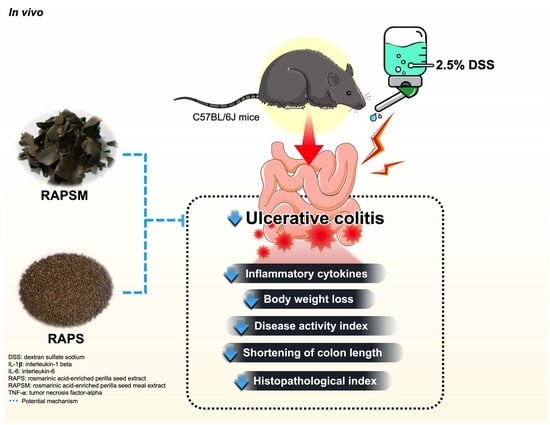
Protective Effect of Perilla Seed Meal and Perilla Seed Extract against Dextran Sulfate Sodium-Induced Ulcerative Colitis through Suppressing Inflammatory Cytokines in Mice
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Determination of PSM and PS Extracts
2.2. Effect of RAPSM and RAPS on Symptomatic Manifestations in DSS-Induced UC Mice
2.3. Effect of RAPSM and RAPS on the Shortening of Colon Length in DSS-Induced UC Mice
2.4. Effect of RAPSM and RAPS on Histopathological Damage in DSS-Induced UC Mice
2.5. Effect of RAPSM and RAPS on Proinflammatory Cytokines in DSS-Induced UC Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of PSM and PS Extracts
4.2. Identification of RA and Phytochemical Analysis
4.3. Animal Experiment and Ethical Approval
4.4. Ulcerative Colitis Severity Assessment
4.5. Histopathological Analysis
4.6. Determination of Proinflammatory Cytokines
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| UC | Ulcerative colitis |
| IBD | Inflammatory bowel disease |
| RA | Rosmarinic acid |
| RAPSM | Rosmarinic acid-enriched perilla seed meal extract |
| RAPS | Rosmarinic acid-enriched perilla seed extract |
| DSS | Dextran sulfate sodium |
| HPLC | High-pressure liquid chromatography |
| TNF-α | Tumor necrosis factor-alpha |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1 beta |
| NF-κB | Nuclear factor-kappa B |
| BW | Body weight |
| SSZ | sulfasalazine |
| PSM | Perilla seed meal |
| PS | Perilla seed |
| Hex | Hexane |
| DCM | Dichloromethane |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| DAI | Disease activity index |
| H&E | Hematoxylin and eosin |
| TLR4 | Toll-like receptor 4 |
| STAT3 | Signal transducer and activator of transcription 3 |
References
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Lynch, W.D.; Hsu, R. Ulcerative Colitis. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H. Update on the epidemiology of inflammatory bowel disease in Asia: Where are we now? Intest. Res. 2022, 20, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Niriella, M.A.; Liyanage, I.K.; Kodisinghe, S.K.; De Silva, A.P.; Jayatissa, A.; Navarathne, N.M.M.; Peiris, R.K.; Kalubovila, U.P.; Kumarasena, S.R.; Jayasekara, R.W.; et al. Changing phenotype, early clinical course and clinical predictors of inflammatory bowel disease in Sri Lanka: A retrospective, tertiary care-based, multi-centre study. BMC Gastroenterol. 2021, 21, 71. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, P.L.; Lakatos, L. Risk for colorectal cancer in ulcerative colitis: Changes, causes and management strategies. World J. Gastroenterol. 2008, 14, 3937–3947. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Siegmund, B.; Le Berre, C.; Wei, S.C.; Ferrante, M.; Shen, B.; Bernstein, C.N.; Danese, S.; Peyrin-Biroulet, L.; Hibi, T. Ulcerative colitis. Nat. Rev. Dis. Prim. 2020, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Mizoguchi, E.; Low, D.; Ezaki, Y.; Okada, T. Recent updates on the basic mechanisms and pathogenesis of inflammatory bowel diseases in experimental animal models. Intest. Res. 2020, 18, 151–167. [Google Scholar] [CrossRef]
- Wang, N.; Kong, R.; Han, W.; Bao, W.; Shi, Y.; Ye, L.; Lu, J. Honokiol alleviates ulcerative colitis by targeting PPAR-γ–TLR4–NF-κB signaling and suppressing gasdermin-D-mediated pyroptosis in vivo and in vitro. Int. Immunopharmacol. 2022, 111, 109058. [Google Scholar] [CrossRef]
- Rogler, G.; Brand, K.; Vogl, D.; Page, S.; Hofmeister, R.; Andus, T.; Knuechel, R.; Baeuerle, P.A.; Schölmerich, J.; Gross, V. Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 1998, 115, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, D.K.; Yi, W.Q.; Ouyang, Q.; Chen, Y.Q.; Gan, H.T. NF-kappaB p65 antisense oligonucleotides may serve as a novel molecular approach for the treatment of patients with ulcerative colitis. Arch. Med. Res. 2008, 39, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Lu, Q.; Li, P.; Zhu, J.; Jiang, J.; Zhao, T.; Hu, Y.; Ding, K.; Zhao, M. Xianglian Pill attenuates ulcerative colitis through TLR4/MyD88/NF-κB signaling pathway. J. Ethnopharmacol. 2023, 300, 115690. [Google Scholar] [CrossRef] [PubMed]
- Das, K.M.; Farag, S.A. Current medical therapy of inflammatory bowel disease. World J. Gastroenterol. 2000, 6, 483–489. [Google Scholar]
- Xu, C.T.; Meng, S.Y.; Pan, B.R. Drug therapy for ulcerative colitis. World J. Gastroenterol. 2004, 10, 2311–2317. [Google Scholar] [CrossRef]
- Pintha, K.; Chaiwangyen, W.; Yodkeeree, S.; Suttajit, M.; Tantipaiboonwong, P. Suppressive Effects of Rosmarinic Acid Rich Fraction from Perilla on Oxidative Stress, Inflammation and Metastasis Ability in A549 Cells Exposed to PM via C-Jun, P-65-Nf-Κb and Akt Signaling Pathways. Biomolecules 2021, 11, 1090. [Google Scholar] [CrossRef]
- Kangwan, N.; Pintha, K.; Khanaree, C.; Kongkarnka, S.; Chewonarin, T.; Suttajit, M. Anti-inflammatory effect of Perilla frutescens seed oil rich in omega-3 fatty acid on dextran sodium sulfate-induced colitis in mice. Res. Pharm. Sci. 2021, 16, 464–473. [Google Scholar] [CrossRef]
- Kangwan, N.; Kongkarnka, S.; Boonkerd, N.; Unban, K.; Shetty, K.; Khanongnuch, C. Protective Effect of Probiotics Isolated from Traditional Fermented Tea Leaves (Miang) from Northern Thailand and Role of Synbiotics in Ameliorating Experimental Ulcerative Colitis in Mice. Nutrients 2022, 14, 227. [Google Scholar] [CrossRef]
- Hou, T.; Netala, V.R.; Zhang, H.; Xing, Y.; Li, H.; Zhang, Z. Perilla frutescens: A Rich Source of Pharmacological Active Compounds. Molecules 2022, 27, 3578. [Google Scholar] [CrossRef]
- Asif, M. Health effects of omega-3,6,9 fatty acids: Perilla frutescens is a good example of plant oils. Orient. Pharm. Exp. Med. 2011, 11, 51–59. [Google Scholar] [CrossRef]
- Kangwan, N.; Pratchayasakul, W.; Kongkaew, A.; Pintha, K.; Chattipakorn, N.; Chattipakorn, S.C. Perilla Seed Oil Alleviates Gut Dysbiosis, Intestinal Inflammation and Metabolic Disturbance in Obese-Insulin-Resistant Rats. Nutrients 2021, 13, 3141. [Google Scholar] [CrossRef] [PubMed]
- Kangwan, N.; Pintha, K.; Lekawanvijit, S.; Suttajit, M. Rosmarinic Acid Enriched Fraction from Perilla frutescens Leaves Strongly Protects Indomethacin-Induced Gastric Ulcer in Rats. Biomed Res. Int. 2019, 2019, 9514703. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.J.; Lee, J.H.; Lee, M.H.; Lee, B.W.; Kwon, H.S.; Park, C.H.; Shim, K.B.; Kim, H.T.; Baek, I.Y.; Jang, D.S. Isolation and identification of phenolic compounds from the seeds of Perilla frutescens (L.) and their inhibitory activities against α-glucosidase and aldose reductase. Food Chem. 2012, 135, 1397–1403. [Google Scholar] [CrossRef]
- Urushima, H.; Nishimura, J.; Mizushima, T.; Hayashi, N.; Maeda, K.; Ito, T. Perilla frutescens extract ameliorates DSS-induced colitis by suppressing proinflammatory cytokines and inducing anti-inflammatory cytokines. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 308, G32–G41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Asada, T.; Sato, A.; Koi, Y.; Nishiwaki, H.; Tamura, H. Rosmarinic acid extract for antioxidant, antiallergic, and α-glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. Food Chem. 2014, 62, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, B.; Kim, S.; Kim, M.S.; Kim, H.; Hwang, S.R.; Kim, K.; Lee, J.H. Characterization of metabolite profiles from the leaves of green perilla (Perilla frutescens) by ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight mass spectrometry and screening for their antioxidant properties. J. Food Drug Anal. 2017, 25, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Osakabe, N.; Sanbongi, C.; Yanagisawa, R.; Inoue, K.; Yasuda, A.; Natsume, M.; Baba, S.; Ichiishi, E.; Yoshikawa, T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp. Biol. Med. 2004, 229, 247–254. [Google Scholar] [CrossRef]
- Choi, U.K.; Lee, O.H.; Lim, S.I.; Kim, Y.C. Optimization of antibacterial activity of Perilla frutescens var. acuta leaf against Pseudomonas aeruginosa using the evolutionary operation-factorial design technique. Int. J. Mol. Sci. 2010, 11, 3922–3932. [Google Scholar] [CrossRef]
- Pintha, K.; Tantipaiboonwong, P.; Yodkeeree, S.; Chaiwangyen, W.; Chumphukam, O.; Khantamat, O.; Khanaree, C.; Kangwan, N.; Thongchuai, B.; Suttajit, M. Thai perilla (Perilla frutescens) leaf extract inhibits human breast cancer invasion and migration. Maejo Int. J. Sci. Technol. 2018, 12, 112–123. [Google Scholar]
- Adam, G.; Robu, S.; Flutur, M.-M.; Cioanca, O.; Vasilache, I.-A.; Adam, A.-M.; Mircea, C.; Nechita, A.; Harabor, V.; Harabor, A.; et al. Applications of Perilla frutescens Extracts in Clinical Practice. Antioxidants 2023, 12, 727. [Google Scholar] [CrossRef]
- Simoniene, G.; Jurkstiene, V.; Jankauskiene, K.; Gailys, V.; Kevelaitis, E.; Venskutonis, P.R. The influence of common perilla (Perilla frutescens (L.) Britton) on non-specific cell-mediated immunity–phagocytosis activity. Medicina 2005, 41, 1042–1047. [Google Scholar] [PubMed]
- Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Anticancer potential of rosmarinic acid and its improved production through biotechnological interventions and functional genomics. Appl. Microbiol. Biotechnol. 2018, 102, 7775–7793. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.R.; Chung, K.S.; Cheon, S.Y.; Lee, M.; Hwang, S.; Noh Hwang, S.; Rhee, K.J.; An, H.J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 2017, 7, 46252. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.A.; Park, C.S.; Ahn, H.J.; Park, Y.S.; Kim, H.M. Effect of Perilla frutescens var. acuta Kudo and rosmarinic acid on allergic inflammatory reactions. Exp. Biol. Med. 2011, 236, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Sanbongi, C.; Takano, H.; Osakabe, N.; Sasa, N.; Natsume, M.; Yanagisawa, R.; Inoue, K.I.; Sadakane, K.; Ichinose, T.; Yoshikawa, T. Rosmarinic acid in perilla extract inhibits allergic inflammation induced by mite allergen, in a mouse model. Clin. Exp. Allergy 2004, 34, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Ku, S.K.; Lee, W.; Lee, S.; Lee, T.; Song, K.S.; Bae, J.S. Barrier protective effects of rosmarinic acid on HMGB1-induced inflammatory responses in vitro and in vivo. J. Cell. Physiol. 2013, 228, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Kim, H.-R.; Woo, E.-R.; Hong, S.-T.; Chae, H.-J.; Chae, S.-W. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochem. Pharmacol. 2005, 70, 1066–1078. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Li, W.; Tsubouchi, R.; Haneda, M.; Murakami, K.; Takeuchi, F.; Nisimoto, Y.; Yoshino, M. Rosmarinic acid inhibits the formation of reactive oxygen and nitrogen species in RAW264.7 macrophages. Free Radic. Res. 2005, 39, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Tantipaiboonwong, P.; Chaiwangyen, W.; Suttajit, M.; Kangwan, N.; Kaowinn, S.; Khanaree, C.; Punfa, W.; Pintha, K. Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-α Induced A549 Lung Adenocarcinoma Cells. Molecules 2021, 26, 6757. [Google Scholar] [CrossRef]
- Phromnoi, K.; Suttajit, M.; Saenjum, C.; Limtrakul, P. Inhibitory Effect of a Rosmarinic Acid-Enriched Fraction Prepared from Nga-Mon (Perilla frutescens) Seed Meal on Osteoclastogenesis through the RANK Signaling Pathway. Antioxidants 2021, 10, 307. [Google Scholar] [CrossRef]
- Tang, W.; Sun, B.; Zhao, Y. Preparative separation and purification of rosmarinic acid from perilla seed meal via combined column chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 947–948, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.11–15.25.14. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.-J.; Cha, Y.-S.; Kim, K.-A. Blackcurrant Alleviates Dextran Sulfate Sodium (DSS)-Induced Colitis in Mice. Foods 2023, 12, 1073. [Google Scholar] [CrossRef] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Ivanov, I.; Zlatev, Z.Z.; Alaniz, R.C.; Weeks, B.R.; Callaway, E.S.; Goldsby, J.S.; Davidson, L.A.; Fan, Y.Y.; Zhou, L.; et al. Dietary fish oil and curcumin combine to modulate colonic cytokinetics and gene expression in dextran sodium sulphate-treated mice. Br. J. Nutr. 2011, 106, 519–529. [Google Scholar] [CrossRef]
- Plosker, G.L.; Croom, K.F. Sulfasalazine. Drugs 2005, 65, 1825–1849. [Google Scholar] [CrossRef] [PubMed]
- Wahl, C.; Liptay, S.; Adler, G.; Schmid, R.M. Sulfasalazine: A potent and specific inhibitor of nuclear factor kappa B. J. Clin. Investig. 1998, 101, 1163–1174. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Ju, J. Perilla frutescens Britton var. frutescens leaves attenuate dextran sulfate sodium-induced acute colitis in mice and lipopolysaccharide-stimulated angiogenic processes in human umbilical vein endothelial cells. Food Sci. Biotechnol. 2020, 29, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M. Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt. Molecules 2018, 24, 102. [Google Scholar] [CrossRef]
- Peng, Y.; Ye, J.; Kong, J. Determination of phenolic compounds in Perilla frutescens L. by capillary electrophoresis with electrochemical detection. J. Agric. Food Chem. 2005, 53, 8141–8147. [Google Scholar] [CrossRef]
- Kang, N.S.; Lee, J.H. Characterisation of phenolic phytochemicals and quality changes related to the harvest times from the leaves of Korean purple perilla (Perilla frutescens). Food Chem. 2011, 124, 556–562. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Ueda, H.; Yamazaki, M. Inhibition of Tumor Necrosis Factor-α Production by Orally Administering a Perilla Leaf Extract. Biosci. Biotechnol. Biochem. 1997, 61, 1292–1295. [Google Scholar] [CrossRef]
- Ueda, H.; Yamazaki, M. Anti-inflammatory and anti-allergic actions by oral administration of a perilla leaf extract in mice. Biosci. Biotechnol. Biochem. 2001, 65, 1673–1675. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.R.; Chung, K.S.; Hwang, S.; Hwang, S.N.; Rhee, K.J.; Lee, M.; An, H.J. Rosmarinic acid represses colitis-associated colon cancer: A pivotal involvement of the TLR4-mediated NF-κB-STAT3 axis. Neoplasia 2021, 23, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Park, D.D.; Yum, H.W.; Zhong, X.; Kim, S.H.; Kim, S.H.; Kim, D.H.; Kim, S.J.; Na, H.K.; Sato, A.; Miura, T.; et al. Perilla frutescens Extracts Protects against Dextran Sulfate Sodium-Induced Murine Colitis: NF-κB, STAT3, and Nrf2 as Putative Targets. Front. Pharmacol. 2017, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Khanaree, C.; Punfa, W.; Tantipaiboonwong, P.; Suttajit, M.; Chewonarin, T.; Pangjit, K.; Pintha, K. The attenuation of TNF-α-mediated inflammatory responses in human lung adenocarcinoma cell line by perilla seed and seed meal extract. Chiang Mai Univ. J. Nat. Sci. 2021, 20, e2021074. [Google Scholar] [CrossRef]
- Zhou, X.J.; Yan, L.L.; Yin, P.P.; Shi, L.L.; Zhang, J.H.; Liu, Y.J.; Ma, C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014, 164, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Jin, X.; Ji, T.; Li, R.; Zhuge, X.; Xu, F.; Quan, Z.; Tong, H.; Yu, W. Luteolin ameliorates DSS-induced colitis in mice via suppressing macrophage activation and chemotaxis. Int. Immunopharmacol. 2023, 124, 110996. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Wang, L.; Meng, Y.; Xue, W.; Liang, J.; Peng, Z.; Meng, J.; Zhang, M. Apigenin remodels the gut microbiota to ameliorate ulcerative colitis. Front. Nutr. 2022, 9, 1062961. [Google Scholar] [CrossRef]
- Kongkeaw, S.; Riebroy, S.; Chaijan, M. Comparative studies on chemical composition, phenolic compounds and antioxidant activities of brown and white perilla (Perilla frutescens) seeds. Chiang Mai J. Sci. 2015, 42, 896–906. [Google Scholar]
- Radácsi, P.; Sárosi, S.; Szomor, L.; Németh-Zámbori, É. Comparison of the production and chemical constituents of five Perilla frutescens (L.) Britt. accessions. Acta Biol. Hung. 2017, 68, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Siriamornpun, S.; Li, D.; Yang, L.; Suttajit, S.; Suttajit, M. Variation of lipid and fatty acid compositions in Thai Perilla seeds grown at different locations. Songklanakarin J. Sci. Technol. 2006, 28, 17–21. [Google Scholar]
- Feuerstein, J.D.; Moss, A.C.; Farraye, F.A. Ulcerative Colitis. Mayo Clin. Proc. 2019, 94, 1357–1373. [Google Scholar] [CrossRef] [PubMed]
- Kangwan, N.; Kongkarnka, S.; Pintha, K.; Saenjum, C.; Suttajit, M. Protective Effect of Red Rice Extract Rich in Proanthocyanidins in a Murine Colitis Model. Biomedicines 2023, 11, 265. [Google Scholar] [CrossRef]
- Murthy, S.N.; Cooper, H.S.; Shim, H.; Shah, R.S.; Ibrahim, S.A.; Sedergran, D.J. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig. Dis. Sci. 1993, 38, 1722–1734. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, H.S.; Kim, E.J.; Won, N.H.; Chi, Y.M.; Kim, B.C.; Lee, K.W. Protective effect of aqueous extract of Perilla frutescens on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in rats. Food Chem. Toxicol. 2007, 45, 1738–1744. [Google Scholar] [CrossRef]
- ten Hove, T.; van den Blink, B.; Pronk, I.; Drillenburg, P.; Peppelenbosch, M.P.; van Deventer, S.J. Dichotomal role of inhibition of p38 MAPK with SB 203580 in experimental colitis. Gut 2002, 50, 507–512. [Google Scholar] [CrossRef]






| Fractions | RAPSM | RAPS |
|---|---|---|
| Hex | 5.81 ± 0.09 | 1.41 ± 0.01 |
| DCM | 4.73 ± 0.05 | 6.31 ± 0.02 |
| Water | 47.41 ± 2.08 | 23.02 ± 0.82 |
| Parameters | Major Findings (vs. N Group) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | DSS | DSS + SSZ | DSS + RAPSM | DSS + RAPS | |||||
| 50 mg/kg | 250 mg/kg | 500 mg/kg | 50 mg/kg | 250 mg/kg | 500 mg/kg | ||||
| Body weight | ↓ | ↓ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |
| DAI score | ↑↑ | ↑ | ↑ | ↑ | ↑ | ↑ * | ↑ * | ↑ * | |
| Colon length | ↓↓ | ↓ | ↓ | ↓ | ↓ | ↓ * | ↓ * | ↓ * | |
| Histopathological index | ↑↑ | ↑ | ↑ | ↑ | ↑ | ↑ * | ↑ * | ↑ * | |
| TNF-α levels | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |
| IL-6 levels | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |
| IL-1β levels | ↑↑ | ↔ | ↔ | ↑ | ↑ | ↔ | ↔ | ↔ | |
| Score | Weight Loss (%) | Stool Consistency | Gross Bleeding |
|---|---|---|---|
| 0 | None | Normal stool, well form pellets | No rectal bleeding |
| 1 | 1.0–5.0 | - | - |
| 2 | 5.1–10.0 | Loose stools, pasty stools that do not stick to the anus | Hemoccult positive |
| 3 | 10.1–15.0 | - | - |
| 4 | >15.0 | Diarrhea, liquid stools that stick to the anus | Visible gross bleeding |
| Lesion Criteria | Score | Descriptive Remarks |
|---|---|---|
| 1. Severity of ulceration/erosion | 0 | Epithelium intact |
| 1 | Involvement of laminar propria | |
| 2 | Involvement of the mucosa | |
| 3 | Into colon wall | |
| 2. Area affected by intestinal inflammation | 0 | None |
| 1 | <10% | |
| 2 | 10% | |
| 3 | 10–50% | |
| 4 | >50% | |
| 3. Extension of follicle aggregation | 0 | None |
| 1 | Mild | |
| 2 | Moderate | |
| 3 | Severe | |
| 4. Edema | 0 | None |
| 1 | Mild | |
| 2 | Moderate | |
| 3 | Severe | |
| 5. Loss of crypt | 0 | None |
| 1 | <10% | |
| 2 | 10% | |
| 3 | 10–50% | |
| 4 | >50% | |
| 6. Infiltration of inflammatory cells | 0 | None |
| 1 | Mild | |
| 2 | Moderate | |
| 3 | Severe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumneang, N.; Pintha, K.; Kongkarnka, S.; Suttajit, M.; Kangwan, N. Protective Effect of Perilla Seed Meal and Perilla Seed Extract against Dextran Sulfate Sodium-Induced Ulcerative Colitis through Suppressing Inflammatory Cytokines in Mice. Molecules 2024, 29, 1940. https://doi.org/10.3390/molecules29091940
Sumneang N, Pintha K, Kongkarnka S, Suttajit M, Kangwan N. Protective Effect of Perilla Seed Meal and Perilla Seed Extract against Dextran Sulfate Sodium-Induced Ulcerative Colitis through Suppressing Inflammatory Cytokines in Mice. Molecules. 2024; 29(9):1940. https://doi.org/10.3390/molecules29091940
Chicago/Turabian StyleSumneang, Natticha, Komsak Pintha, Sarawut Kongkarnka, Maitree Suttajit, and Napapan Kangwan. 2024. "Protective Effect of Perilla Seed Meal and Perilla Seed Extract against Dextran Sulfate Sodium-Induced Ulcerative Colitis through Suppressing Inflammatory Cytokines in Mice" Molecules 29, no. 9: 1940. https://doi.org/10.3390/molecules29091940