Immobilization of Perylenetetracarboxylic Dianhydride on Al2O3 for Efficiently Photocatalytic Sulfide Oxidation
Abstract
:1. Introduction
2. Results and Discussion
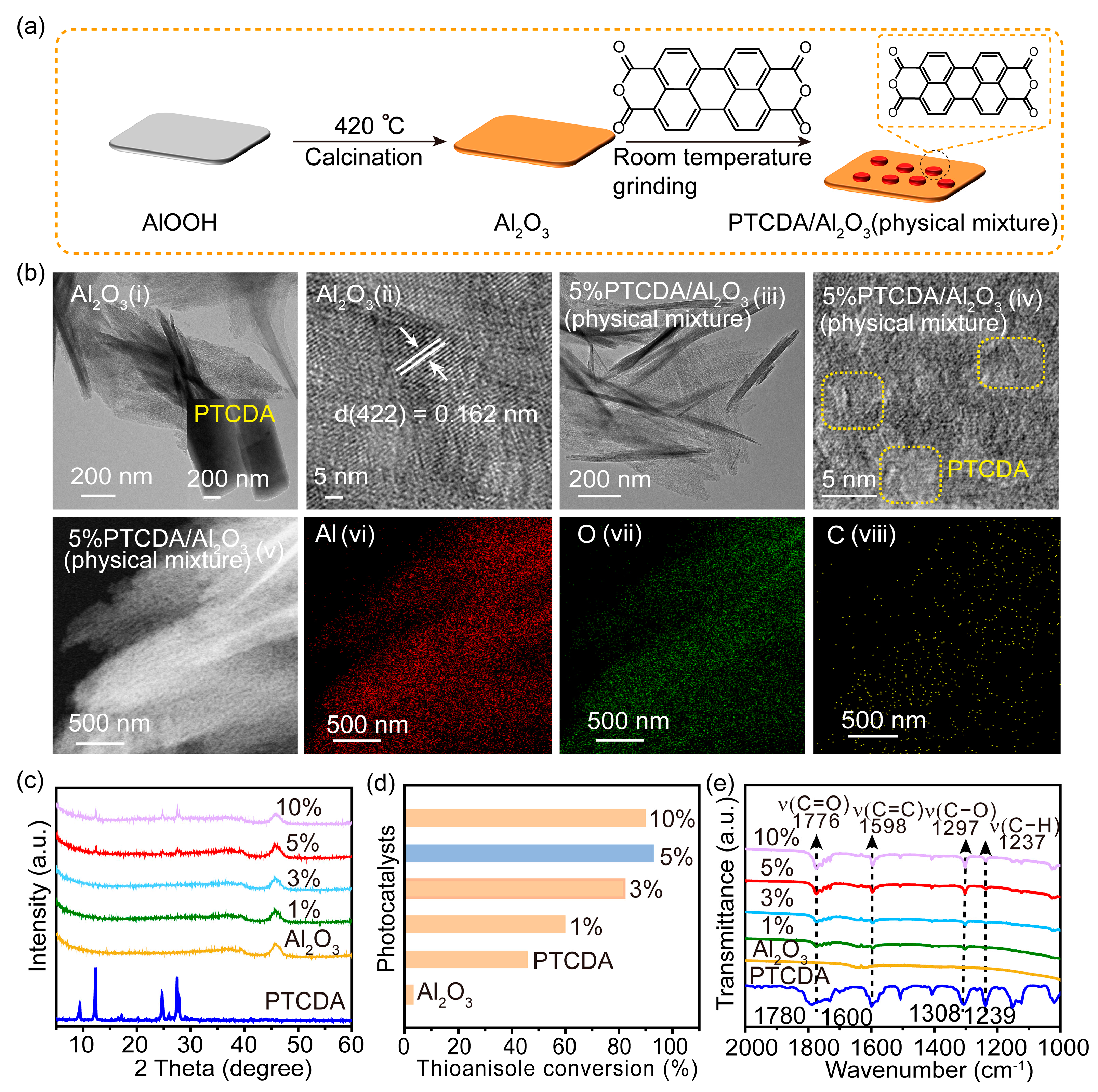
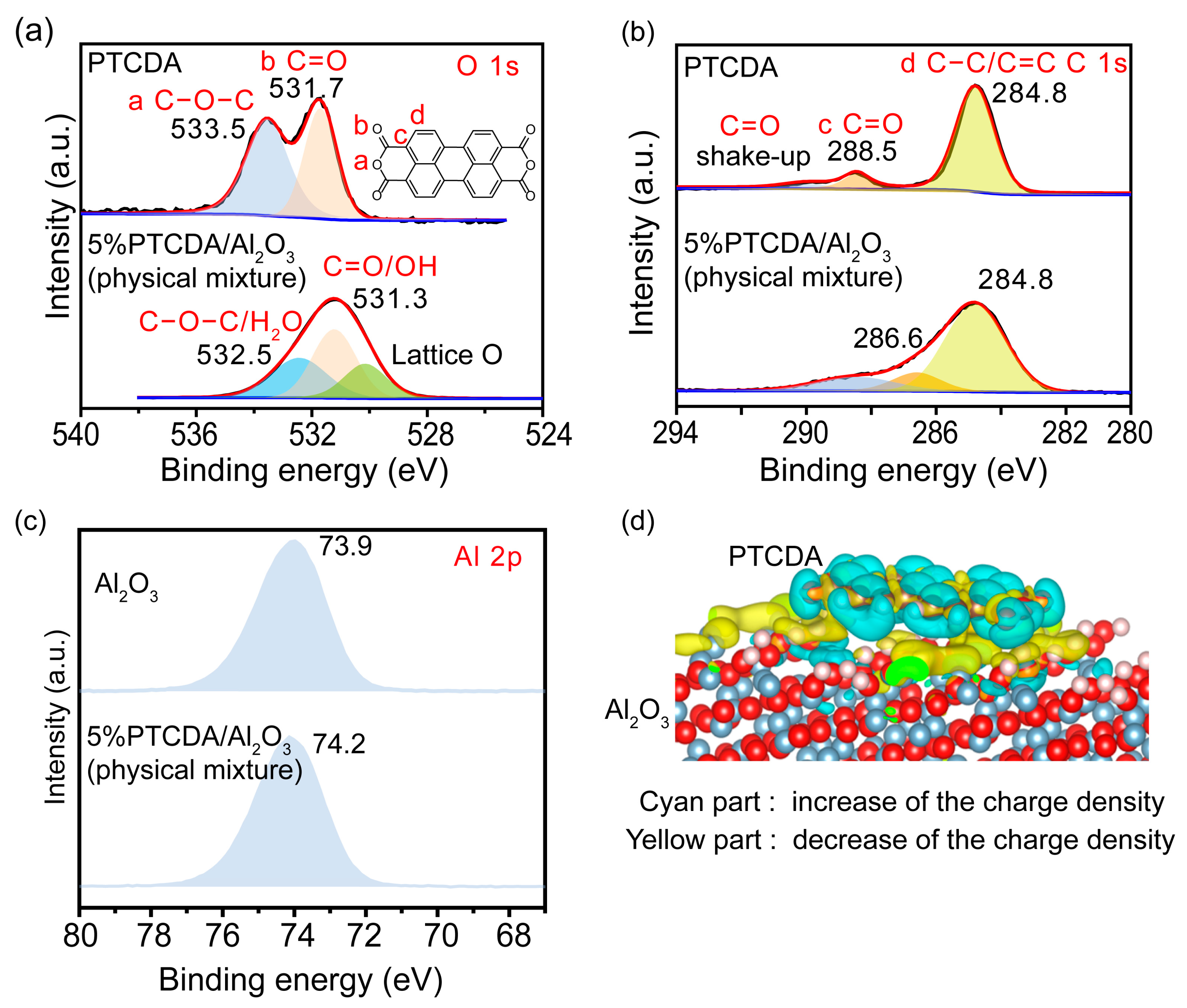
2.1. Synthesis, Activity Evaluation, and Interaction Study of PTCDA/Al2O3 Mixture
2.2. Exploration of the Mechanism
3. Materials and Methods
3.1. Reagents and Solvents
3.2. Preparation of 2D Al2O3 Nanosheets as Precursor
3.3. Preparation of PTCDA/Al2O3(Physical Mixture)
3.4. General Procedure for the Photocatalytic Selective Oxidation of Sulfide
3.5. Characterizations
3.6. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghosh, I.; Ghosh, T.; Bardagi, J.I.; Konig, B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. Science 2014, 346, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, N.; Shanmugam, S.; Xu, J.; Boyer, C. Photocatalysis in organic and polymer synthesis. Chem. Soc. Rev. 2016, 45, 6165–6212. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhou, Q.; Nan, J.; Shi, W.; Cui, F.; Zhu, Y. Perylenetetracarboxylic acid nanosheets with internal electric fields and anisotropic charge migration for photocatalytic hydrogen evolution. Nat. Commun. 2022, 13, 2067. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Meng, J.; Yang, X.; Hu, A.; Yang, Y.; Guo, Y. Fabrication of a perylene tetracarboxylic diimide-graphitic carbon nitride heterojunction photocatalyst for efficient degradation of aqueous organic pollutants. ACS Appl. Mater. Interfaces 2019, 11, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wonneberger, H. Perylene imides for organic photovoltaics: Yesterday, today, and tomorrow. Adv. Mater. 2012, 24, 613–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, X.; Zhang, H.; Liu, W.; Zhu, W.; Zhu, Y. A highly crystalline perylene imide polymer with the robust built-in electric field for efficient photocatalytic water oxidation. Adv. Mater. 2020, 32, e1907746. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Blaney, L.; Cagnetta, G.; Huang, J.; Wang, B.; Wang, Y.; Deng, S.; Yu, G. Degradation of ofloxacin by perylene diimide supramolecular nanofiber sunlight-driven photocatalysis. Environ. Sci. Technol. 2019, 53, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, C.C.; Li, Y.; Wang, P.; Wei, Q. The Z-scheme NH2-UiO-66/PTCDA composite for enhanced photocatalytic Cr(VI) reduction under low-power LED visible light. Chemosphere 2021, 280, 130734. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Li, W.; Xu, L.; Zhu, Y. High photocatalytic oxygen evolution via strong built-in electric field induced by high crystallinity of perylene imide supramolecule. Adv. Mater. 2022, 34, 2102354. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Liu, W.; Lou, Y.; Zhang, Y.; Dong, Y.; Xu, J.; Pan, C.; Zhu, Y. Create a strong internal electric-field on PDI photocatalysts for boosting phenols degradation via preferentially exposing π-conjugated planes up to 100%. Appl. Catal. B Environ. 2022, 300, 120762. [Google Scholar] [CrossRef]
- Ronconi, F.; Syrgiannis, Z.; Bonasera, A.; Prato, M.; Argazzi, R.; Caramori, S.; Cristino, V.; Bignozzi, C.A. Modification of nanocrystalline WO3 with a d Perylene bisimide: Applications to molecular level solar water splitting. J. Am. Chem. Soc. 2015, 137, 4630–4633. [Google Scholar] [CrossRef]
- Godlewski, S.; Prauzner-Bechcicki, J.S.; Glatzel, T.; Meyer, E.; Szymoński, M. Transformations of PTCDA structures on rutile TiO2 induced by thermal annealing and intermolecular forces. Beilstein J. Nanotechnol. 2015, 6, 1498–1507. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Huang, S.; Xu, J.; Liu, L.; Li, J.; Jing, J.; Zhu, Y. Electron-enriched supramolecular PDI-SiO2 promoting PDS activation for enhanced photocatalytic advanced oxidation. Appl. Catal. B Environ. 2024, 340, 123262. [Google Scholar] [CrossRef]
- Liu, X.; Ding, X.; An, S.; Wang, X.; Xue, Y.; Zhang, X.; Tian, J. S-scheme enhanced photocatalysis on graphitic carbon nitride functionalized with perylene tetracarboxylic diimide. Appl. Surf. Sci. 2023, 614, 156273. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, X.; Wang, Y.; Gong, Y.; Cao, D.; Qiao, M. Peroxymonosulfate-enhanced visible light photocatalytic degradation of bisphenol A by perylene imide-modified g-C3N4. Appl. Catal. B Environ. 2018, 237, 976–985. [Google Scholar] [CrossRef]
- Wei, J.; Chen, X.; Ren, X.; Tian, S.; Bai, F. Facile construction of intramolecular g-CN-PTCDA donor-acceptor system for efficient CO2 photoreduction. Catalysts 2023, 13, 600. [Google Scholar] [CrossRef]
- Lv, J.G.; Wang, D.; Peng, L.M.; Guo, X.F.; Ding, W.P.; Yang, W.M. Ethanol dehydration to ethylene over high-energy facets exposed gamma alumina. Catalysts 2023, 13, 994. [Google Scholar] [CrossRef]
- Li, S.-Q.; Liu, Y.; Li, Y.-L.; Hao, Y.-J.; Liu, R.-H.; Chen, L.-J.; Li, F.-T. Development of γ-Al2O3 with oxygen vacancies induced by amorphous structures for photocatalytic reduction of CO2. Chem. Commun. 2022, 58, 11649–11652. [Google Scholar] [CrossRef]
- Su, K.; Zhang, C.; Wang, Y.; Zhang, J.; Guo, Q.; Gao, Z.; Wang, F. Unveiling the highly disordered NbO6 units as electron-transfer sites in Nb2O5 photocatalysis with N-hydroxyphthalimide under visible light irradiation. Chin. J. Catal. 2022, 43, 1894–1905. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, S.; Zhao, H.; Li, A.; Luo, L.; Guo, L. Subnano-FeOx clusters anchored in an ultrathin amorphous Al2O3 nanosheet for styrene epoxidation. ACS Catal. 2021, 11, 11542–11550. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Chen, D.; Lei, X.; Wang, Y.; Zhong, S.; Liu, G.; Xu, B.; Ouyang, C. Tunable electronic structures in BP/MoSSe van der Waals heterostructures by external electric field and strain. Appl. Surf. Sci. 2019, 497, 143809. [Google Scholar] [CrossRef]

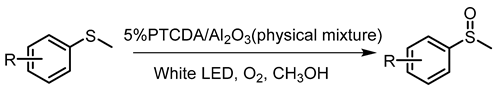
| Entry | Substrates | Products | Time (h) | Conv. (%) | Sulfoxide Yield(%) |
|---|---|---|---|---|---|
| 1 |  | 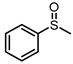 | 6 | 93 | 68 |
| 2 | 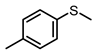 |  | 6 | 94 | 69 |
| 3 | 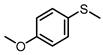 |  | 6 | 99 | 94 |
| 4 |  |  | 6 | 91 | 72 |
| 5 | 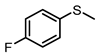 | 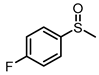 | 6 | 94 | 79 |
| 6 | 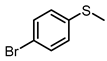 |  | 6 | 96 | 79 |
| 7 |  | 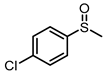 | 8 | 91 | 47 |
| 8 |  |  | 8 | 96 | 64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Wang, J.; Hou, H.; Xu, Q.; Liu, W.; Su, C.; Sun, H. Immobilization of Perylenetetracarboxylic Dianhydride on Al2O3 for Efficiently Photocatalytic Sulfide Oxidation. Molecules 2024, 29, 1934. https://doi.org/10.3390/molecules29091934
Liang J, Wang J, Hou H, Xu Q, Liu W, Su C, Sun H. Immobilization of Perylenetetracarboxylic Dianhydride on Al2O3 for Efficiently Photocatalytic Sulfide Oxidation. Molecules. 2024; 29(9):1934. https://doi.org/10.3390/molecules29091934
Chicago/Turabian StyleLiang, Jiahao, Jie Wang, Hao Hou, Qingzhu Xu, Wei Liu, Chenliang Su, and Hongli Sun. 2024. "Immobilization of Perylenetetracarboxylic Dianhydride on Al2O3 for Efficiently Photocatalytic Sulfide Oxidation" Molecules 29, no. 9: 1934. https://doi.org/10.3390/molecules29091934





