The Simultaneous Detection of Dopamine and Uric Acid In Vivo Based on a 3D Reduced Graphene Oxide–MXene Composite Electrode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Direct Electrochemical Behavior of the 3D rGO-Ti3C2 Electrode
2.3. Electrocatalytic Oxidation of DA and UA by the 3D rGO-Ti3C2 Electrode
2.4. Anti-Interference, Stability, and Repeatability of the 3D rGO-Ti3C2 Electrode
2.5. Fetal Bovine Serum
2.6. Detection of DA in Rat Brains
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of the 3D rGO-Ti3C2 Electrode
3.3. Electrochemical Measurements
3.4. Animals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Q.; Bao, J.; Huo, D.; Yang, M.; Wu, H.; Hou, C.; Zhao, Y.; Luo, X.; Fa, H. 3DGH-Fc based electrochemical sensor for the simultaneous determination of ascorbic acid, dopamine and uric acid. J. Electroanal. Chem. 2017, 799, 459–467. [Google Scholar] [CrossRef]
- Wang, L.; Chen, J.; Wang, J.; Li, H.; Chen, C.; Feng, J.; Guo, Y.; Yu, H.; Sun, X.; Peng, H. Flexible dopamine-sensing fiber based on potentiometric method for long-term detection in vivo. Sci. China Chem. 2021, 64, 1763–1769. [Google Scholar] [CrossRef]
- Setoudeh, N.; Jahani, S.; Kazemipour, M.; Foroughi, M.M.; Hassani Nadiki, H. Zeolitic imidazolate frameworks and cobalt-tannic acid nanocomposite modified carbon paste electrode for simultaneous determination of dopamine, uric acid, acetaminophen and tryptophan: Investigation of kinetic parameters of surface electrode and its analytical performance. J. Electroanal. Chem. 2020, 863, 114045. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajheidari, N. ZnO–CuxO/polypyrrole nanocomposite modified electrode for simultaneous determination of ascorbic acid, dopamine, and uric acid. Anal. Biochem. 2015, 473, 53–62. [Google Scholar] [CrossRef]
- Zhu, Q.; Bao, J.; Huo, D.; Yang, M.; Hou, C.; Guo, J.; Chen, M.; Fa, H.; Luo, X.; Ma, Y. 3D Graphene hydrogel—Gold nanoparticles nanocomposite modified glassy carbon electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2017, 238, 1316–1323. [Google Scholar] [CrossRef]
- Iranmanesh, T.; Foroughi, M.M.; Jahani, S.; Shahidi Zandi, M.; Hassani Nadiki, H. Green and facile microwave solvent-free synthesis of CeO2 nanoparticle-decorated CNTs as a quadruplet electrochemical platform for ultrasensitive and simultaneous detection of ascorbic acid, dopamine, uric acid and acetaminophen. Talanta 2020, 207, 120318. [Google Scholar] [CrossRef]
- Zhao, L.; Li, H.; Gao, S.; Li, M.; Xu, S.; Li, C.; Guo, W.; Qu, C.; Yang, B. MgO nanobelt-modified graphene-tantalum wire electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Electrochim. Acta 2015, 168, 191–198. [Google Scholar] [CrossRef]
- Moccia, M.; Pappatà, S.; Erro, R.; Picillo, M.; Vitale, C.; Amboni, M.; Longo, K.; Palladino, R.; Barone, P.; Pellecchia, M.T. Uric acid relates to dopamine transporter availability in Parkinson’s disease. Acta Neurol. Scand. 2015, 131, 127–131. [Google Scholar] [CrossRef]
- Oh, Y.S.; Kim, J.S.; Yoo, S.W.; Hwang, E.J.; Lyoo, C.H.; Lee, K.S. Gender difference in the effect of uric acid on striatal dopamine in early Parkinson’s disease. Eur. J. Neurol. 2019, 27, 258–264. [Google Scholar] [CrossRef]
- O’Neill, R.D. Uric acid levels and dopamine transmission in rat striatum: Diurnal changes and effects of drugs. Brain Res. 1990, 507, 267–272. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Huang, J.; You, T. Simultaneous determination of dopamine, ascorbic acid and uric acid at electrochemically reduced graphene oxide modified electrode. Sens. Actuators B Chem. 2014, 193, 166–172. [Google Scholar] [CrossRef]
- Maseed, H.; Reddy Yenugu, V.M.; Devarakonda, S.S.; Petnikota, S.; Gajulapalli, M.; Srikanth, V.V.S.S. Peroxidase-like Fe3O4 Nanoparticle/Few-Layered Graphene Composite for Electrochemical Detection of Dopamine, Ascorbic Acid, and Uric Acid. ACS Appl. Nano Mater. 2023, 6, 18531–18538. [Google Scholar] [CrossRef]
- Tukimin, N.; Abdullah, J.; Sulaiman, Y. Review—Electrochemical Detection of Uric Acid, Dopamine and Ascorbic Acid. J. Electrochem. Soc. 2018, 165, B258–B267. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, P.; Gao, B.; Yuan, M.; Yu, J.; Wang, Z.; Chen, X. Self-reduction of bimetallic nanoparticles on flexible MXene-graphene electrodes for simultaneous detection of ascorbic acid, dopamine, and uric acid. Microchem. J. 2023, 185, 108177. [Google Scholar] [CrossRef]
- Jiang, J.; Ding, D.; Wang, J.; Lin, X.; Diao, G. Three-dimensional nitrogen-doped graphene-based metal-free electrochemical sensors for simultaneous determination of ascorbic acid, dopamine, uric acid, and acetaminophen. Analyst 2021, 146, 964–970. [Google Scholar] [CrossRef]
- Zheng, J.; Diao, J.; Jin, Y.; Ding, A.; Wang, B.; Wu, L.; Weng, B.; Chen, J. An Inkjet Printed Ti3C2-GO Electrode for the Electrochemical Sensing of Hydrogen Peroxide. J. Electrochem. Soc. 2018, 165, B227–B231. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Samavat, S.; Deganello, D.; Teng, K.S. Vertically Aligned Graphene Prepared by Photonic Annealing for Ultrasensitive Biosensors. ACS Appl. Mater. Interfaces 2020, 12, 35328–35336. [Google Scholar] [CrossRef]
- Chen, S.; Shi, M.; Xu, Q.; Xu, J.; Duan, X.; Gao, Y.; Lu, L.; Gao, F.; Wang, X.; Yu, Y. Ti3C2Tx MXene/nitrogen-doped reduced graphene oxide composite: A high-performance electrochemical sensing platform for adrenaline detection. Nanotechnology 2021, 32, abef94. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Biswas, K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene, the new nanocarbon. J. Mater. Chem. 2009, 19, 2457–2469. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, P.; Tian, Y.; Feng, J.; Xiao, J.; Li, J.; Liu, J.; Li, G.; He, Q. Simultaneous and sensitive determination of ascorbic acid, dopamine and uric acid via an electrochemical sensor based on PVP-graphene composite. J. Nanobiotechnol. 2020, 18, 112. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, C.; Wang, Y.; Tomsia, A.P.; Li, M.; Saiz, E.; Fang, S.; Baughman, R.H.; Jiang, L.; Cheng, Q. Super-tough MXene-functionalized graphene sheets. Nat. Commun. 2020, 11, 2077. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, X.; Deng, W.; Lei, D.; Liu, Z. 3D Porous MXene (Ti3C2)/Reduced Graphene Oxide Hybrid Films for Advanced Lithium Storage. ACS Appl. Mater. Interfaces 2018, 10, 3634–3643. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; Wang, E.; Yang, T.; Wang, H.; Hou, X. Ti3C2Tx (MXene)/Pt nanoparticle electrode for the accurate detection of DA coexisting with AA and UA. Dalton Trans. 2022, 51, 4549–4559. [Google Scholar] [CrossRef]
- Wang, A.; Chen, Y.; Liu, L.; Liu, X.; Wang, Z.; Zhang, Y. Sulfur nanoparticles/Ti3C2TxMXene with an optimum sulfur content as a cathode for highly stable lithium–sulfur batteries. Dalton Trans. 2021, 50, 5574–5581. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, B.; Ding, A.; Weng, B.; Chen, J. Synthesis of MXene/DNA/Pd/Pt nanocomposite for sensitive detection of dopamine. J. Electroanal. Chem. 2018, 816, 189–194. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Xie, X.; Ren, C.E.; Makaryan, T.; Anasori, B.; Wang, G.; Gogotsi, Y. Hollow MXene Spheres and 3D Macroporous MXene Frameworks for Na-Ion Storage. Adv. Mater. 2017, 29, 1702410. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Wu, F.; Cao, X.; Li, Z.; Alharbi, M.; Abbas, A.N.; Amer, M.R.; Zhou, C. Highly Sensitive and Wearable In2O3 Nanoribbon Transistor Biosensors with Integrated On-Chip Gate for Glucose Monitoring in Body Fluids. ACS Nano 2018, 12, 1170–1178. [Google Scholar] [CrossRef]
- Schultz, T.; Frey, N.C.; Hantanasirisakul, K.; Park, S.; May, S.J.; Shenoy, V.B.; Gogotsi, Y.; Koch, N. Surface Termination Dependent Work Function and Electronic Properties of Ti3C2Tx MXene. Chem. Mater. 2019, 31, 6590–6597. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Q.; Huang, Y.; Li, F.; Zhang, W.; Wei, C.; Chen, J.; Dai, P.; Huang, L.; Huang, Z.; et al. Graphitic carbon nitride nanosheets doped graphene oxide for electrochemical simultaneous determination of ascorbic acid, dopamine and uric acid. Electrochim. Acta 2014, 142, 125–131. [Google Scholar] [CrossRef]
- Gao, W.; Alemany, L.B.; Ci, L.; Ajayan, P.M. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Wang, H.; Xie, A.; Li, S.; Wang, J.; Chen, K.; Su, Z.; Song, N.; Luo, S. Three-dimensional g-C3N4/MWNTs/GO hybrid electrode as electrochemical sensor for simultaneous determination of ascorbic acid, dopamine and uric acid. Anal. Chim. Acta 2022, 1211, 339907. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, W. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2014, 56, 300–306. [Google Scholar] [CrossRef]
- Feng, S.; Yu, L.; Yan, M.; Ye, J.; Huang, J.; Yang, X. Holey nitrogen-doped graphene aerogel for simultaneously electrochemical determination of ascorbic acid, dopamine and uric acid. Talanta 2021, 224, 121851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.-C.; Ma, L.-X. One-pot facile fabrication of graphene-zinc oxide composite and its enhanced sensitivity for simultaneous electrochemical detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2016, 227, 488–496. [Google Scholar] [CrossRef]
- Zhou, L.; Hou, H.; Wei, H.; Yao, L.; Sun, L.; Yu, P.; Su, B.; Mao, L. In Vivo Monitoring of Oxygen in Rat Brain by Carbon Fiber Microelectrode Modified with Antifouling Nanoporous Membrane. Anal. Chem. 2019, 91, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Or, S.W. Self-assembled three-dimensional macroscopic graphene/MXene-based hydrogel as electrode for supercapacitor. APL Mater. 2020, 8, 091101. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, B.; Deng, Y.; Cheng, H.; Li, X.; Yan, L.; Li, G.; Sun, W. Reduced graphene oxide/titanium carbide MXene nanocomposite-modified electrode for electrochemical hemoglobin biosensor. J. Chin. Chem. Soc. 2021, 68, 2326–2336. [Google Scholar] [CrossRef]



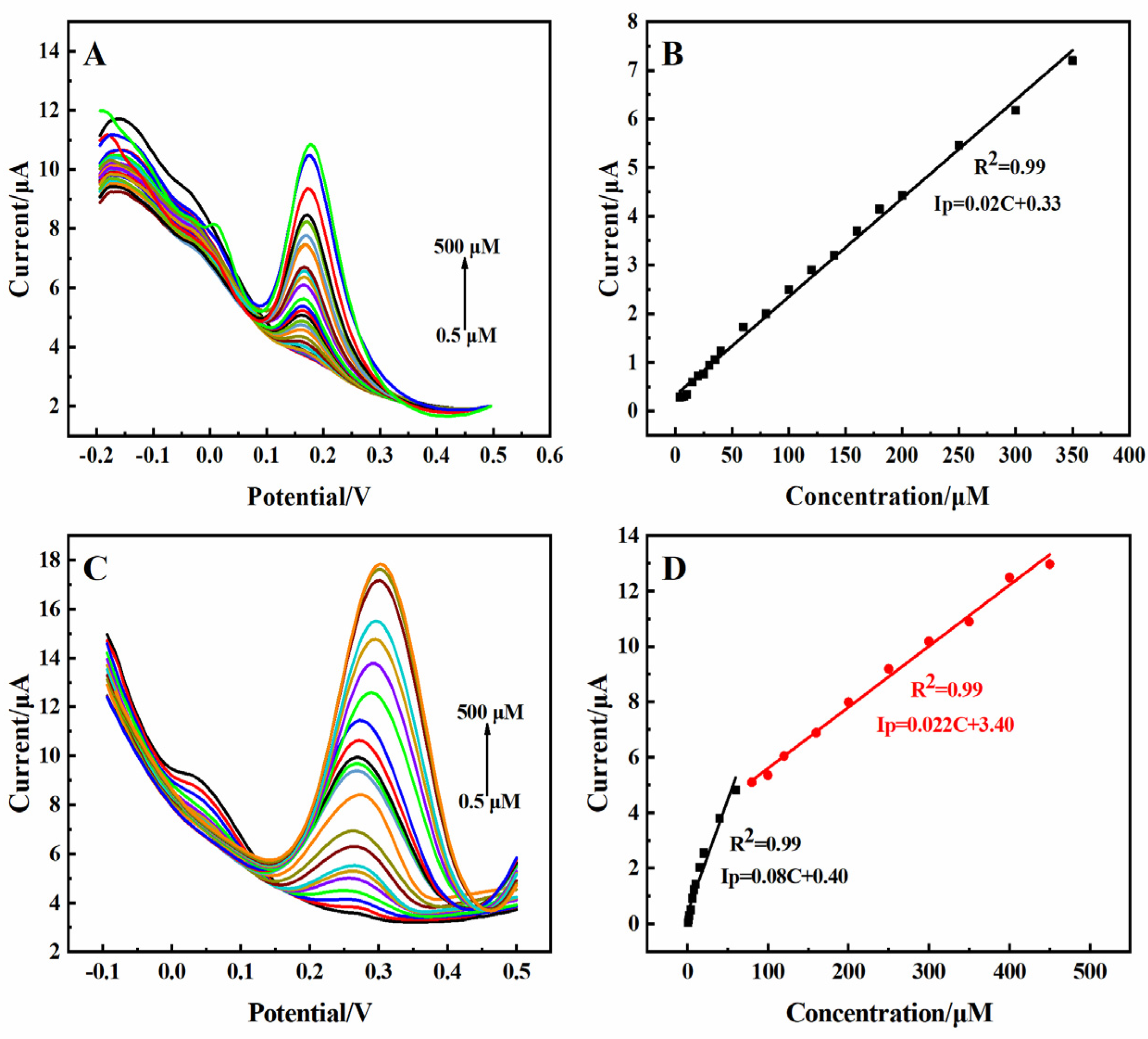
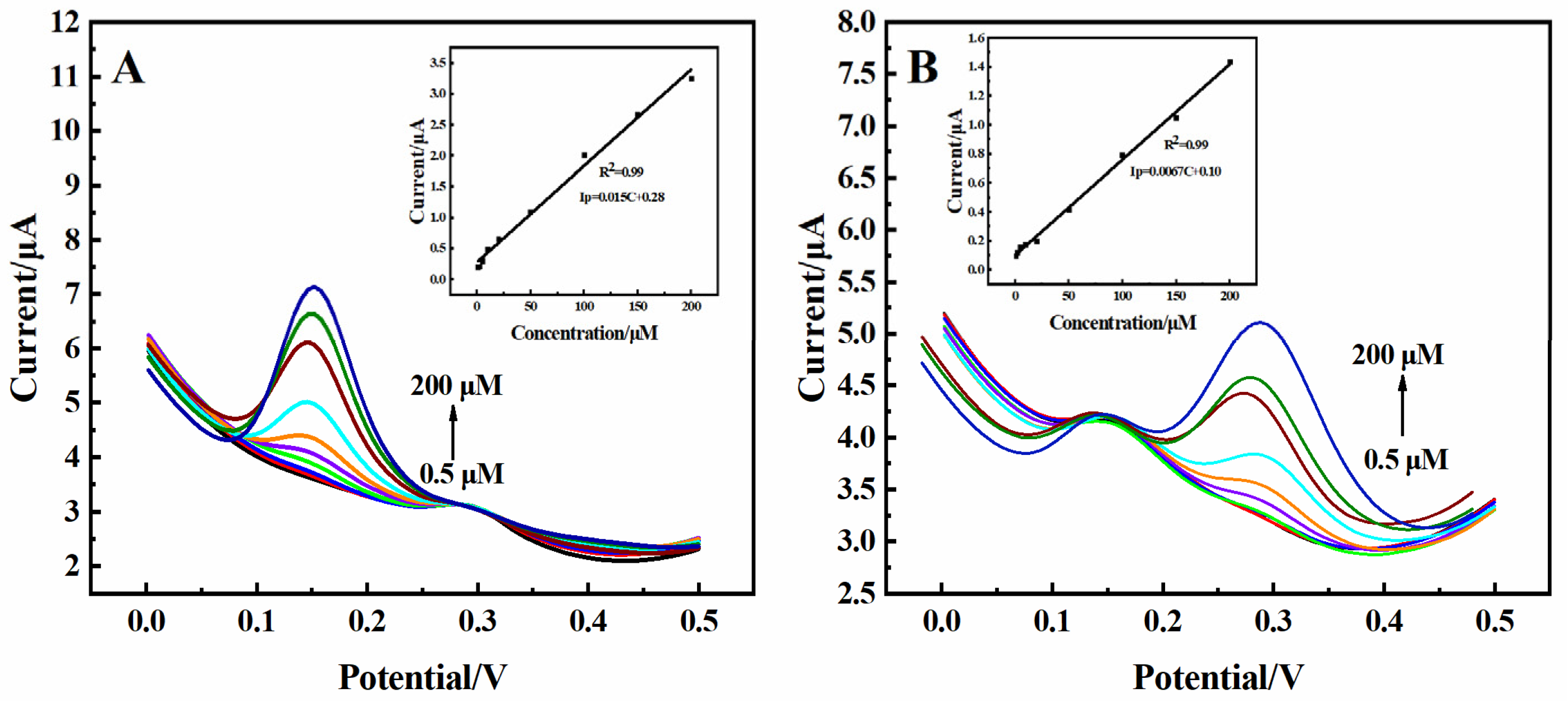


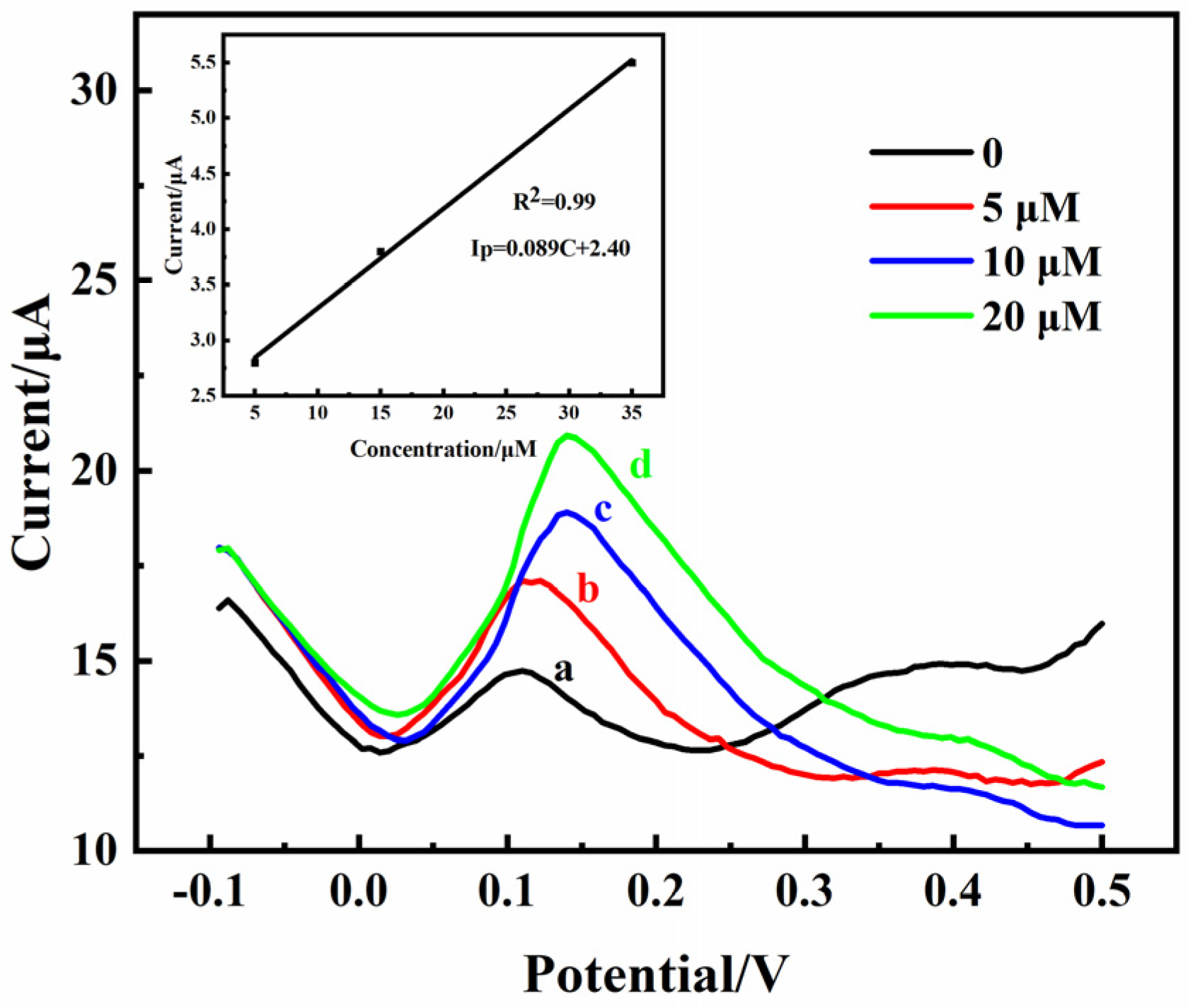
| Samples | Electrochemical Method | Detection Object | Linear Range (µM) | Detection Limit (µM) | Sensitivity (µA·µM−1·cm−2) |
|---|---|---|---|---|---|
| 0.01 M PBS | DPV | DA | 0.5–500 | 0.061 | 0.74 |
| UA | 0.5–60, 80–450 | 0.086 | 2.96, 0.81 | ||
| Amperometric | DA | 0.5–260 | 0.065 | 0.74 | |
| UA | 0.5–105 | 0.088 | 0.7 | ||
| 10% FBS | DPV | DA | 0.5–500 | 0.091 | 0.41 |
| UA | 2–500 | 0.6 | 0.11 | ||
| Amperometric | DA | 0.5–8, 10–80 | 0.087 | 0.61, 0.41 | |
| UA | 0.5–50 | 0.25 | 0.107 |
| Electrode Materials | Linear Range (µM) | LOD (µM) | References | ||
|---|---|---|---|---|---|
| DA | UA | DA | UA | ||
| 3DGH-Fc/GCE | 10–180 | 8–400 | 0.042 | 0.067 | [1] |
| MgO/Gr/Ta | 0.1–7 | 1–70 | 0.15 | 0.12 | [7] |
| Au-Pd/MXene/LSG | 12–240 | 8–100 | 0.13 | 1.47 | [14] |
| CNNS-GO | 1–20 | 10–100 | 0.096 | 0.228 | [29] |
| fg-C3N4/MWNTs/GO | 2–100 | 4–200 | 0.22 | 1.36 | [31] |
| CTAB-GO/MWNT/GCE | 5–300 | 3–60 | 1.5 | 1 | [32] |
| HNGA/GCE | 0.6–75 | 0.4–50 | 0.22 | 0.12 | [33] |
| RGO-ZnO/GCE | 1–70 | 3–330 | 0.33 | 1.08 | [34] |
| 3D rGO-Ti3C2 | 0.5–500 | 0.5–450 | 0.061 | 0.085 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, L.; Li, R.; Li, H.; Yu, S.; Sun, X.; Yu, Y.; Ren, Q. The Simultaneous Detection of Dopamine and Uric Acid In Vivo Based on a 3D Reduced Graphene Oxide–MXene Composite Electrode. Molecules 2024, 29, 1936. https://doi.org/10.3390/molecules29091936
Shang L, Li R, Li H, Yu S, Sun X, Yu Y, Ren Q. The Simultaneous Detection of Dopamine and Uric Acid In Vivo Based on a 3D Reduced Graphene Oxide–MXene Composite Electrode. Molecules. 2024; 29(9):1936. https://doi.org/10.3390/molecules29091936
Chicago/Turabian StyleShang, Lingjun, Ruijiao Li, Haojie Li, Shuaiqun Yu, Xuming Sun, Yi Yu, and Qiongqiong Ren. 2024. "The Simultaneous Detection of Dopamine and Uric Acid In Vivo Based on a 3D Reduced Graphene Oxide–MXene Composite Electrode" Molecules 29, no. 9: 1936. https://doi.org/10.3390/molecules29091936
APA StyleShang, L., Li, R., Li, H., Yu, S., Sun, X., Yu, Y., & Ren, Q. (2024). The Simultaneous Detection of Dopamine and Uric Acid In Vivo Based on a 3D Reduced Graphene Oxide–MXene Composite Electrode. Molecules, 29(9), 1936. https://doi.org/10.3390/molecules29091936








