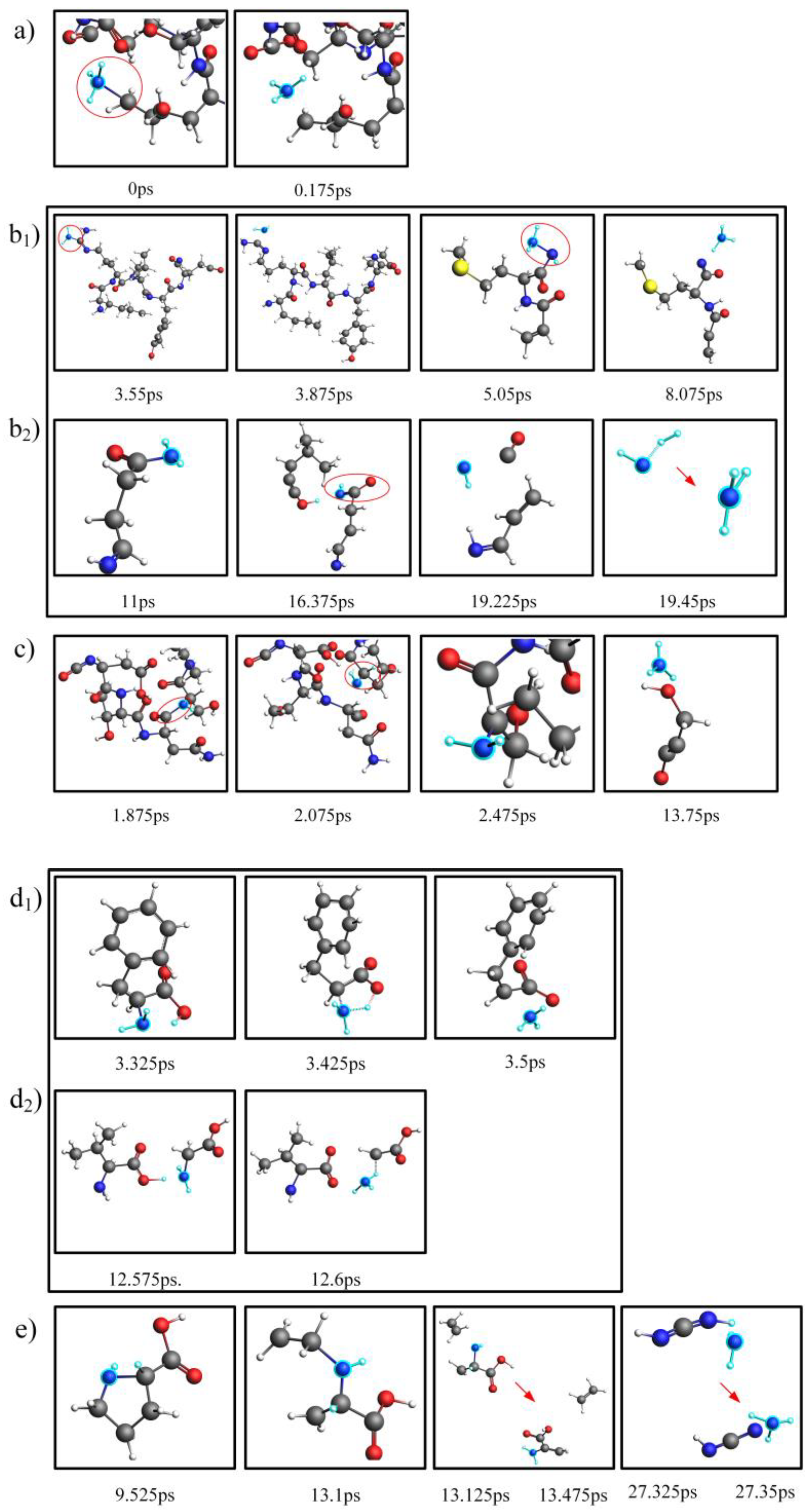
2.3. Comparison and Analysis of the NH3 Production Pathways for Actual Proteins and Their Assembled Amino Acids
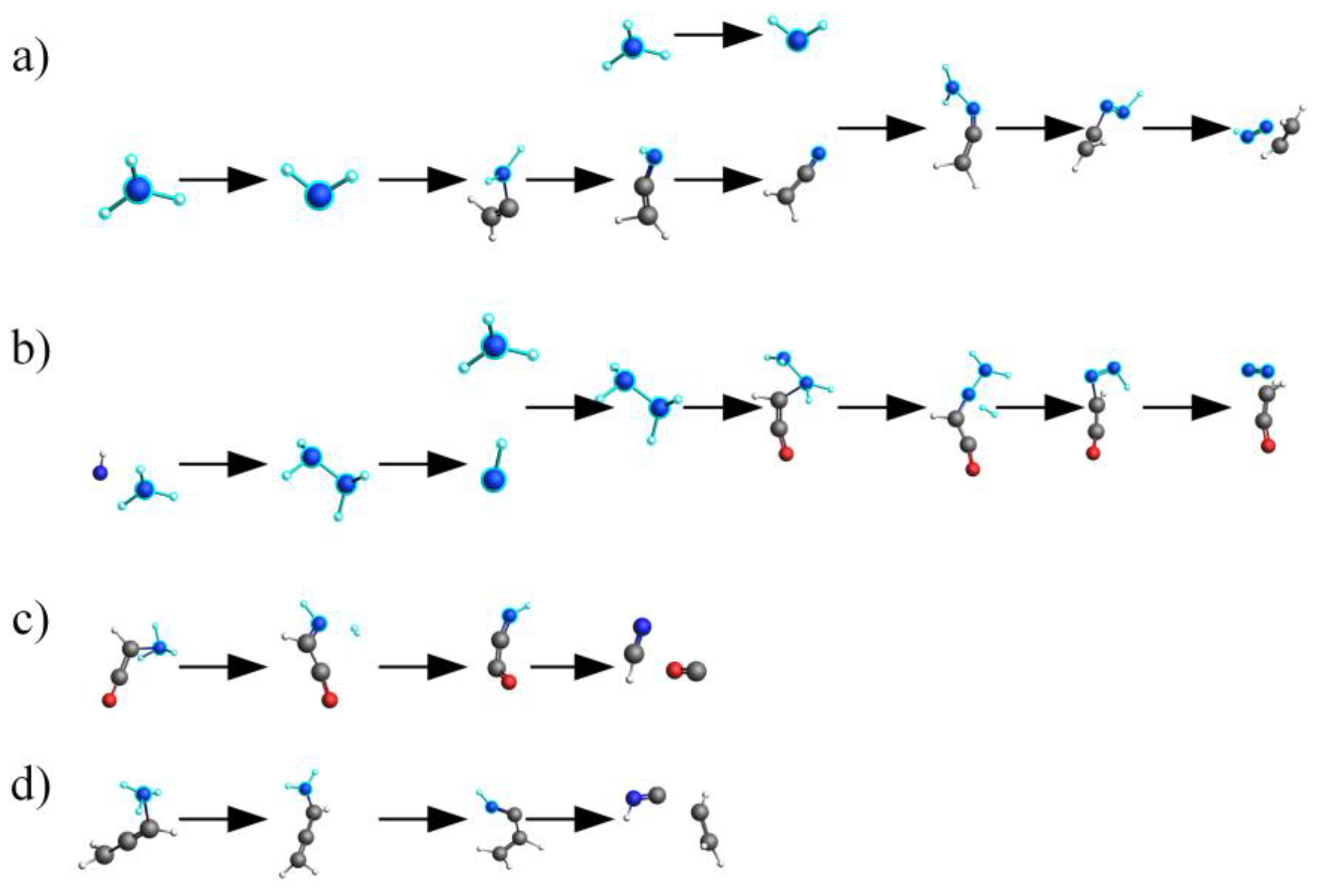
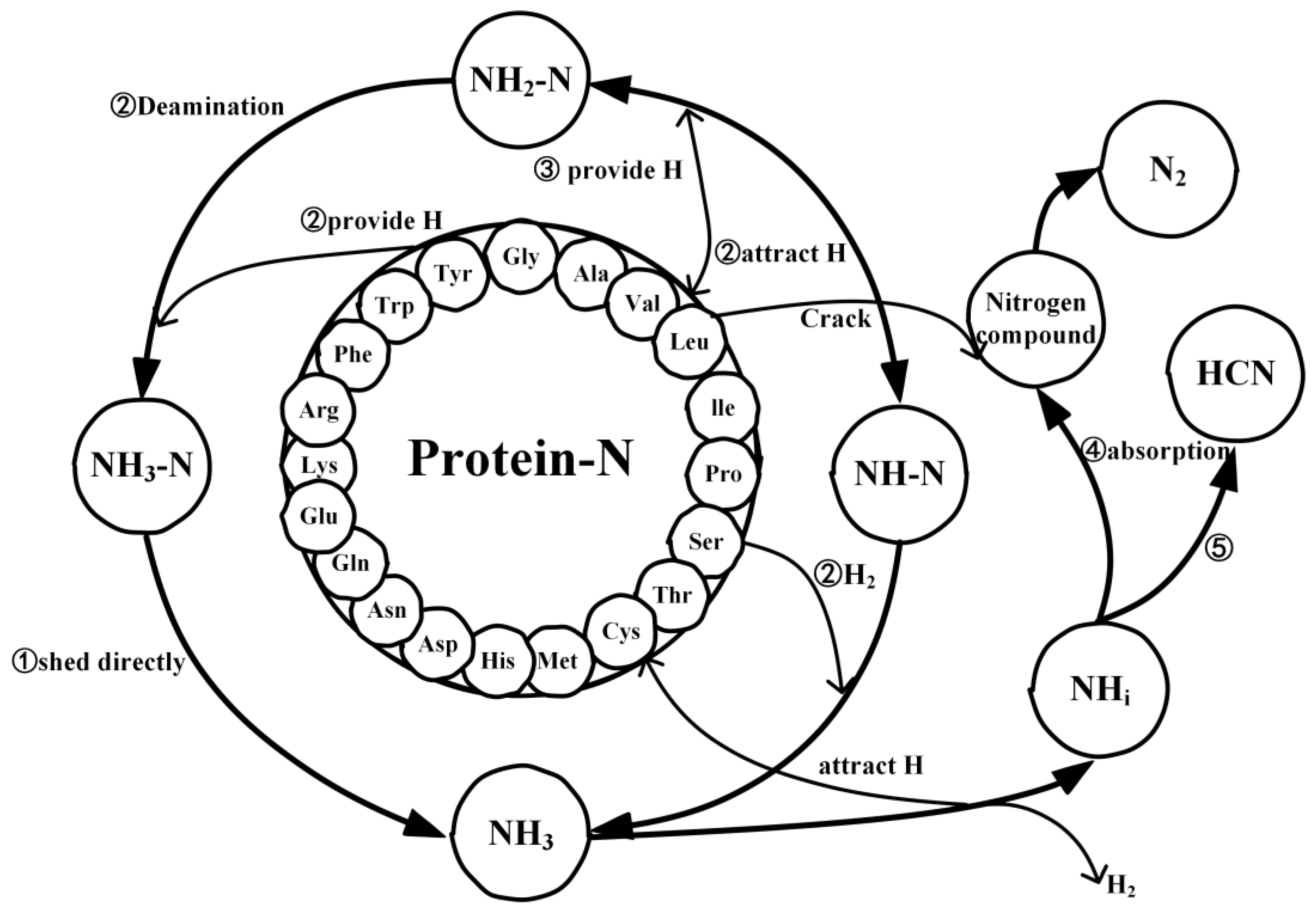
2.4. Mechanistic Analysis of the Actual Protein’s Generation of NH3
3. Materials and Methods
4. Conclusions
- Protein thermal decomposition occurs in two stages. The first stage involves the thermal rupture of unstable weak bonds in the protein’s large molecular structure, causing the gradual transfer of carbon and nitrogen to gas and tar fragments. The second stage is characterized by the thermal decomposition of tar, resulting in a notable migration of nitrogen from tar to the gas phase during the decomposition reaction.
- In our study, the actual protein and its assembled amino acids exhibited significant temperature-dependent variations in their maximum conversion rate during thermal decomposition. Specifically, at 1000 K, 1500 K, 2000 K, and 2500 K, the maximum conversion rates of the actual protein were 5%, 16%, 22%, and 21%, respectively. In contrast, the maximum conversion rate from the thermal decomposition of the assembled amino acids was relatively high, measuring 3%, 29%, 44%, and 47% at the corresponding temperatures. This phenomenon can be explained by the fact that the actual protein must overcome an energy barrier to break weak bonds, thereby exposing nitrogen and forming NH3. This process is more challenging than the direct deamination of the assembled amino acids to produce NH3.
- Under conditions of 2000 K, the formation of NH3 was primarily influenced by hydrogen radicals, causing the conversion of the nitrogen in the protein (NH3-N, NH2-N, NH-N) into NH3. Furthermore, the decrease in the quantity of NH3 in the later stages of thermal decomposition was attributed to its reconversion as a reactant, predominantly forming HCN and N2.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Wu, Y.; Gao, Y.; Chen, X.; Liu, D.; Fan, M. High-Performance Mesoporous (AlN/Al2O3) for Enhanced NH3 Yield during Chemical Looping Ammonia Generation Technology. Int. J. Hydrogen Energy 2020, 45, 9903–9913. [Google Scholar] [CrossRef]
- Weng, Q.; Toan, S.; Ai, R.; Sun, Z.; Sun, Z. Ammonia Production from Biomass via a Chemical Looping–Based Hybrid System. J. Clean. Prod. 2021, 289, 125749. [Google Scholar] [CrossRef]
- Wang, B.; Guo, H.; Yin, X.; Shen, L. N-Sorption Capability of Al2O3-Supported Mn-/Fe-Based Nitrogen Carriers during Chemical Looping Ammonia Synthesis Technology. Energy Fuels 2020, 34, 10247–10255. [Google Scholar] [CrossRef]
- Wang, H.; Mao, Q.; Yu, H.; Wang, S.; Xu, Y.; Li, X.; Wang, Z.; Wang, L. Enhanced Electrocatalytic Performance of Mesoporous Au-Rh Bimetallic Films for Ammonia Synthesis. Chem. Eng. J. 2021, 418, 129493. [Google Scholar] [CrossRef]
- Chen, J.G.; Crooks, R.M.; Seefeldt, L.C.; Bren, K.L.; Morris Bullock, R.; Darensbourg, M.Y.; Holland, P.L.; Hoffman, B.; Janik, M.J.; Jones, A.K. Beyond Fossil Fuel–Driven Nitrogen Transformations. Science 2018, 360, eaar6611. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chang, F.; Gao, W.; Guo, J.; Wu, G.; He, T.; Chen, P. Breaking Scaling Relations to Achieve Low-Temperature Ammonia Synthesis through LiH-Mediated Nitrogen Transfer and Hydrogenation. Nat. Chem. 2017, 9, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Gil-Lalaguna, N.; Afailal, Z.; Aznar, M.; Fonts, I. Exploring the Sustainable Production of Ammonia by Recycling N and H in Biological Residues: Evolution of Fuel-N during Glutamic Acid Gasification. J. Clean. Prod. 2021, 282, 124417. [Google Scholar] [CrossRef]
- Wang, P.; Xu, P.; Wang, B.; Shen, C.; Shen, L. Green Ammonia Production via Microalgae Steam Catalytic Gasification Process over LaFeO3 Perovskite. Fuel 2022, 318, 123322. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Wang, B.; Shen, L.; Song, T. Green Production of Ammonia from Nitrogen-Rich Biomass Pyrolysis: Evolution of Fuel-N under H2-Rich Atmosphere. Fuel Process. Technol. 2022, 227, 107126. [Google Scholar] [CrossRef]
- Zhao, Z.; Feng, S.; Zhao, Y.; Wang, Z.; Ma, J.; Xu, L.; Yang, J.; Shen, B. Investigation on the Fuel Quality and Hydrophobicity of Upgraded Rice Husk Derived from Various Inert and Oxidative Torrefaction Conditions. Renew. Energy 2022, 189, 1234–1248. [Google Scholar] [CrossRef]
- Wang, K.; Brown, R.C. Erratum: Catalytic Pyrolysis of Microalgae for Production of Aromatics and Ammonia. Green Chem. 2013, 15, 3486–3489. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, H.; Wang, Y.; Wang, F.; Wang, H.; Liu, H.; Liu, H. Influence mechanism of K on cellulose pyrolysis by stepwise isothermal method in-situ DRIFTS method. Fuel 2024, 360, 130601. [Google Scholar] [CrossRef]
- Guo, S.; Liu, T.; Hui, J.; Che, D.; Li, X.; Sun, B.; Li, S. Effects of Calcium Oxide on Nitrogen Oxide Precursor Formation during Sludge Protein Pyrolysis. Energy 2019, 189, 116217. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Yang, X.; Hu, L.; Liu, Y.; Wang, C. Evaluate the Pyrolysis Pathway of Glycine and Glycylglycine by TG-FTIR. J. Anal. Appl. Pyrolysis 2007, 80, 247–253. [Google Scholar] [CrossRef]
- Jie, L.; Yuwen, L.; Jingyan, S.; Zhiyong, W.; Ling, H.; Xi, Y.; Cunxin, W. The Investigation of Thermal Decomposition Pathways of Phenylalanine and Tyrosine by TG-FTIR. Thermochim. Acta 2007, 467, 20–29. [Google Scholar] [CrossRef]
- Bhoi, S.; Banerjee, T.; Mohanty, K. Insights on the Combustion and Pyrolysis Behavior of Three Different Ranks of Coals Using Reactive Molecular Dynamics Simulation. RSC Adv. 2016, 6, 2559–2570. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Z.; Li, X.; Qiao, X.; Song, W.; Guo, L. Initial Reaction Mechanisms of Cellulose Pyrolysis Revealed by ReaxFF Molecular Dynamics. Fuel 2016, 177, 130–141. [Google Scholar] [CrossRef]
- Su, J.; Zhao, D.; Feng, H.; Wu, T.; Liu, H.; Wang, H.; Guo, S.; Liu, H. Using Molecular Dynamics Simulations to Study the Non-Thermal Effects of Microwave Radiation on the Mechanism of Char Gasification. Renew. Energy 2023, 202, 784–796. [Google Scholar] [CrossRef]
- Zhao, D.; Shu, S.; Zhao, J.; Liang, Y.; Wang, H.; Liu, H.; Li, L.; Wang, D. Interactions of H2O and O2 with Char during Gasification in Mixed Atmosphere Analyzed by Isotope Tracer Method and In-Situ DRIFTS. Fuel 2023, 337, 127173. [Google Scholar] [CrossRef]
- Castro-Marcano, F.; Kamat, A.M.; Russo, M.F.; van Duin, A.C.T.; Mathews, J.P. Combustion of an Illinois No. 6 Coal Char Simulated Using an Atomistic Char Representation and the ReaxFF Reactive Force Field. Combust. Flame 2012, 159, 1272–1285. [Google Scholar] [CrossRef]
- Castro-Marcano, F.; Lobodin, V.V.; Rodgers, R.P.; McKenna, A.M.; Marshall, A.G.; Mathews, J.P. A Molecular Model for Illinois No. 6 Argonne Premium Coal: Moving toward Capturing the Continuum Structure. Fuel 2012, 95, 35–49. [Google Scholar] [CrossRef]
- Castro-Marcano, F.; Russo, M.F.; Van Duin, A.C.T.; Mathews, J.P. Pyrolysis of a Large-Scale Molecular Model for Illinois No. 6 Coal Using the ReaxFF Reactive Force Field. J. Anal. Appl. Pyrolysis 2014, 109, 79–89. [Google Scholar] [CrossRef]
- Zheng, M.; Li, X.; Liu, J.; Wang, Z.; Gong, X.; Guo, L.; Song, W. Pyrolysis of Liulin Coal Simulated by GPU-Based ReaxFF MD with Cheminformatics Analysis. Energy Fuels 2014, 28, 522–534. [Google Scholar] [CrossRef]
- Hong, D.; Gao, P.; Wang, C. A Comprehensive Understanding of the Synergistic Effect during Co-Pyrolysis of Polyvinyl Chloride (PVC) and Coal. Energy 2022, 239, 122258. [Google Scholar] [CrossRef]
- Chen, H.; Lin, G.; Chen, Y.; Chen, W.; Yang, H. Biomass Pyrolytic Polygeneration of Tobacco Waste: Product Characteristics and Nitrogen Transformation. Energy Fuels 2016, 30, 1579–1588. [Google Scholar] [CrossRef]
- Xu, T.; Hong, D.; Wang, C.; Zhang, Y.; Li, Y. Investigation of N Migration during Municipal Sludge/Coal Co-Pyrolysis via ReaxFF Molecular Dynamics. Energy Fuels 2023, 37, 12776–12787. [Google Scholar] [CrossRef]
- Leng, L.; Yang, L.; Zu, H.; Yang, J.; Ai, Z.; Zhang, W.; Peng, H.; Zhan, H.; Li, H.; Zhong, Q. Insights into Glycine Pyrolysis Mechanisms: Integrated Experimental and Molecular Dynamics/DFT Simulation Studies. Fuel 2023, 351, 128949. [Google Scholar] [CrossRef]
- Yi, L.; Liu, H.; Lu, G.; Zhang, Q.; Wang, J.; Hu, H.; Yao, H. Effect of Mixed Fe/Ca Additives on Nitrogen Transformation during Protein and Amino Acid Pyrolysis. Energy Fuels 2017, 31, 9484–9490. [Google Scholar] [CrossRef]
- Li, H.; Mou, H.; Zhao, N.; Yu, Y.; Hong, Q.; Philbert, M.; Zhou, Y.; Dizaji, H.B.; Dong, R. Nitrogen Migration during Pyrolysis of Raw and Acid Leached Maize Straw. Sustainability 2021, 13, 3786. [Google Scholar] [CrossRef]
- Gao, P.; Guo, D.; Liang, C.; Liu, G.; Yang, S. Nitrogen Conversion during the Rapid Pyrolysis of Raw/Torrefied Wheat Straw. Fuel 2020, 259, 116227. [Google Scholar] [CrossRef]
- Mullen, C.A.; Boateng, A.A. Production and Analysis of Fast Pyrolysis Oils from Proteinaceous Biomass. Bioenergy Res. 2011, 4, 303–311. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, C.; Chen, X.; Duan, L.; Li, Y.; Ma, C. NOx and N2O Precursors (NH3 and HCN) from Biomass Pyrolysis: Co-Pyrolysis of Amino Acids and Cellulose, Hemicellulose and Lignin. Proc. Combust. Inst. 2011, 33, 1715–1722. [Google Scholar] [CrossRef]
- Yang, L.; Wang, G.; Liu, T.; Wan, Y.; Peng, H.; Leng, L.; Zhong, Q. Experimental and ReaxFF Molecular Dynamic Study on Pyrolysis of Phenylalanine. Fuel 2022, 324, 124690. [Google Scholar] [CrossRef]
- Xiao, K.; Guan, R.; Yang, J.; Li, H.; Yu, Z.; Liang, S.; Yu, W.; Hu, J.; Hou, H.; Liu, B. Effects of Red Mud on Emission Control of NO x Precursors during Sludge Pyrolysis: A Protein Model Compound Study. Waste Manag. 2019, 85, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, P.; Shen, L. Fast Pyrolysis of Tea Waste under a Hydrogen-Rich Atmosphere: A Study on Nitrogen Evolution and Green Ammonia Production. Energy Fuels 2022, 36, 14304–14314. [Google Scholar] [CrossRef]
- Döntgen, M.; Przybylski-Freund, M.-D.; Kröger, L.C.; Kopp, W.A.; Ismail, A.E.; Leonhard, K. Automated Discovery of Reaction Pathways, Rate Constants, and Transition States Using Reactive Molecular Dynamics Simulations. J. Chem. Theory Comput. 2015, 11, 2517–2524. [Google Scholar] [CrossRef]
- Yuan, S.; Zhou, Z.-J.; Li, J.; Wang, F.-C. Nitrogen Conversion during Rapid Pyrolysis of Coal and Petroleum Coke in a High-Frequency Furnace. Appl. Energy 2012, 92, 854–859. [Google Scholar] [CrossRef]
- Tan, L.L.; Li, C.Z. Formation of NOx and SOx Precursors during the Pyrolysis of Coal and Biomass. Part I. Effects of Reactor Configuration on the Determined Yields of HCN and NH3 during Pyrolysis. Fuel 2000, 79, 1883–1889. [Google Scholar] [CrossRef]
- Tan, L.L.; Li, C.Z. Formation of NOx and SOx Precursors during the Pyrolysis of Coal and Biomass. Part II. Effects of Experimental Conditions on the Yields of NOx and SOx Precursors from the Pyrolysis of a Victorian Brown Coal. Fuel 2000, 79, 1891–1897. [Google Scholar] [CrossRef]
- Li, C.Z.; Tan, L.L. Formation of NOx and SOx Precursors during the Pyrolysis of Coal and Biomass. Part III. Further Discussion on the Formation of HCN and NH3 during Pyrolysis. Fuel 2000, 79, 1899–1906. [Google Scholar] [CrossRef]
- Liu, G.; Wright, M.M.; Zhao, Q.; Brown, R.C.; Wang, K.; Xue, Y. Catalytic Pyrolysis of Amino Acids: Comparison of Aliphatic Amino Acid and Cyclic Amino Acid. Energy Convers. Manag. 2016, 112, 220–225. [Google Scholar] [CrossRef]
- Available online: https://www.cusabio.cn/Recombinant-Proteins/Recombinant-Nicotiana-tabacum-Adenosylhomocysteinase(SAHH)-128088.html (accessed on 1 January 2023).
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Chenoweth, K.; Van Duin, A.C.T.; Goddard, W.A. ReaxFF Reactive Force Field for Molecular Dynamics Simulations of Hydrocarbon Oxidation. J. Phys. Chem. A 2008, 112, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- ReaxFF 202*, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands. Available online: http://www.scm.com (accessed on 1 January 2023).
- Zhang, L.; Zybin, S.V.; Van Duin, A.C.T.; Dasgupta, S.; Goddard, W.A.; Kober, E.M. Carbon Cluster Formation during Thermal Decomposition of Octahydro-l,3,5,7-Tetranitro-l,3,5,7-Tetrazocine and l,3,5-Triamino-2,4,6- Trinitrobenzene High Explosives from ReaxFF Reactive Molecular Dynamics Simulations. J. Phys. Chem. A 2009, 113, 10619–10640. [Google Scholar] [CrossRef] [PubMed]
- Senftle, T.P.; Hong, S.; Islam, M.M.; Kylasa, S.B.; Zheng, Y.; Shin, Y.K.; Junkermeier, C.; Engel-Herbert, R.; Janik, M.J.; Aktulga, H.M.; et al. The ReaxFF Reactive Force-Field: Development, Applications and Future Directions. NPJ Comput. Mater. 2016, 2, 15011. [Google Scholar] [CrossRef]
- Sørensen, M.R.; Voter, A.F. Temperature-Accelerated Dynamics for Simulation of Infrequent Events. J. Chem. Phys. 2000, 112, 9599–9606. [Google Scholar] [CrossRef]

| Amino Acids | Ratio% |
|---|---|
| Glycine | 8.25 |
| Alanine | 8.87 |
| Valine | 7.63 |
| Leucine | 9.28 |
| Isoleucine | 5.77 |
| Phenylalanine | 2.89 |
| Proline | 3.92 |
| Tryptophan | 1.44 |
| Serine | 4.54 |
| Tyrosine | 2.68 |
| Cysteine | 2.06 |
| Methionine | 3.30 |
| Asparagine | 3.51 |
| Glutamine | 3.09 |
| Threonine | 7.42 |
| Aspartic acid | 6.19 |
| Glutamic acid | 6.60 |
| Lysine | 7.22 |
| Arginine | 3.30 |
| Histidine | 2.06 |
| ∑ | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Wang, Y.; Zhu, S.; Qu, H.; Zhao, D.; Li, X.; Zhao, Y. Reactive Force Field Molecular Dynamics Investigation of NH3 Generation Mechanism during Protein Pyrolysis Process. Molecules 2024, 29, 2016. https://doi.org/10.3390/molecules29092016
Guo S, Wang Y, Zhu S, Qu H, Zhao D, Li X, Zhao Y. Reactive Force Field Molecular Dynamics Investigation of NH3 Generation Mechanism during Protein Pyrolysis Process. Molecules. 2024; 29(9):2016. https://doi.org/10.3390/molecules29092016
Chicago/Turabian StyleGuo, Shuai, Yu Wang, Shujun Zhu, Hongwei Qu, Deng Zhao, Xingcan Li, and Yan Zhao. 2024. "Reactive Force Field Molecular Dynamics Investigation of NH3 Generation Mechanism during Protein Pyrolysis Process" Molecules 29, no. 9: 2016. https://doi.org/10.3390/molecules29092016





