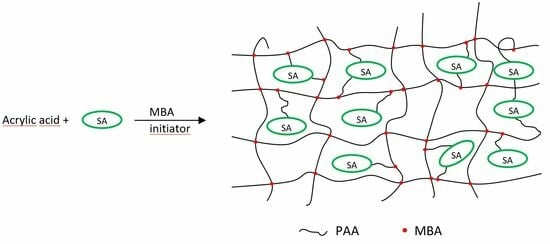
Structure Effects on Swelling Properties of Hydrogels Based on Sodium Alginate and Acrylic Polymers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Swelling Properties of PAA/SA Hydrogels
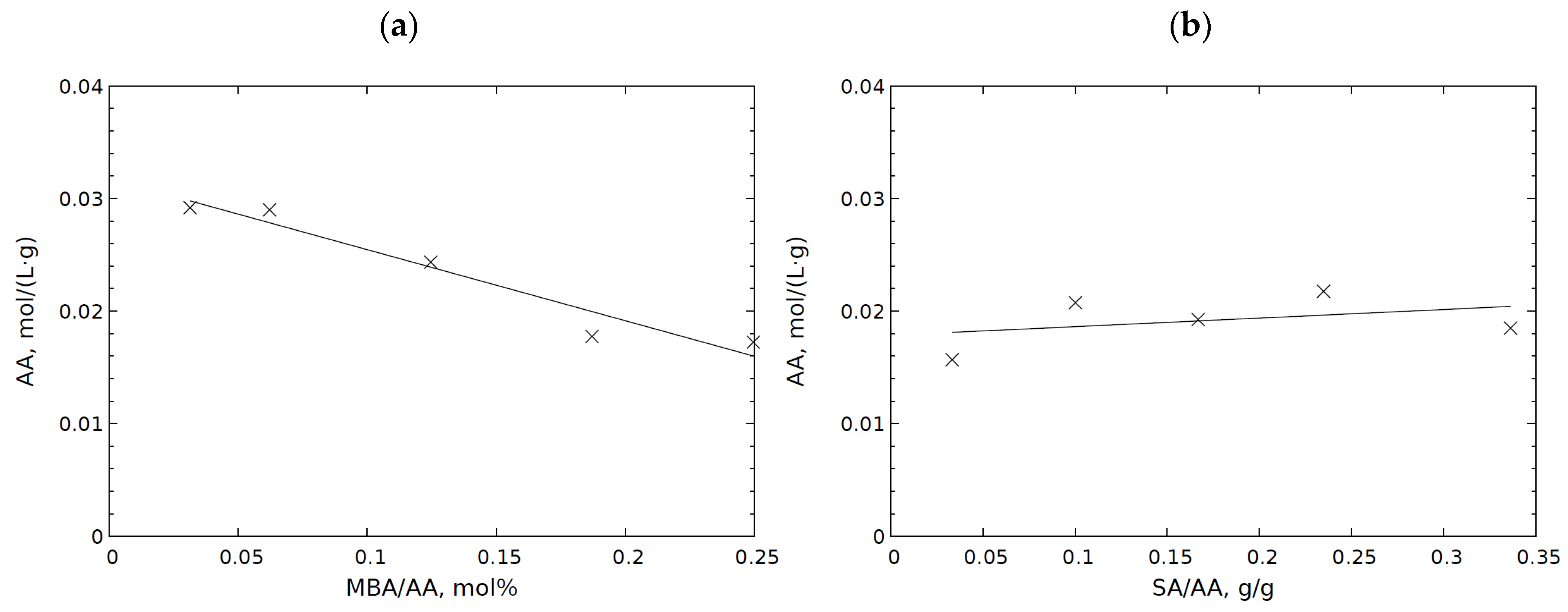
2.2. Analysis of Sol Fraction Content in SA Based Hydrogels
2.3. The FT-IR Spectra of PAA/SA Hydrogels
2.4. Molecular Weight Distribution of Hydrogel Soluble Fractions
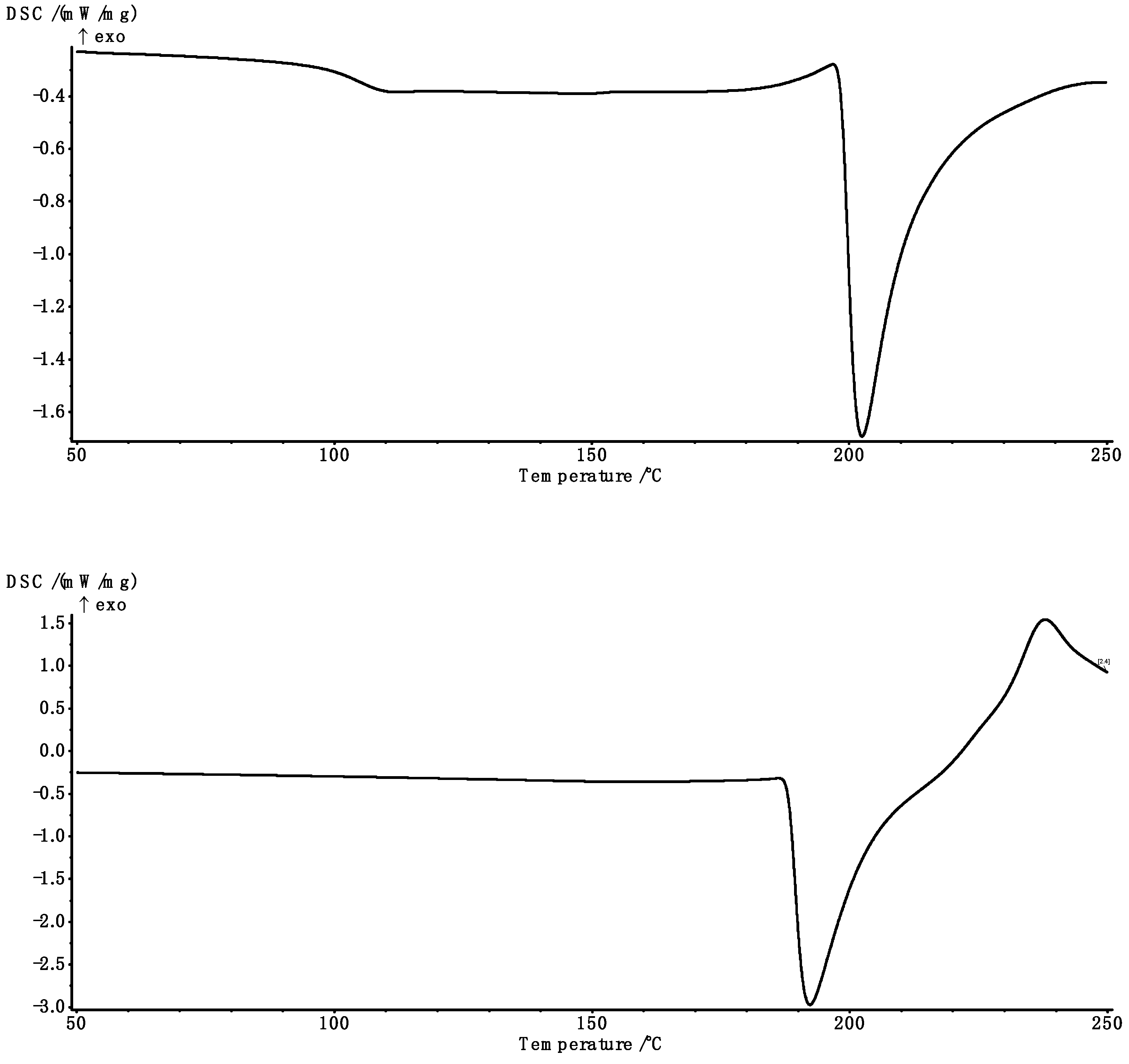
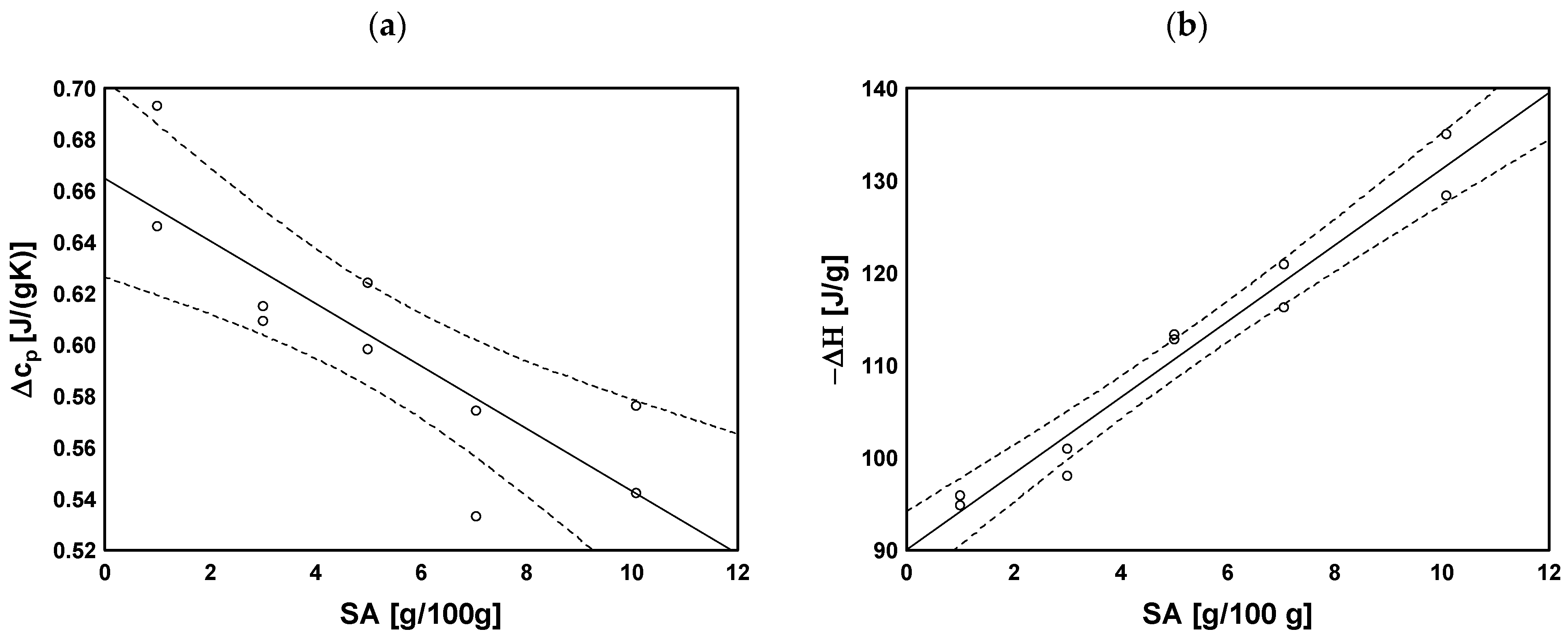
2.5. Thermal Analysis
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Alginate-Based Hydrogels
3.3. Determination of Unreacted Fraction
3.4. GPC Measurements of Soluble Hydrogel Fractions
3.5. FT-IR Spectroscopy
3.6. Swelling Properties
3.7. Thermal Characterisation
3.8. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, K.A.; Anseth, K.S. Controlled Release from Crosslinked Degradable Networks. Crit. Rev. Ther. Drug Carrier Syst. 2002, 19, 385–424. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, K.; Omidian, H.; Zohuriaan-Mehr, M.J.; Doroudiani, S. Superabsorbent Hydrogel Composites and Nanocomposites: A Review. Polym. Compos. 2011, 32, 277–289. [Google Scholar] [CrossRef]
- Murali Mohan, Y.; Keshava Murthy, P.S.; Mohana Raju, K. Synthesis, Characterization and Effect of Reaction Parameters on Swelling Properties of Acrylamide–Sodium Methacrylate Superabsorbent Copolymers. React. Funct. Polym. 2005, 63, 11–26. [Google Scholar] [CrossRef]
- Amine, K.M.; Champagne, C.P.; Salmieri, S.; Britten, M.; St-Gelais, D.; Fustier, P.; Lacroix, M. Effect of Palmitoylated Alginate Microencapsulation on Viability of Bifidobacterium Longum during Freeze-Drying. LWT—Food Sci. Technol. 2014, 56, 111–117. [Google Scholar] [CrossRef]
- Kulicke, W.-M.; Eidam, D.; Kath, F.; Kix, M.; Kull, A.H. Hydrocolloids and Rheology: Regulation of Visco-Elastic Characteristics of Waxy Rice Starch in Mixtures with Galactomannans. Starch-Starke 1996, 48, 105–114. [Google Scholar] [CrossRef]
- Mandal, B.; Ray, S.K. Synthesis of Interpenetrating Network Hydrogel from Poly(Acrylic Acid-Co-Hydroxyethyl Methacrylate) and Sodium Alginate: Modeling and Kinetics Study for Removal of Synthetic Dyes from Water. Carbohydr. Polym. 2013, 98, 257–269. [Google Scholar] [CrossRef]
- Murphy, S.V.; Skardal, A.; Atala, A. Evaluation of Hydrogels for Bio-Printing Applications. J. Biomed. Mater. Res. Part A 2013, 101A, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Tomić, S.L.; Mićić, M.M.; Dobić, S.N.; Filipović, J.M.; Suljovrujić, E.H. Smart Poly(2-Hydroxyethyl Methacrylate/Itaconic Acid) Hydrogels for Biomedical Application. Radiat. Phys. Chem. 2010, 79, 643–649. [Google Scholar] [CrossRef]
- Manaila, E.; Craciun, G.; Calina, I.C. Sodium Alginate-g-Acrylamide/Acrylic Acid Hydrogels Obtained by Electron Beam Irradiation for Soil Conditioning. Int. J. Mol. Sci. 2022, 24, 104. [Google Scholar] [CrossRef]
- Naeem, A.; Yu, C.; Hetonghui; Zang, Z.; Zhu, W.; Guan, Y. β-Cyclodextrin/Chitosan-Based (Polyvinyl Alcohol-Co-Acrylic Acid) Interpenetrating Hydrogels for Oral Drug Delivery. Int. J. Biol. Macromol. 2023, 242, 125149. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Shen, H.-X.; Zhao, W.; Li, Q.; Wang, C.-F.; Chen, S. Rapid Synthesis of Robust Antibacterial and Biodegradable Hydrogels via Frontal Polymerization. Gels 2023, 9, 920. [Google Scholar] [CrossRef] [PubMed]
- Manaila, E.; Demeter, M.; Calina, I.C.; Craciun, G. NaAlg-g-AA Hydrogels: Candidates in Sustainable Agriculture Applications. Gels 2023, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Tian, Z.; Yu, Y.; Wang, P.; Zhou, M.; Wang, Q. Enzymatic Synthesis of Sodium Alginate-g-poly (Acrylic Acid) Grafting Copolymers as a Novel Printing Thickener. Color. Technol. 2022, 138, 278–290. [Google Scholar] [CrossRef]
- Chang, A. pH-Sensitive Starch-g-Poly(Acrylic Acid)/Sodium Alginate Hydrogels for Controlled Release of Diclofenac Sodium. Iran. Polym. J. 2015, 24, 161–169. [Google Scholar] [CrossRef]
- Athawale, V.D.; Lele, V. Recent Trends in Hydrogels Based on Starchgraft-Acrylic Acid: A Review. Starch-Stärke 2001, 53, 7–13. [Google Scholar] [CrossRef]
- Kowalski, G.; Kijowska, K.; Witczak, M.; Kuterasiński, Ł.; Łukasiewicz, M. Synthesis and Effect of Structure on Swelling Properties of Hydrogels Based on High Methylated Pectin and Acrylic Polymers. Polymers 2019, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Kalendova, P.; Svoboda, L.; Hroch, J.; Honcova, P.; Drobna, H.; Slang, S. Hydrogels Based on Starch from Various Natural Sources: Synthesis and Characterization. Starch-Stärke 2021, 73, 2100051. [Google Scholar] [CrossRef]
- Martínez-Salcedo, S.L.; Torres-Rendón, J.G.; García-Enriquez, S.; Anzaldo-Hernández, J.; Silva-Guzmán, J.A.; De Muniz, G.I.B.; Lomelí-Ramírez, M.G. Physicomechanical Characterization of Poly(Acrylic Acid-Co-Acrylamide) Hydrogels Reinforced with TEMPO-Oxidized Blue Agave Cellulose Nanofibers. Fibers Polym. 2022, 23, 1161–1170. [Google Scholar] [CrossRef]
- Kowalski, G.; Ptaszek, P. The Effect of Swelling Time on Rheological Properties of Hydrogels, Consisting of High -Amylose Carboxymethyl Corn Starch and Acrylic Polymers: Starch Acrylic Hydrogels. Starch-Stärke 2016, 68, 381–388. [Google Scholar] [CrossRef]
- Toledo, P.V.O.; Limeira, D.P.C.; Siqueira, N.C.; Petri, D.F.S. Carboxymethyl Cellulose/Poly(Acrylic Acid) Interpenetrating Polymer Network Hydrogels as Multifunctional Adsorbents. Cellulose 2019, 26, 597–615. [Google Scholar] [CrossRef]
- Zhai, X.; Hu, H.; Hu, M.; Ji, S.; Lei, T.; Wang, X.; Zhu, Z.; Dong, W.; Teng, C.; Wei, W. A Nano-Composite Hyaluronic Acid-Based Hydrogel Efficiently Antibacterial and Scavenges ROS for Promoting Infected Diabetic Wound Healing. Carbohydr. Polym. 2024, 334, 122064. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Sharma, M. Synthesis and Characterization of Graft Copolymers of Sago Starch and Acrylic Acid. Starch-Stärke 2012, 64, 441–451. [Google Scholar] [CrossRef]
- Parvathy, P.C.; Jyothi, A.N. Water Sorption Kinetics of Superabsorbent Hydrogels of Saponified Cassava Starch-Graft-Poly(Acrylamide). Starch-Stärke 2012, 64, 803–812. [Google Scholar] [CrossRef]
- Loureiro Dos Santos, L.A. Natural Polymeric Biomaterials: Processing and Properties. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017; p. 9780128035818022530. ISBN 978-0-12-803581-8. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.T.; Jiang, Z. Alginate Oligosaccharides: Production, Biological Activities, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef]
- Jeon, Y.S.; Lei, J.; Kim, J.-H. Dye Adsorption Characteristics of Alginate/Polyaspartate Hydrogels. J. Ind. Eng. Chem. 2008, 14, 726–731. [Google Scholar] [CrossRef]
- Mancini, M.; Moresi, M.; Rancini, R. Mechanical Properties of Alginate Gels: Empirical Characterisation. J. Food Eng. 1999, 39, 369–378. [Google Scholar] [CrossRef]
- Reakasame, S.; Boccaccini, A.R. Oxidized Alginate-Based Hydrogels for Tissue Engineering Applications: A Review. Biomacromolecules 2018, 19, 3–21. [Google Scholar] [CrossRef]
- Tan, J.; Luo, Y.; Guo, Y.; Zhou, Y.; Liao, X.; Li, D.; Lai, X.; Liu, Y. Development of Alginate-Based Hydrogels: Crosslinking Strategies and Biomedical Applications. Int. J. Biol. Macromol. 2023, 239, 124275. [Google Scholar] [CrossRef]
- Yang, Q.; Gao, C.; Zhang, X.; Tsou, C.; Zhao, X.; De Guzman, M.R.; Pu, Z.; Li, X.; Lu, Y.; Zeng, C.; et al. A Dual Physical Cross-Linking Strategy to Construct Tough Hydrogels with High Strength, Excellent Fatigue Resistance, and Stretching-Induced Strengthening Effect. Macromol. Mater. Eng. 2021, 306, 2100093. [Google Scholar] [CrossRef]
- Mohamadinia, P.; Anarjan, N.; Jafarizadeh-Malmiri, H. Preparation and Characterization of Sodium Alginate/Acrylic Acid Composite Hydrogels Conjugated to Silver Nanoparticles as an Antibiotic Delivery System. Green Process. Synth. 2021, 10, 860–873. [Google Scholar] [CrossRef]
- Yahşi, A.; Şahin, F.; Demirel, G.; Tümtürk, H. Binary Immobilization of Tyrosinase by Using Alginate Gel Beads and Poly(Acrylamide-Co-Acrylic Acid) Hydrogels. Int. J. Biol. Macromol. 2005, 36, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Fei, L.; Tang, C.; Yin, C. Synthesis, Characterization, Mechanical Properties and Biocompatibility of Interpenetrating Polymer Network–Super-porous Hydrogel Containing Sodium Alginate. Polym. Int. 2007, 56, 1563–1571. [Google Scholar] [CrossRef]
- Tally, M.; Atassi, Y. Synthesis and Characterization of pH-Sensitive Superabsorbent Hydrogels Based on Sodium Alginate-g-Poly(Acrylic Acid-Co-Acrylamide) Obtained via an Anionic Surfactant Micelle Templating under Microwave Irradiation. Polym. Bull. 2016, 73, 3183–3208. [Google Scholar] [CrossRef]
- Manaila, E.; Craciun, G. Biodegradable Hydrogels Based on Acrylamide, Acrylic Acid and Sodium Alginate Synthesized by Electron Beam Irradiation. Acta Phys. Pol. A 2019, 135, 1063–1064. [Google Scholar] [CrossRef]
- Khalid, I.; Ahmad, M.; Usman Minhas, M.; Barkat, K.; Sohail, M. Cross-Linked Sodium Alginate-g-poly(Acrylic Acid) Structure: A Potential Hydrogel Network for Controlled Delivery of Loxoprofen Sodium. Adv. Polym. Technol. 2018, 37, 985–995. [Google Scholar] [CrossRef]
- Yin, Y.; Ji, X.; Dong, H.; Ying, Y.; Zheng, H. Study of the Swelling Dynamics with Overshooting Effect of Hydrogels Based on Sodium Alginate-g-Acrylic Acid. Carbohydr. Polym. 2008, 71, 682–689. [Google Scholar] [CrossRef]
- Thakur, S.; Arotiba, O.A. Synthesis, Swelling and Adsorption Studies of a pH-Responsive Sodium Alginate–Poly(Acrylic Acid) Superabsorbent Hydrogel. Polym. Bull. 2018, 75, 4587–4606. [Google Scholar] [CrossRef]
- Lin, H.; Zhou, J.; Yingde, C.; Gunasekaran, S. Synthesis and Characterization of pH- and Salt-responsive Hydrogels Based on Etherificated Sodium Alginate. J. Appl. Polym. Sci. 2010, 115, 3161–3167. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, P.; Zhao, L.; Chen, Y. Preparation and Swelling Properties of a Starch-g-Poly(Acrylic Acid)/Organo-Mordenite Hydrogel Composite. Front. Chem. Sci. Eng. 2016, 10, 147–161. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical Mechanics of Cross-Linked Polymer Networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Peppas, N.A. (Ed.) Hydrogels in Medicine and Pharmacy; CRC Press: Boca Raton, FL, USA, 1986; ISBN 978-0-8493-5546-2. [Google Scholar]
- Pourjavadi, A.; Barzegar, S.; Zeidabadi, F. Synthesis and Properties of Biodegradable Hydrogels of κ-Carrageenan Grafted Acrylic Acid-Co-2-Acrylamido-2-Methylpropanesulfonic Acid as Candidates for Drug Delivery Systems. React. Funct. Polym. 2007, 67, 644–654. [Google Scholar] [CrossRef]
- Scranton, A.B.; Klier, J.; Peppas, N.A. Soluble Chain Fractions in Hydrophilic Polymer Networks: Origin and Effect on Dynamic Uptake Overshoots. Polymer 1990, 31, 1288–1293. [Google Scholar] [CrossRef]
- Smith, M.J.; Peppas, N.A. Effect of the Degree of Crosslinking on Penetrant Transport in Polystyrene. Polymer 1985, 26, 569–574. [Google Scholar] [CrossRef]
- Urdahl, K.G.; Peppas, N.A. Anomalous Penetrant Transport in Glassy Polymers V. Cyclohexane Transport in Polystyrene. J. Appl. Polym. Sci. 1987, 33, 2669–2687. [Google Scholar] [CrossRef]
- Valencia, J.; Piérola, I.F. Swelling Kinetics of Poly( N.-vinylimidazole-Co.-sodium Styrenesulfonate) Hydrogels. J. Appl. Polymer Sci. 2002, 83, 191–200. [Google Scholar] [CrossRef]
- Díez-Peña, E.; Quijada-Garrido, I.; Barrales-Rienda, J.M. On the Water Swelling Behaviour of Poly(N-Isopropylacrylamide) [P(N-iPAAm)], Poly(Methacrylic Acid) [P(MAA)], Their Random Copolymers and Sequential Interpenetrating Polymer Networks (IPNs). Polymer 2002, 43, 4341–4348. [Google Scholar] [CrossRef]
- Brzeźnicki, S.; Bonczarowska, M.; Gromiec, J. Oznaczanie kwasu akrylowego w powietrzu środowiska pracy metodą wysokosprawnej chromatografii cieczowej. Podstawy I Metod. Oceny Sr. Pr. 2013, 75, 139–151. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Ali, G.; Delattre, C.; Laroche, C.; Petit, E.; Wadouachi, A.; Michaud, P. Extraction and Characterization of an Alginate from the Brown Seaweed Sargassum Turbinarioides Grunow. J. Appl. Phycol. 2010, 22, 131–137. [Google Scholar] [CrossRef]
- Helmiyati; Aprilliza, M. Characterization and Properties of Sodium Alginate from Brown Algae Used as an Ecofriendly Superabsorbent. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012019. [Google Scholar] [CrossRef]
- Wasikiewicz, J.M.; Yoshii, F.; Nagasawa, N.; Wach, R.A.; Mitomo, H. Degradation of Chitosan and Sodium Alginate by Gamma Radiation, Sonochemical and Ultraviolet Methods. Radiat. Phys. Chem. 2005, 73, 287–295. [Google Scholar] [CrossRef]
- Pereira, R.; Tojeira, A.; Vaz, D.C.; Mendes, A.; Bártolo, P. Preparation and Characterization of Films Based on Alginate and Aloe Vera. Int. J. Polym. Anal. Charact. 2011, 16, 449–464. [Google Scholar] [CrossRef]
- Król, Ż.; Malik, M.; Marycz, K.; Jarmoluk, A. Characteristic of Gelatine, Carrageenan and Sodium Alginate Hydrosols Treated by Direct Electric Current. Polymers 2016, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data, 4th ed.; Springer: Berlin, Germany, 2009; ISBN 978-3-540-93809-5. [Google Scholar]
- Sinitsya, A.; Čopíková, J.; Prutyanov, V.; Skoblya, S.; Machovič, V. Amidation of Highly Methoxylated Citrus Pectin with Primary Amines. Carbohydr. Polym. 2000, 42, 359–368. [Google Scholar] [CrossRef]
- Qiao, D.; Yu, L.; Bao, X.; Zhang, B.; Jiang, F. Understanding the Microstructure and Absorption Rate of Starch-Based Superabsorbent Polymers Prepared under High Starch Concentration. Carbohydr. Polym. 2017, 175, 141–148. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, B.; Hu, X.; Jin, Z.; Xu, X.; Tian, Y. Preparation and Characterization of Carboxymethyl Starch Microgel with Different Crosslinking Densities. Carbohydr. Polym. 2015, 124, 245–253. [Google Scholar] [CrossRef]






| Sample | SA [g] | AA [g] | MBA [g] | K2S2O8 [g] | NaOH [g] |
|---|---|---|---|---|---|
| ALG-AA | 5 | 30 | 0.0149 | 0.2 | 5 |
| ALG-A | 5 | 30 | 0.0201 | 0.2 | 5 |
| ALG-B | 5 | 30 | 0.0399 | 0.2 | 5 |
| ALG-C | 5 | 30 | 0.0800 | 0.2 | 5 |
| ALG-D | 5 | 30 | 0.1201 | 0.2 | 5 |
| ALG-E | 5 | 30 | 0.1603 | 0.2 | 5 |
| ALG-D1 | 1 | 30 | 0.1200 | 0.2 | 5 |
| ALG-D3 | 3 | 30 | 0.1200 | 0.2 | 5 |
| ALG-D5 | 5 | 30 | 0.1200 | 0.2 | 5 |
| ALG-D7 | 7 | 30 | 0.1200 | 0.2 | 5 |
| ALG-D10 | 10 | 30 | 0.1200 | 0.2 | 5 |
| Sample | Mn × 10−5 [g∙mol−1] | Mw × 10−5 [g∙mol−1] | pd |
|---|---|---|---|
| SA | 1.10 | 6.49 | 5.9 |
| ALG-AA | 1.00 | 10.20 | 10.2 |
| ALG-A | 0.98 | 9.02 | 9.2 |
| ALG-B | 0.77 | 4.86 | 6.3 |
| ALG-C | 0.51 | 3.29 | 6.5 |
| ALG-D | 0.53 | 3.30 | 6.2 |
| ALG-E | 0.41 | 2.14 | 5.2 |
| ALG-D1 | 0.55 | 2.64 | 4.8 |
| ALG-D3 | 0.89 | 3.91 | 4.4 |
| ALG-D5 | 0.85 | 3.83 | 4.5 |
| ALG-D7 | 0.94 | 4.49 | 4.8 |
| ALG-D10 | 0.97 | 3.49 | 5.2 |
| Sample | ALG [g] | MBA [g] | Ton [°C] | Tmid [°C] | Tinf [°C] | Tend [°C] | Tend–Ton [°C] | Δcp [J·g−1·K−1] |
|---|---|---|---|---|---|---|---|---|
| SA | - | - | nd | nd | nd | nd | nd | nd |
| ALG-AA | 5 | 0.016 | 103.5 ± 0.35 h | 109.7 ± 0.28 i | 111.9 ± 0.14 f | 115.6 ± 0.07 g | 12.1 ± 0.28 ab | 0.593 ± 0.0240 abc |
| ALG-A | 5 | 0.020 | 101.3 ± 0.07 g | 107.5 ± 0.07 h | 109.6 ± 0.14 e | 113.1 ± 0.14 f | 11.9 ± 0.21 ab | 0.592 ± 0.0156 abc |
| ALG-B | 5 | 0.040 | 100.4 ± 0.07 f | 106.7 ± 0.07 g | 109.3 ± 0.00 e | 112.6 ± 0.14 ef | 12.3 ± 0.21 bc | 0.633 ± 0.0042 cd |
| ALG-C | 5 | 0.080 | 100.0 ± 0.07 ef | 106.8 ± 0.07 g | 109.5 ± 0.21 e | 113.3 ± 0.07 f | 13.3 ± 0.14 cd | 0.628 ± 0.0417 cd |
| ALG-D | 5 | 0.120 | 99.6 ± 0.21 e | 106.0 ± 0.35 f | 108.1 ± 0.07 d | 111.9 ± 0.21 de | 12.3 ± 0.00 bc | 0.611 ± 0.0184 abc |
| ALG-E | 5 | 0.160 | 97.5 ± 0.28 c | 104.9 ± 0.28 e | 107.8 ± 0.35 d | 111.6 ± 0.42 d | 14.1 ± 0.14 d | 0.592 ± 0.0106 abc |
| ALG-D1 | 1 | 0.120 | 97.2 ± 0.21 bc | 102.9 ± 0.07 b | 105.3 ± 0.07 b | 108.3 ± 0.14 b | 11.2 ± 0.35 a | 0.670 ± 0.0332 d |
| ALG-D3 | 3 | 0.120 | 98.0 ± 0.14 d | 104.1 ± 0.07 d | 106.7 ± 0.14 c | 109.9 ± 0.21 c | 11.9 ± 0.35 ab | 0.612 ± 0.0042 bc |
| ALG-D5 | 5 | 0.120 | 99.6 ± 0.21 e | 106.0 ± 0.35 f | 108.1 ± 0.07 d | 111.9 ± 0.21 de | 12.3 ± 0.00 bc | 0.611 ± 0.0184 abc |
| ALG-D7 | 7 | 0.120 | 97.0 ± 0.28 b | 103.5 ± 0.49 c | 104.6 ± 1.34 b | 109.3 ± 0.92 c | 12.3 ± 1.20 bc | 0.554 ± 0.0290 a |
| ALG-D10 | 10 | 0.120 | 93.4 ± 0.21 a | 99.7 ± 0.35 a | 102.5 ± 0.21 a | 105.8 ± 0.35 a | 12.4 ± 0.14 bc | 0.559 ± 0.0240 ab |
| One-way ANOVA—p | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 | 0.016 | ||
| Sample | ALG [g] | MBA [g] | Ton [°C] | Tp [°C] | Tend [°C] | Tend—Ton [°C] | ΔH [J·g−1] |
|---|---|---|---|---|---|---|---|
| SA | - | 0 | 187.8 ± 0.49 a | 192.1 ± 0.35 a | 203.0 ± 0.21 a | 15.2 ± 0.71 | 179.4 ± 8.06 e |
| ALG-AA | 5 | 0.016 | 202.0 ± 0.14 bcd | 205.4 ± 0.07 bcde | 213.6 ± 0.21 bc | 11.6 ± 0.35 | 108.4 ± 8.91 abc |
| ALG-A | 5 | 0.020 | 202.2 ± 2.69 cd | 205.7 ± 2.90 cde | 214.8 ± 4.17 bc | 12.6 ± 1.48 | 108.3 ± 9.40 abc |
| ALG-B | 5 | 0.040 | 205.2 ± 0.21 d | 208.8 ± 0.21 e | 217.8 ± 0.35 c | 12.6 ± 0.14 | 108.8 ± 3.75 bc |
| ALG-C | 5 | 0.080 | 203.7 ± 0.49 d | 207.1 ± 0.07 de | 215.2 ± 1.48 bc | 11.5 ± 1.98 | 113.3 ± 6.36 c |
| ALG-D | 5 | 0.120 | 200.4 ± 3.04 bcd | 204.4 ± 2.62 bcde | 215.7 ± 0.49 bc | 15.3 ± 2.55 | 113.0 ± 0.42 c |
| ALG-E | 5 | 0.160 | 201.2 ± 3.68 bcd | 204.7 ± 3.75 bcde | 213.1 ± 4.03 bc | 11.9 ± 0.35 | 113.1 ± 3.25 c |
| ALG-D1 | 1 | 0.120 | 203.9 ± 0.28 d | 207.3 ± 0.07 de | 215.9 ± 0.49 bc | 12.0 ± 0.78 | 95.3 ± 0.71 a |
| ALG-D3 | 3 | 0.120 | 196.8 ± 5.23 bc | 201.3 ± 4.31 bc | 213.1 ± 0.85 bc | 16.3 ± 4.38 | 99.4 ± 2.08 ab |
| ALG-D5 | 5 | 0.120 | 200.4 ± 3.04 bcd | 204.4 ± 2.62 bcde | 215.7 ± 0.49 bc | 15.3 ± 2.55 | 113.0 ± 0.42 c |
| ALG-D7 | 7 | 0.120 | 199.7 ± 0.21 bcd | 203.4 ± 0.42 bcd | 213.7 ± 0.71 bc | 14.1 ± 0.49 | 118.6 ± 3.32 c |
| ALG-D10 | 10 | 0.120 | 196.4 ± 0.99 b | 200.5 ± 0.35 b | 211.7 ± 1.48 b | 15.3 ± 2.47 | 131.6 ± 4.67 d |
| One-way ANOVA—p | 0.001 | <0.001 | 0.001 | 0.180 | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalski, G.; Witczak, M.; Kuterasiński, Ł. Structure Effects on Swelling Properties of Hydrogels Based on Sodium Alginate and Acrylic Polymers. Molecules 2024, 29, 1937. https://doi.org/10.3390/molecules29091937
Kowalski G, Witczak M, Kuterasiński Ł. Structure Effects on Swelling Properties of Hydrogels Based on Sodium Alginate and Acrylic Polymers. Molecules. 2024; 29(9):1937. https://doi.org/10.3390/molecules29091937
Chicago/Turabian StyleKowalski, Grzegorz, Mariusz Witczak, and Łukasz Kuterasiński. 2024. "Structure Effects on Swelling Properties of Hydrogels Based on Sodium Alginate and Acrylic Polymers" Molecules 29, no. 9: 1937. https://doi.org/10.3390/molecules29091937