Temperature-Caused Changes in Raman Pattern and Protein Profiles of Winter Triticale (x Triticosecale, Wittm.) Field-Grown Seedlings
Abstract
:1. Introduction
2. Results
2.1. Field Cultivation Conditions
2.2. Analysis of Chlorophyll a Fluorescence
2.3. Antioxidant Activity
2.4. Immunodetection of Proteins
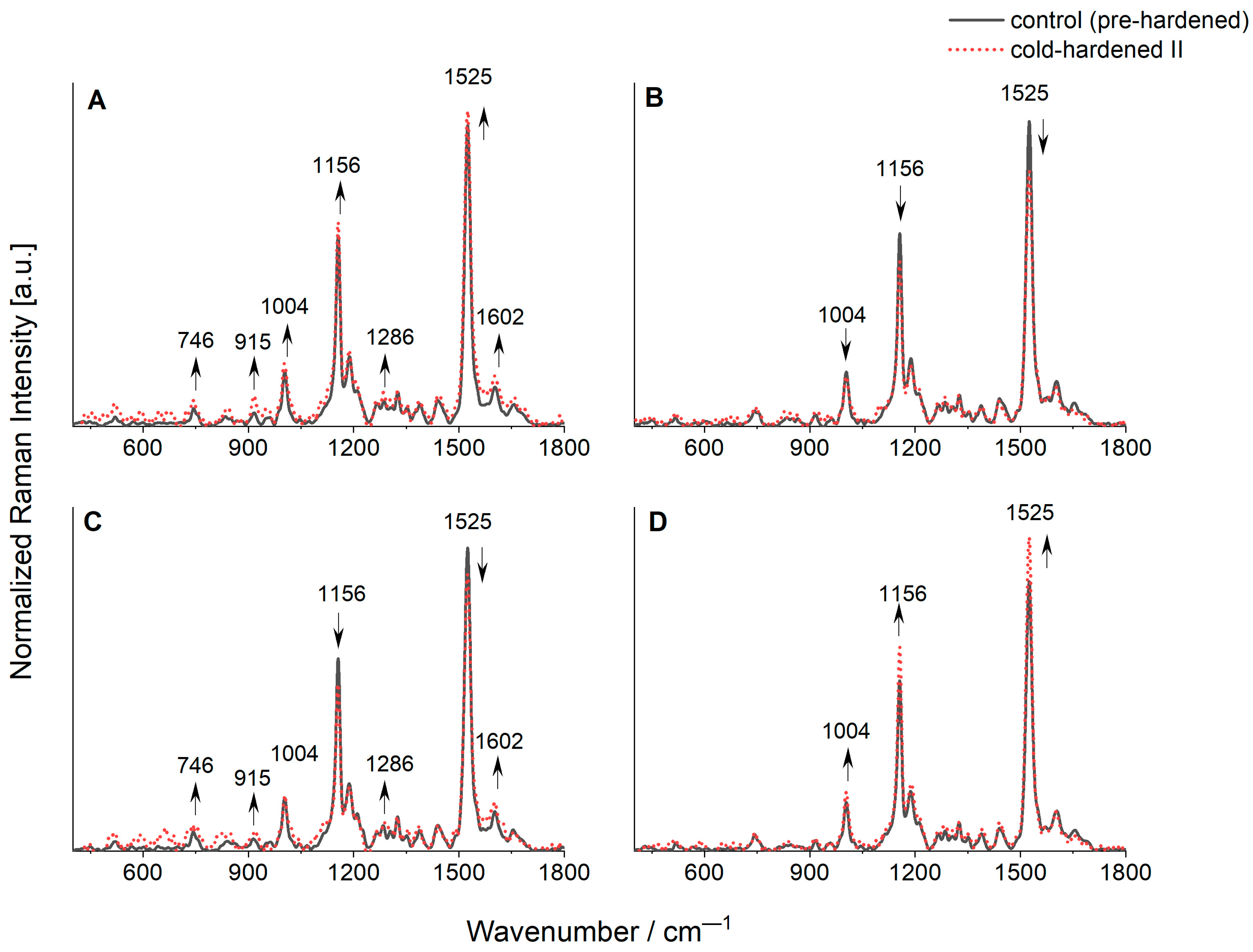
2.5. FT-Raman Studies
2.6. Correlation between the Values of Measured Parameters
2.7. Location of Coding Genes and Protein Conformation
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Design
4.2. Sampling
4.3. Analysis of Chlorophyll a Fluorescence
4.4. Enzymatic Assays
4.5. Western Blot
4.6. FT-Raman Spectroscopy
4.7. Statistical Analysis
4.8. Bioinformatic Analysis
5. Conclusions
- The analysis of chlorophyll a fluorescence confirmed that PSII efficiency (Fv/Fm) was influenced by seedling genotype, cultivation conditions, and the interaction of these factors.
- For DH1 and DH2 lines, PSII adapts more efficiently to changing temperature conditions, including cold periods and freeze–thawing cycles, compared to DH3 and cv. Hewo seedlings. In DH1 and DH2 lines, PrxQ may have a protective function against oxidative stress.
- Both the DH1 and DH2 lines had the highest PsbC abundance after the cold-hardening period II, as well as the highest PsbD abundance after the cold-hardening period I.
- For the DH1 and DH3 lines, after the cold-hardening period II, an additional band typical for chlorophyll b (chl b) was observed (possible influence of the cold-hardening period II on the formation of chl b in plastids)
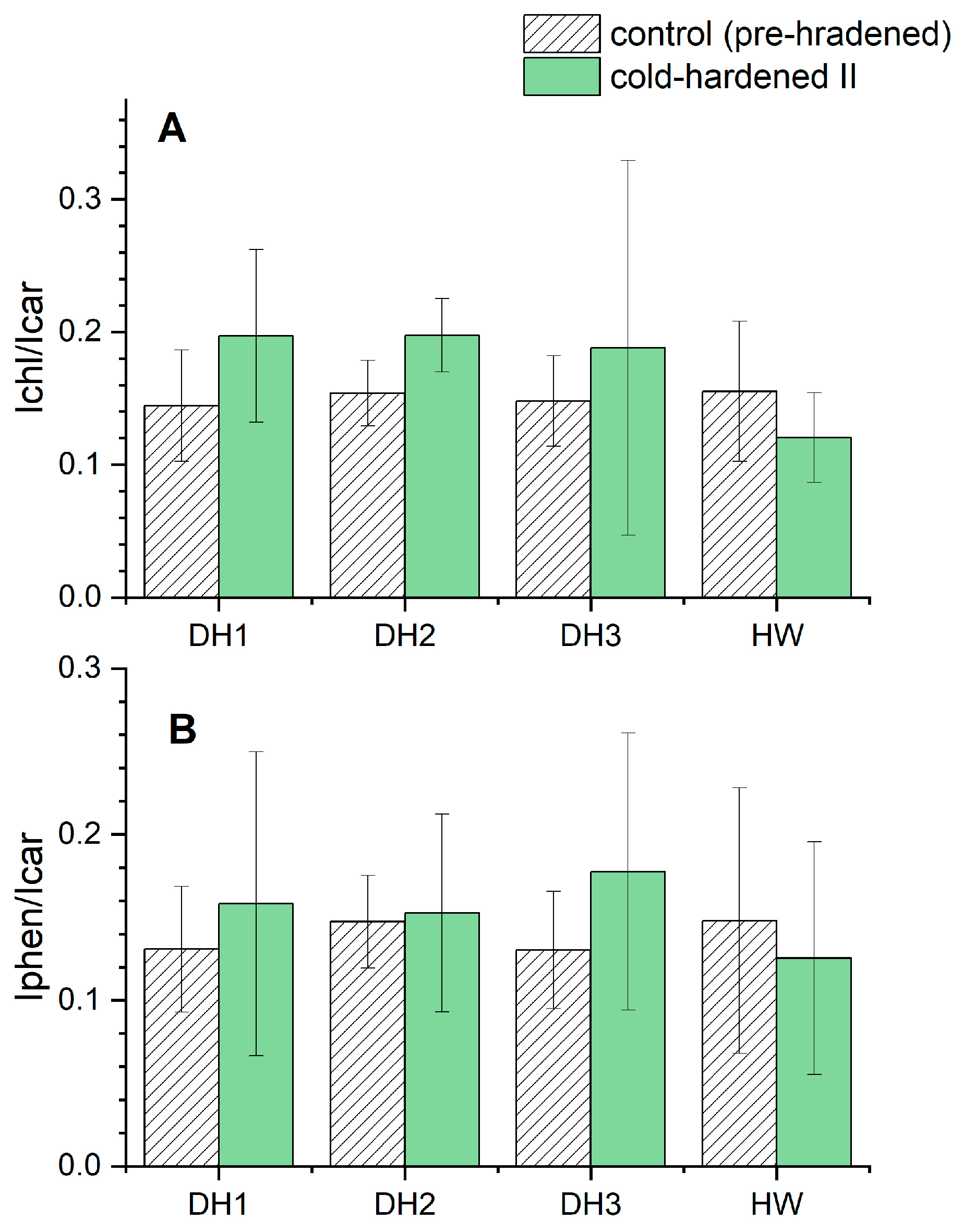
- An increasing content of phenolic compounds compared to carotenoid dyes after the cold-hardening period II observed for the DH lines can be connected with a protective role of the former against stress factors.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hura, T.; Tyrka, M.; Hura, K.; Ostrowska, A.; Dziurka, K. QTLs for cell wall-bound phenolics in relation to the photosynthetic apparatus activity and leaf water status under drought stress at different growth stages of triticale. Mol. Genet. Genom. 2017, 292, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowska, G.; Dyda, M.; Wajdzik, K. Quantitative trait loci and candidate genes associated with cold-acclimation and Microdochium nivale tolerance/susceptibility in winter triticale (×Triticosecale). Plants 2021, 10, 2678. [Google Scholar] [CrossRef] [PubMed]
- Wąsek, I.; Dyda, M.; Gołębiowska, G.; Tyrka, M.; Rapacz, M.; Szechyńska-Hebda, M.; Wędzony, M. Quantitative trait loci and candidate genes associated with freezing tolerance of winter triticale (×Triticosecale Wittmack). J. Appl. Genet. 2022, 63, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Dyda, M.; Tyrka, M.; Gołębiowska, G.; Rapacz, M.; Wędzony, M. Mapping of QTL and candidate genes associated with powdery mildew resistance in triticale (×Triticosecale Wittm.). Plant Growth Regul. 2022, 98, 103–115. [Google Scholar] [CrossRef]
- Dyda, M.; Wąsek, I.; Tyrka, M.; Wędzony, M.; Szechyńska-Hebda, M. Local and systemic regulation of PSII efficiency in triticale infected by the hemibiotrophic pathogen Microdochium nivale. Physiol. Plant. 2019, 165, 711–727. [Google Scholar] [CrossRef] [PubMed]
- Kosová, K.; Klíma, M.; Prášil, I.T.; Vítámvás, P. COR/LEA proteins as indicators of frost tolerance in Triticeae: A comparison of controlled versus field conditions. Plants 2021, 10, 789. [Google Scholar] [CrossRef] [PubMed]
- Båga, M.; Bahrani, H.; Larsen, J.; Hackauf, B.; Graf, R.J.; Laroche, A.; Chibbar, R.N. Association mapping of autumn-seeded rye (Secale cereale L.) reveals genetic linkages between genes controlling winter hardiness and plant development. Sci. Rep. 2022, 12, 5793. [Google Scholar] [CrossRef] [PubMed]
- Tyrka, M.; Chelkowski, J. Enhancing the resistance of triticale by using genes from wheat and rye. J. Appl. Genet. 2004, 45, 283–296. [Google Scholar] [PubMed]
- Gołębiowska, G.; Wędzony, M.; Płażek, A. The responses of pro-and antioxidative systems to cold-hardening and pathogenesis differ in triticale (×Triticosecale Wittm.) seedlings susceptible or resistant to pink snow mould (Microdochium nivale Fr., Samuels & Hallett). J. Phytopathol. 2011, 159, 19–27. [Google Scholar] [CrossRef]
- Gołębiowska-Pikania, G.; Golemiec, E. Cold-enhanced gene expression of the foliar thiol-specific antioxidant protein in triticale (×Triticosecale Wittm.) seedlings resistant to Microdochium nivale (Samuels and IC Hallett) infection. Zesz. Naukowe. Acta Biol. Uniw. Szczeciński 2015, 22, 97–117. [Google Scholar] [CrossRef]
- Gołębiowska-Pikania, G.; Gawronska, K. The effects of cold-hardening and Microdochium nivale infection on oxidative stress and antioxidative protection of the two contrasting genotypes of winter triticale. Eur. Food Res. Technol. 2016, 242, 1267–1276. [Google Scholar] [CrossRef]
- Gołębiowska, G.J.; Bonar, E.; Emami, K.; Wędzony, M. Cold-modulated small proteins abundance in winter triticale (×Triticosecale, Wittm.) seedlings tolerant to the pink snow mold (Microdochium nivale, Samuels and Hallett) infection. Acta Biochim. Pol. 2019, 66, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowska-Pikania, G.; Dziurka, M.; Wąsek, I.; Wajdzik, K.; Dyda, M.; Wędzony, M. Changes in phenolic acid abundance involved in low temperature and Microdochium nivale (Samuels and Hallett) cross-tolerance in winter triticale (×Triticosecale Wittmack). Acta Physiol. Plant. 2019, 41, 38. [Google Scholar] [CrossRef]
- Gołębiowska, G.; Stawoska, I.; Wesełucha-Birczyńska, A. Cold-modulated leaf compounds in winter triticale DH lines tolerant to freezing and Microdochium nivale infection: LC-MS and Raman study. Funct. Plant Biol. 2022, 49, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Szechyńska-Hebda, M.; Wędzony, M.; Tyrka, M.; Gołębiowska, G.; Chrupek, M.; Czyczyło-Mysza, I.; Golemiec, E. Identifying QTLs for cold-induced resistance to Microdochium nivale in winter triticale. Plant Genet. Resour. 2011, 9, 296–299. [Google Scholar] [CrossRef]
- Niedziela, A.; Bednarek, P.T.; Cichy, H.; Budzianowski, G.; Kilian, A.; Anioł, A. Aluminum tolerance association mapping in triticale. BMC Genom. 2012, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Zhang, Z.; Yan, G.; Wang, F.; Zhao, L.; Liu, N.; Shi, S. Salt-responsive transcriptome analysis of triticale reveals candidate genes involved in the key metabolic pathway in response to salt stress. Sci. Rep. 2020, 10, 20669. [Google Scholar] [CrossRef] [PubMed]
- Mergoum, M.; Sapkota, S.; El Doliefy, A.E.A.; Naraghi, S.M.; Pirseyedi, S.; Alamri, M.S.; Abu Hammad, W. Triticale (×Triticosecale Wittmack) Breeding. In Advances in Plant Breeding Strategies: Cereals; Springer: Cham, Switzerland, 2019; pp. 405–451. [Google Scholar] [CrossRef]
- Rapacz, M.; Macko-Podgórni, A.; Jurczyk, B.; Kuchar, L. Modeling wheat and triticale winter hardiness under current and predicted winter scenarios for Central Europe: A focus on deacclimation. Agric. For. Meteorol. 2022, 313, 108739. [Google Scholar] [CrossRef]
- Rys, M.; Pociecha, E.; Oliwa, J.; Ostrowska, A.; Jurczyk, B.; Saja, D.; Janeczko, A. Deacclimation of winter oilseed rape—Insight into physiological changes. Agronomy 2020, 10, 1565. [Google Scholar] [CrossRef]
- Rapacz, M.; Sasal, M.; Gut, M. Chlorophyll fluorescence-based studies of frost damage and the tolerance for cold-induced photoinhibition in freezing tolerance analysis of Triticale (×Triticosecale Wittmack). J. Agron. Crop Sci. 2011, 197, 378–389. [Google Scholar] [CrossRef]
- Rapacz, M.; Sasal, M.; Kalaji, H.M.; Kościelniak, J. Is the OJIP test a reliable indicator of winter hardiness and freezing tolerance of common wheat and triticale under variable winter environments? PLoS ONE 2015, 10, e013482. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, A.; Gruszka, D.; Pociecha, E.; Dziurka, M.; Filek, M.; Jurczyk, B.; Waligórski, P. Physiological and biochemical characterisation of watered and drought-stressed barley mutants in the HvDWARF gene encoding C6-oxidase involved in brassinosteroid biosynthesis. Plant Physiol. Biochem. 2016, 99, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Stawoska, I.; Staszak, A.M.; Ciereszko, I.; Oliwa, J.; Skoczowski, A. Using isothermal calorimetry and FT-Raman spectroscopy for step-by-step monitoring of maize seed germination: Case study. J Therm Anal Calorim 2020, 142, 755–763. [Google Scholar] [CrossRef]
- Stawoska, I.; Weselucha-Birczynska, A.; Regonesi, M.E.; Riva, M.; Tortora, P.; Stochel, G. Interaction of selected divalent metal ions with human ataxin-3 Q36. J. Biol. Inorg. Chem. 2009, 14, 1175–1185. [Google Scholar] [CrossRef]
- Adamczyk, A.; Nowakowska, A.M.; Jakubowska, J.; Zabczynska, M.; Bartoszek, M.; Kashyrskaya, S.; Fatla, A.; Stawoski, K.; Siakala, K.; Pastorczak, A.; et al. Raman classification of selected subtypes of acute lymphoblastic leukemia (ALL). Analyst 2024, 149, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Stawoska, I.; Waga, J.; Wesełucha-Birczyńska, A.; Dziurka, M.; Podolska, G.; Aleksandrowicz, E.; Skoczowski, A. Does nitrogen fertilization affect the secondary structures of gliadin proteins in hypoallergenic wheat? Molecules 2022, 27, 5684. [Google Scholar] [CrossRef]
- Stawoska, I.; Wesełucha-Birczyńska, A.; Skoczowski, A.; Dziurka, M.; Waga, J. FT-Raman Spectroscopy as a Tool to Study the Secondary Structures of Wheat Gliadin Proteins. Molecules 2021, 26, 5388. [Google Scholar] [CrossRef]
- Lukaszuk, E.; Rys, M.; Mozdzen, K.; Stawoska, I.; Skoczowski, A.; Ciereszko, I. Photosynthesis and sucrose metabolism in leaves of Arabidopsis thaliana aos, ein4 and rcd1 mutants as affected by wounding. Acta Physiol. Plant. 2017, 39, 12. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M.; Baranski, R. Potential of NIR-FT-Raman spectroscopy in natural carotenoid analysis. Biopolymers 2005, 77, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Takatsuka, I.; Nakata, M.; Tasumi, M. Raman and infrared-spectra of the all-trans, 7-cis, 9-cis, 13-cis and 15-cis isomers of beta-carotene—Key bands distinguishing stretched or terminal-bent configurations from central-bent configurations. J. Raman Spectrosc. 1988, 19, 37–49. [Google Scholar] [CrossRef]
- Gołębiowska-Pikania, G.; Kopeć, P.; Surówka, E.; Krzewska, M.; Dubas, E.; Nowicka, A.; Żur, I. Changes in protein abundance and activity involved in freezing tolerance acquisition in winter barley (Hordeum vulgare L.). J. Proteom. 2017, 169, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Gołębiowska-Pikania, G.; Kopeć, P.; Surówka, E.; Janowiak, F.; Krzewska, M.; Dubas, E.; Żur, I. Changes in protein abundance and activity induced by drought during generative development of winter barley (Hordeum vulgare L.). J. Proteom. 2017, 169, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Golebiowska-Paluch, G.; Dyda, M. The genome regions associated with abiotic and biotic stress tolerance, as well as other important breeding traits in triticale. Plants 2023, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Withnall, R.; Chowdhry, B.Z.; Silver, J.; Edwards, H.G.M.; de Oliveira, L.F.C. Raman spectra of carotenoids in natural products. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.E.; Castro, H.V.; Edwards, H.G.M.; de Oliveira, L.F.C. Carotenes and carotenoids in natural biological samples: A Raman spectroscopic analysis. J. Raman Spectrosc. 2010, 41, 642–650. [Google Scholar] [CrossRef]
- Vitek, P.; Novotna, K.; Hodanova, P.; Rapantova, B.; Klem, K. Detection of herbicide effects on pigment composition and PSII photochemistry in Helianthus annuus by Raman spectroscopy and chlorophyll a fluorescence. Spectrochim. Acta Part A-Molec. Biomolec. Spectr. 2017, 170, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Filek, M.; Łabanowska, M.; Kurdziel, M.; Wesełucha-Birczyńska, A.; Bednarska-Kozakiewicz, E. Structural and biochemical response of chloroplasts in tolerant and sensitive barley genotypes to drought stress. J. Plant Physiol. 2016, 207, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Mandrile, L.; Rotunno, S.; Miozzi, L.; Vaira, A.M.; Giovannozzi, A.M.; Rossi, A.M.; Noris, E. Nondestructive raman spectroscopy as a tool for early detection and discrimination of the infection of tomato plants by two economically important viruses. Anal. Chem. 2019, 91, 9025–9031. [Google Scholar] [CrossRef]
- Schrader, B.; Klump, H.H.; Schenzel, K.; Schulz, H. Non-destructive NIR FT Raman analysis of plants. J. Mol. Struct. 1999, 509, 201–212. [Google Scholar] [CrossRef]
- Wesełucha-Birczyńska, A.; Łabanowska, M.; Kurdziel, M.; Filek, M. Resonance Raman and EPR spectroscopy studies of untreated spring wheat leaves. Vib. Spectrosc. 2012, 60, 113–117. [Google Scholar] [CrossRef]
- Pascal, A.; Peterman, E.; Gradinaru, C.; van Amerongen, H.; van Grondelle, R.; Robert, B. Structure and interactions of the chlorophyll a molecules in the higher plant Lhcb4 antenna protein. J. Phys. Chem. B 2000, 104, 9317–9321. [Google Scholar] [CrossRef]
- Koyama, Y.; Umemoto, Y.; Akamatsu, A.; Uehara, K.; Tanaka, M. Raman-spectra of chlorophyll forms. J. Mol. Struct. 1986, 146, 273–287. [Google Scholar] [CrossRef]
- Pursiheimo, S.; Rintamäki, E.; Baena-Gonzalez, E.; Aro, E.M. Thylakoid protein phosphorylation in evolutionally divergent species with oxygenic photosynthesis. Febs Lett. 1998, 423, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Lamkemeyer, P.; Laxa, M.; Collin, V.; Li, W.; Finkemeier, I.; Schöttler, M.A.; Holtkamp, V.; Tognetti, V.B.; Issakidis-Bourguet, E.; Kandlbinder, A.; et al. Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. Plant J. 2006, 45, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Saski, C.; Lee, S.B.; Fjellheim, S. Complete chloroplast genome sequences of Hordeum vulgare, Sorghum bicolor and Agrostis stolonifera, and comparative analyses with other grass genomes. Theor. Appl. Genet. 2007, 115, 571–590. [Google Scholar]
- Wędzony, M. Protocol for anther culture in hexaploid triticale (×Triticosecale Wittm.). In Doubled Haploid Production in Crop Plants; Springer: Dordrecht, The Netherlands, 2003; pp. 123–128. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.V. Peroxidases. In Methods in Enzymatic Analysis; Bergmeyer, H.V., Ed.; Academic Press: New York, NY, USA, 1965; pp. 384–388. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Yao, E.; Blake, V.C.; Cooper, L.; Wight, C.P.; Michel, S.; Cagirici, H.B.; Lazo, G.R.; Birkett, C.L.; Waring, D.J.; Jannink, J.-L.; et al. GrainGenes: A Data-Rich Repository for Small Grains Genetics and Genomics. Database 2022, 2022, baac034. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]




| Parameter | Genotype | Cultivation Conditions 1–3 | Genotype × Conditions 1–3 | |
|---|---|---|---|---|
| Chlorophyll a fluorescence | Fv/Fm | ***** | ***** | **** |
| NPQ | ***** | ns | * | |
| Enzyme activity | Cu/Zn SOD I | **** | *** | **** |
| Fe/Mn SOD | ** | ** | ns | |
| Protein abundance | PrxQ | *** | * | ***** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stawoska, I.; Wesełucha-Birczyńska, A.; Golebiowska-Paluch, G. Temperature-Caused Changes in Raman Pattern and Protein Profiles of Winter Triticale (x Triticosecale, Wittm.) Field-Grown Seedlings. Molecules 2024, 29, 1933. https://doi.org/10.3390/molecules29091933
Stawoska I, Wesełucha-Birczyńska A, Golebiowska-Paluch G. Temperature-Caused Changes in Raman Pattern and Protein Profiles of Winter Triticale (x Triticosecale, Wittm.) Field-Grown Seedlings. Molecules. 2024; 29(9):1933. https://doi.org/10.3390/molecules29091933
Chicago/Turabian StyleStawoska, Iwona, Aleksandra Wesełucha-Birczyńska, and Gabriela Golebiowska-Paluch. 2024. "Temperature-Caused Changes in Raman Pattern and Protein Profiles of Winter Triticale (x Triticosecale, Wittm.) Field-Grown Seedlings" Molecules 29, no. 9: 1933. https://doi.org/10.3390/molecules29091933






