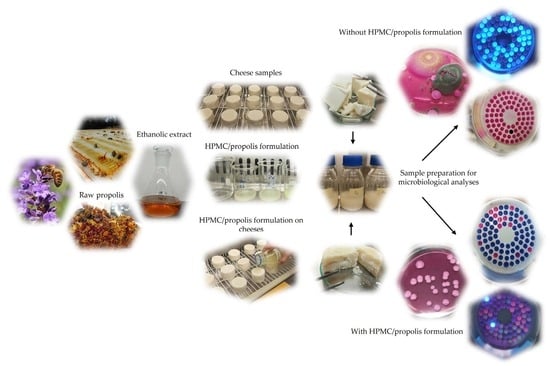
Microbiological and Physicochemical Evaluation of Hydroxypropyl Methylcellulose (HPMC) and Propolis Film Coatings for Cheese Preservation
Abstract
:1. Introduction
2. Results
2.1. Microbiological Analysis
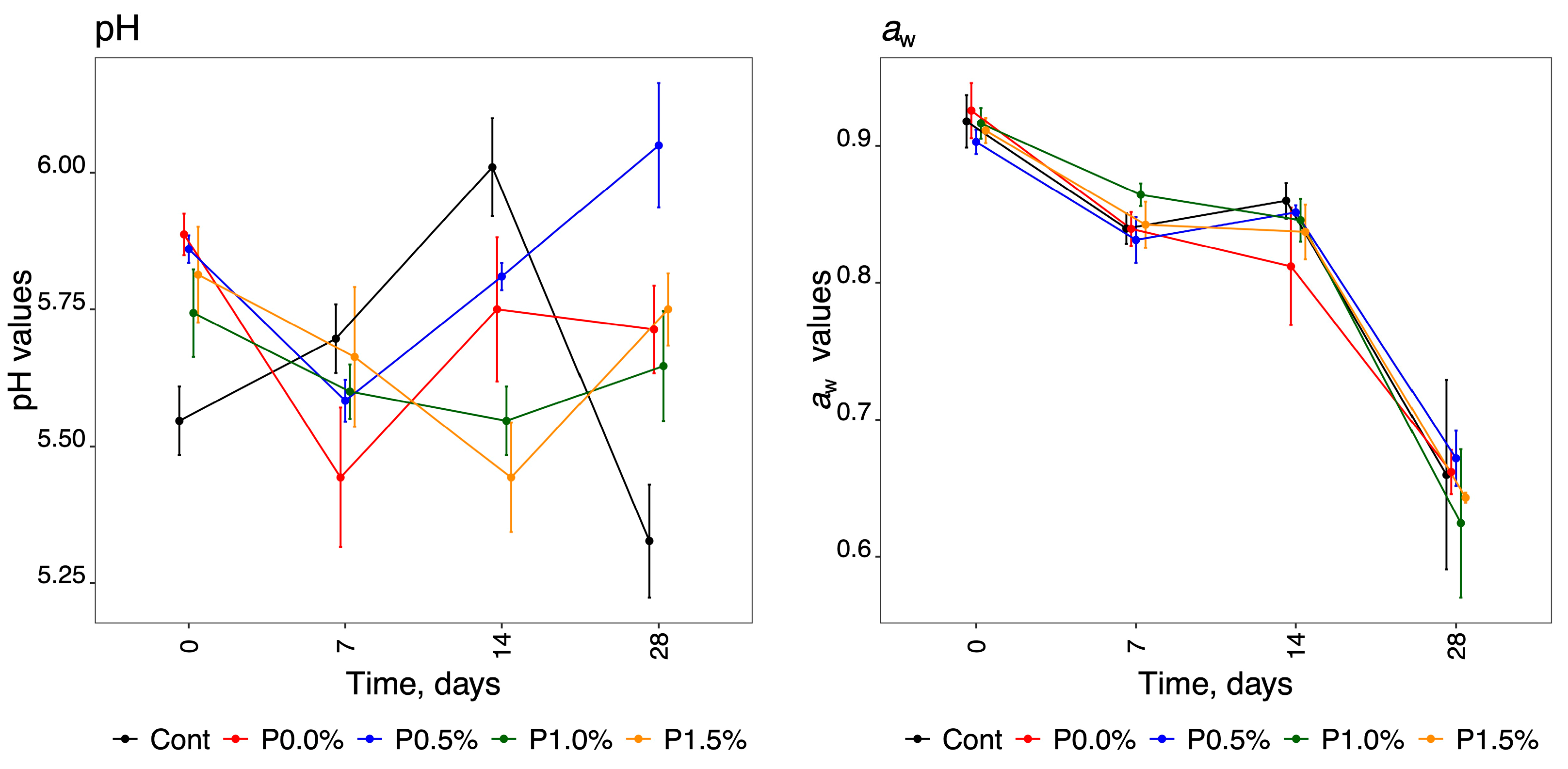
2.2. Determination of pH, Water Activity, and Weight Loss
2.3. Colour Determination
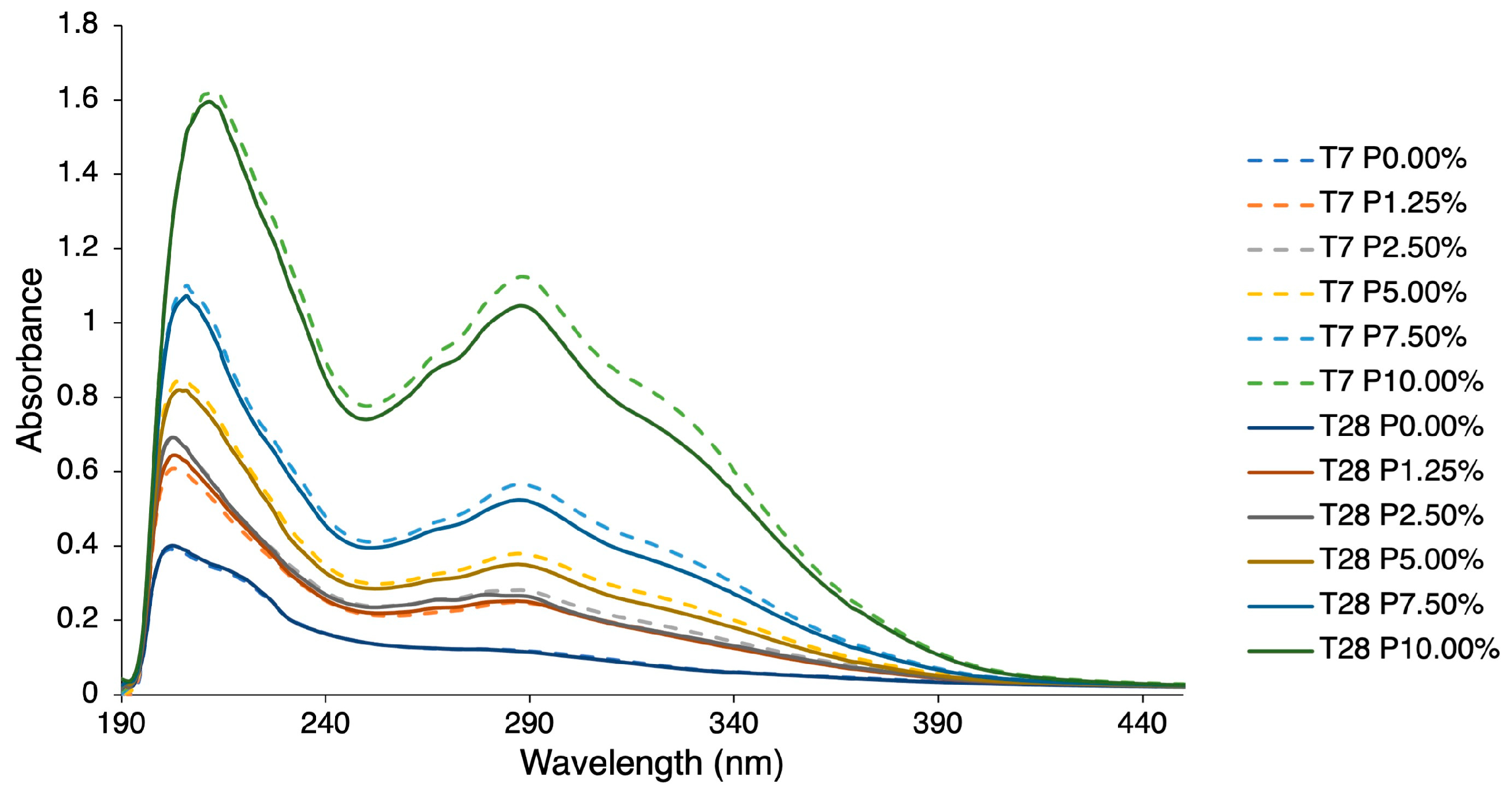
2.4. Quantification of Phenolic Compounds
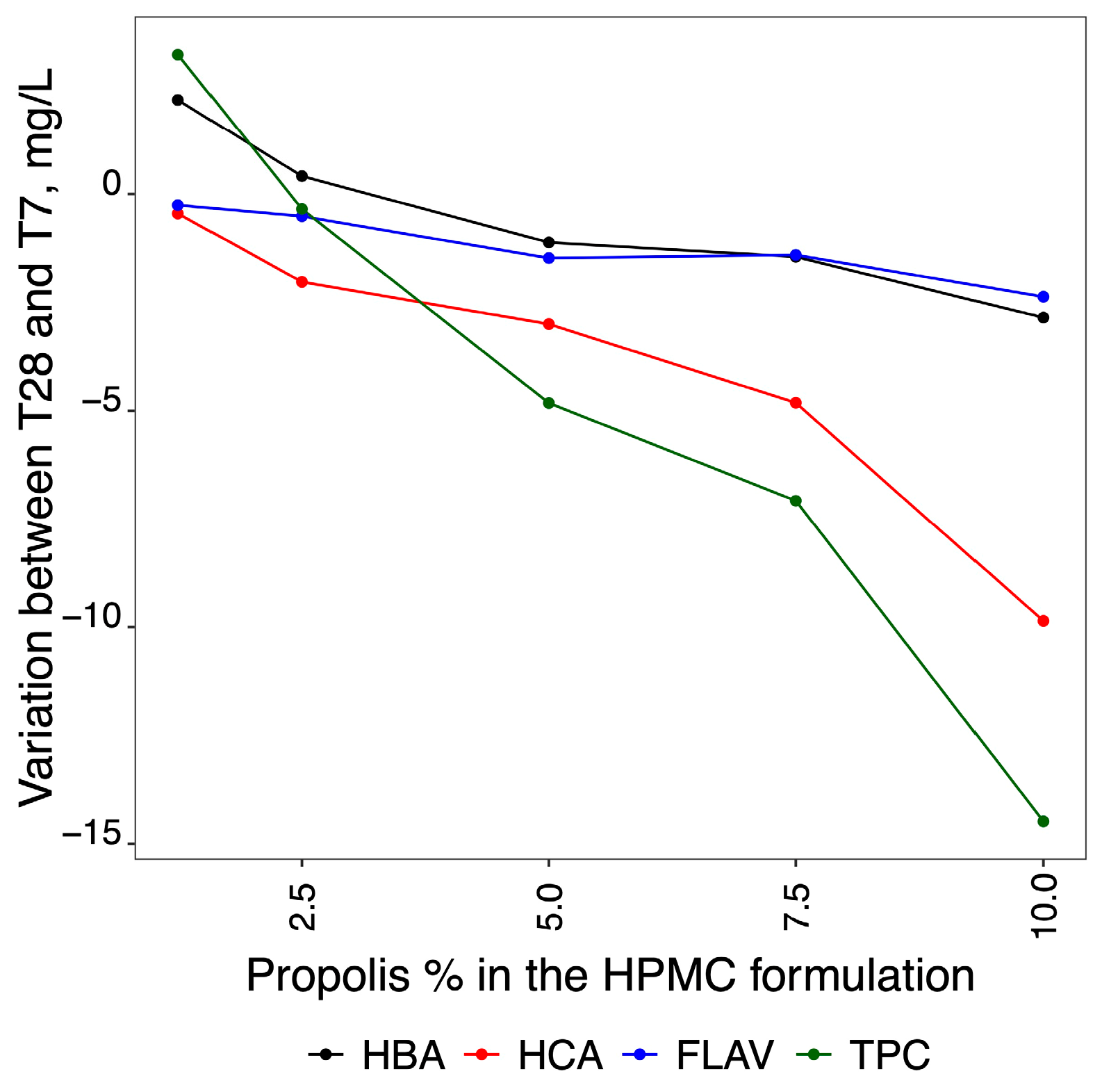
2.5. Quantification of the Variation of Phenolic Compounds
3. Discussion
3.1. Microbiological Analysis
3.2. pH, Water Activity and Weight Loss
3.3. Colour
3.4. Phenolic Compounds
3.5. Variation of Phenolic Compounds over Time
4. Materials and Methods
4.1. Reagents and Samples
4.2. Film Preparation
4.3. Cheese Application
4.3.1. Microbiological Analysis
4.3.2. Determination of pH, Water Activity and Cheese Weight
4.3.3. Colour Determination
4.4. Quantification of Phenolic Compounds
4.5. Quantification of the Variation of Phenolic Compounds over Time
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kure, C.F.; Skaar, I. The fungal problem in cheese industry. Curr. Opin. Food Sci. 2019, 29, 14–19. [Google Scholar] [CrossRef]
- Trmčić, A.; Ralyea, R.; Meunier-Goddik, L.; Donnelly, C.; Glass, K.; D’amico, D.; Meredith, E.; Kehler, M.; Tranchina, N.; McCue, C.; et al. Consensus categorization of cheese based on water activity and pH—A rational approach to systemizing cheese diversity. J. Dairy Sci. 2017, 100, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Qiu, C.; Ji, H.; Li, X.; Sang, S.; McClements, D.J.; Jiao, A.; Wang, J.; Jin, Z. Recent advances in biomolecule-based films and coatings for active and smart food packaging applications. Food Biosci. 2023, 52, 102378. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [PubMed]
- Garnier, L.; Valence, F.; Mounier, J. Diversity and control of spoilage fungi in dairy products: An update. Microorganisms 2017, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, S.; Salehabadi, A.; Nafchi, A.M.; Oladzadabbasabadi, N.; Jafari, S.M. Cheese packaging by edible coatings and biodegradable nanocomposites; improvement in shelf life, physicochemical and sensory properties. Trends Food Sci. Technol. 2021, 116, 218–231. [Google Scholar] [CrossRef]
- Mei, L.X.; Nafchi, A.M.; Ghasemipour, F.; Easa, A.M.; Jafarzadeh, S.; Al-Hassan, A.A. Characterization of pH sensitive sago starch films enriched with anthocyanin-rich torch ginger extract. Int. J. Biol. Macromol. 2020, 164, 4603–4612. [Google Scholar]
- Tilocca, B.; Costanzo, N.; Morittu, V.M.; Spina, A.A.; Soggiu, A.; Britti, D.; Roncada, P.; Piras, C. Milk microbiota: Characterization methods and role in cheese production. J. Proteom. 2020, 210, 103534. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Costa, M.J.; Maciel, L.C.; Teixeira, J.A.; Vicente, A.A.; Cerqueira, M.A. Use of edible films and coatings in cheese preservation: Opportunities and challenges. Food Res. Int. 2018, 107, 84–92. [Google Scholar] [CrossRef]
- Yildirim, S.C.; Ates, F. Antimicrobial Edible Cellulose-Based (CB) Films and Coatings for Enhancing Microbial Safety of White Cheese During Storage. Emir. J. Food Agric. 2022, 34, 1061–1071. [Google Scholar] [CrossRef]
- Ollé Resa, C.P.; Gerschenson, L.N.; Jagus, R.J. Effect of natamycin on physical properties of starch edible films and their effect on Saccharomyces cerevisiae activity. Food Bioprocess Technol. 2013, 6, 3124–3133. [Google Scholar] [CrossRef]
- Zhong, Y.; Cavender, G.; Zhao, Y. Investigation of different coating application methods on the performance of edible coatings on Mozzarella cheese. LWT-Food Sci. Technol. 2014, 56, 1–8. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Zúñiga, R.N.; Osorio, F.; Pedreschi, F. Physical properties of emulsion-based hydroxypropyl methylcellulose/whey protein isolate (HPMC/WPI) edible films. Carbohydr. Polym. 2015, 123, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Polo, J.; Silva-Weiss, A.; Zamorano, M.; Osorio, F.A. Humectability and physical properties of hydroxypropyl methylcellulose coatings with liposome-cellulose nanofibers: Food application. Carbohydr. Polym. 2020, 231, 115702. [Google Scholar] [CrossRef]
- Negi, P. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Santos, M.S.; Estevinho, M.L.M.F.; de Carvalho, C.A.L.; Magalhães-Guedes, K.T.; Schwan, R.F.; de Castro Almeida, R.C. Propolis as natural additive: A systematic review. Afr. J. Biotechnol. 2018, 17, 1282–1291. [Google Scholar]
- Thamnopoulos, I.A.I.; Michailidis, G.F.; Fletouris, D.J.; Badeka, A.; Kontominas, M.G.; Angelidis, A.S. Inhibitory activity of propolis against Listeria monocytogenes in milk stored under refrigeration. Food Microbiol. 2018, 73, 168–176. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. New emerging fields of application of propolis. Maced. J. Chem. Chem. Eng. 2016, 35, 1–11. [Google Scholar] [CrossRef]
- Segueni, N.; Boutaghane, N.; Asma, S.T.; Tas, N.; Acaroz, U.; Arslan-Acaroz, D.; Shah, S.R.A.; Abdellatieff, H.A.; Akkal, S.; Peñalver, R.; et al. Review on Propolis Applications in Food Preservation and Active Packaging. Plants 2023, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality—A review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Tumbarski, Y.D.; Todorova, M.M.; Topuzova, M.G.; Georgieva, P.I.; Ganeva, Z.A.; Mihov, R.B.; Yanakieva, V.B. Antifungal Activity of Carboxymethyl Cellulose Edible Films Enriched with Propolis Extracts and Their Role in Improvement of the Storage Life of Kashkaval Cheese. Curr. Res. Nutr. Food Sci. 2021, 9, 487–499. [Google Scholar] [CrossRef]
- El-Deeb, A.; Omar, S. Effect of Propolis Extract as a Natural Preservative on the Microbial Content of Kareish Cheese. J. Food Dairy Sci. 2017, 8, 295–302. [Google Scholar] [CrossRef]
- Guirguis, E. Extending the Shelf-Life of Processed Cheese Spread Using Propolis Extract. Int. J. Case Stud. 2019, 8, 82–88. [Google Scholar]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef] [PubMed]
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Poplar-type propolis: Chemical composition, botanical origin and biological activity. Nat. Prod. Commun. 2015, 10, 1934578X1501001117. [Google Scholar] [CrossRef]
- Silici, S.; Ünlü, M.; Vardar-Ünlü, G. Antibacterial activity and phytochemical evidence for the plant origin of Turkish propolis from different regions. World J. Microbiol. Biotechnol. 2007, 23, 1797–1803. [Google Scholar] [CrossRef]
- Banzato, T.P.; Gubiani, J.R.; Bernardi, D.I.; Nogueira, C.R.; Monteiro, A.F.; Juliano, F.F.; Alencar, S.M.; Pilli, R.A.; Lima, C.A.; Longato, G.B.; et al. Antiproliferative flavanoid dimers isolated from Brazilian red propolis. J. Nat. Prod. 2020, 83, 1784–1793. [Google Scholar] [CrossRef]
- Touzani, S.; Embaslat, W.; Imtara, H.; Kmail, A.; Kadan, S.; Zaid, H.; ElArabi, I.; Badiaa, L.; Saad, B. In vitro evaluation of the potential use of propolis as a multitarget therapeutic product: Physicochemical properties, chemical composition, and immunomodulatory, antibacterial, and anticancer properties. BioMed Res. Int. 2019, 2019, 4836378. [Google Scholar] [CrossRef]
- Sokeng, S.D.; Talla, E.; Sakava, P.; Fokam Tagne, M.A.; Henoumont, C.; Sophie, L.; Mbafor, J.T.; Tchuenguem Fohouo, F.-N. Anti-inflammatory and analgesic effect of arachic acid ethyl ester isolated from propolis. BioMed Res. Int. 2020, 2020, 8797284. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, C.; Núñez, O.; Torres, A.; Guzmán, L.; Wehinger, S. Antitumor activity of propolis: Recent advances in celular perspectives, animal models and possible applications. Food Rev. Int. 2020, 36, 429–455. [Google Scholar] [CrossRef]
- Utispan, K.; Chitkul, B.; Koontongkaew, S. Cytotoxic Activity of Propolis Extracts from the Stingless Bee Trigona Sirindhornae Against Primary and Metastatic Head and Neck Cancer Cell Lines. Asian Pac. J. Cancer Prev. 2017, 18, 1051–1055. [Google Scholar] [PubMed]
- Bodini, R.B.; Sobral, P.D.A.; Fávaro-Trindade, C.S.; Carvalho, R.D. Properties of gelatin-based films with added ethanol–propolis extract. LWT-Food Sci. Technol. 2013, 51, 104–110. [Google Scholar] [CrossRef]
- Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Onopiuk, A.; Zalewska, M.; Poltorak, A. Application of propolis extract in gelatin coatings as environmentally friendly method for extending the shelf life of pork loin. Coatings 2021, 11, 979. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of chitosan-based biodegradable active films using bio-waste enriched with polyphenol propolis extract envisaging food packaging applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Pastor, C.; Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and antifungal properties of hydroxypropylmethylcellulose based films containing propolis as affected by moisture content. Carbohydr. Polym. 2010, 82, 1174–1183. [Google Scholar] [CrossRef]
- de Araújo, G.K.P.; de Souza, S.J.; da Silva, M.V.; Yamashita, F.; Gonçalves, O.H.; Leimann, F.V.; Shirai, M.A. Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. Int. J. Food Sci. 2015, 50, 2080–2087. [Google Scholar] [CrossRef]
- Busch, V.M.; Pereyra-Gonzalez, A.; Šegatin, N.; Santagapita, P.R.; Ulrih, N.P.; Buera, M.D.P. Propolis encapsulation by spray drying: Characterization and stability. LWT-Food Sci. Technol. 2017, 75, 227–235. [Google Scholar] [CrossRef]
- Irigoiti, Y.; Navarro, A.; Yamul, D.; Libonatti, C.; Tabera, A.; Basualdo, M. The use of propolis as a functional food ingredient: A review. Trends Food Sci. Technol. 2021, 115, 297–306. [Google Scholar] [CrossRef]
- Paulo, F.; Paula, V.; Estevinho, L.M.; Santos, L. Propolis microencapsulation by double emulsion solvent evaporation approach: Comparison of different polymeric matrices and extract to polymer ratio. Food Bioprod. Process. 2021, 127, 408–425. [Google Scholar] [CrossRef]
- Yang, W.; Wu, Z.; Huang, Z.Y.; Miao, X. Preservation of orange juice using propolis. J. Food Sci. Technol. 2017, 54, 3375–3383. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.R. Combined effect of bioactive compounds and storage temperature on sensory quality and safety of minimally processed celery, leek and butternut squash. J. Food Saf. 2015, 35, 560–574. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ.; Okatan, V.; Wan, C. Biochemical composition of propolis and its efficacy in maintaining postharvest storability of fresh fruits and vegetables. J. Food Qual. 2020, 2020, 8869624. [Google Scholar] [CrossRef]
- Casquete, R.; Castro, S.M.; Teixeira, P. Antimicrobial activity of ethanolic extract of propolis in “Alheira”, a fermented meat sausage. Cogent Food Agric. 2016, 2, 1125774. [Google Scholar] [CrossRef]
- Gunhan, R.; Keskin, S.; Telli, N.; Takma, C.; Kolayli, S. Effect of Encapsulated Propolis on Microbial Quality and Antioxidant Activity of Yoghurt. Prog. Nutr. 2022, 24. [Google Scholar] [CrossRef]
- Santos, M.S.; Estevinho, L.M.; Carvalho, C.A.L.; Morais, J.S.; Conceição, A.L.S.; Paula, V.B.; Magalhães-Guedes, K.; Almeida, R.C. Probiotic yogurt with Brazilian red propolis: Physicochemical and bioactive properties, stability, and shelf life. J. Food Sci. 2019, 84, 3429–3436. [Google Scholar] [CrossRef]
- Tumbarski, Y.; Yanakieva, V.; Nikolova, R.; Mineva, G.; Deseva, I.; Mihaylova, D.; Ivanov, I. Application of a bacteriocin isolated from Bacillus methylotrophicus BM47 as a biopreservative in raw cow’s milk. Ind. Technol. 2018, 5, 20–25. [Google Scholar]
- Karanth, S.; Feng, S.; Patra, D.; Pradhan, A.K. Linking microbial contamination to food spoilage and food waste: The role of smart packaging, spoilage risk assessments, and date labeling. Front. Microbiol. 2023, 14, 1198124. [Google Scholar] [CrossRef]
- Fernández, M.; Hudson, J.A.; Korpela, R.; de los Reyes-Gavilán, C.G. Impact on human health of microorganisms present in fermented dairy products: An overview. BioMed Res. Int. 2015, 2015, 412714. [Google Scholar] [CrossRef]
- Quigley, L.; O’Sullivan, O.; Stanton, C.; Beresford, T.P.; Ross, R.P.; Fitzgerald, G.F.; Cotter, P.D. The complex microbiota of raw milk. FEMS Microbiol. Rev. 2013, 37, 664–698. [Google Scholar] [CrossRef]
- Pereira, V.; Lopes, C.; Castro, A.; Silva, J.; Gibbs, P.; Teixeira, P. Characterization for enterotoxin production, virulence factors, and antibiotic susceptibility of Staphylococcus aureus isolates from various foods in Portugal. Food Microbiol. 2009, 26, 278–282. [Google Scholar] [CrossRef]
- Vrdoljak, J.; Dobranić, V.; Filipović, I.; Zdolec, N. Microbiological quality of soft, semi-hard and hard cheeses during the shelf-life. Maced. Vet. Rev. 2016, 39, 59–64. [Google Scholar] [CrossRef]
- Forsythe, S.J. Food Safety Microbiology; Artmed: Porto Alegre, Brazil, 2002. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2007, 322, 12–29. [Google Scholar]
- Saraiva, M.; Correia, C.B.; Cunha, I.C.; Maia, C.; Bonito, C.C.; Furtado, R.; Calhau, M.A. Interpretação dos Resultados de Ensaios Microbiológicos em Alimentos Prontos para Consumo e em Superfícies do Ambiente de Produção e Distribuição Alimentar: Valores-Guia; Instituto Nacional de Saúde Doutor Ricardo Jorge, IP: Lisbon, Portugal, 2019. [Google Scholar]
- Kavas, G.; Kinik, O.; Uysal, H.; Kilic, S.; Celikel, N.; Akbulut, N. Characterisation of yeasts isolated from artisanal Turkish dairy products. Int. J. Dairy Sci. 2010, 5, 185–191. [Google Scholar] [CrossRef]
- Roostita, R.; Fleet, G.H. Growth of yeasts in milk and associated changes to milk composition. Int. J. Food Microbiol. 1996, 31, 205–219. [Google Scholar] [CrossRef]
- Goncalves Dos Santos, M.T.P.; Benito, M.J.; de Guia Cordoba, M.; Alvarenga, N.; Ruiz-Moyano Seco de Herrera, S. Yeast community in traditional Portuguese Serpa cheese by culture-dependent and-independent DNA approaches. Int. J. Food Microbiol. 2017, 262, 63–70. [Google Scholar] [CrossRef]
- Fröhlich-Wyder, M.T.; Arias-Roth, E.; Jakob, E. Cheese yeasts. Yeast 2019, 36, 129–141. [Google Scholar] [CrossRef]
- Bintsis, T. Yeasts in different types of cheese. AIMS Microbiol. 2021, 7, 447–470. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, X.; Wang, B. A review on the general cheese processing technology, flavor biochemical pathways and the influence of yeasts in cheese. Front. Microbiol. 2021, 12, 703284. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast Spoilage of Foods and Beverages. In The Yeasts, a Taxonomic Study, 5th ed.; Fell, C.P.K.C.J.W., Boekhout, T., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; pp. 53–63. [Google Scholar]
- Monnet, C.; Landaud, S.; Bonnarme, P.; Swennen, D. Growth and adaptation of microorganisms on the cheese surface. FEMS Microbiol. Lett. 2014, 263, 1–9. [Google Scholar] [CrossRef]
- Padilla, B.; Manzanares, P.; Belloch, C. Yeast species and genetic heterogeneity within Debaryomyces hansenii along the ripening process of traditional ewes’ and goats’ cheeses. Food Microbiol. 2014, 38, 160–166. [Google Scholar] [CrossRef]
- Riešutė, R.; Šalomskienė, J.; Moreno, D.S.; Gustienė, S. Effect of yeasts on food quality and safety and possibilities of their inhibition. Trends Food Sci. Technol. 2021, 108, 1–10. [Google Scholar] [CrossRef]
- Cortés-Higareda, M.; de Lorena Ramos-García, M.; Correa-Pacheco, Z.N.; Del Río-García, J.C.; Bautista-Baños, S. Nanostructured Chitosan/Propolis Formulations: Characterization and Effect on the Growth of Aspergillus flavus and Production of Aflatoxins. Heliyon 2019, 5, e01776. [Google Scholar] [CrossRef]
- Franchin, M.; Cólon, D.F.; Castanheira, F.V.S.; da Cunha, M.G.; Bueno-Silva, B.; Alencar, S.M.; Cunha, T.M.; Rosalen, P.L. Vestitol Isolated from Brazilian Red Propolis Inhibits Neutrophils Migration in the Inflammatory Process: Elucidation of the Mechanism of Action. J. Nat. Prod. 2016, 79, 954–960. [Google Scholar] [CrossRef]
- Jia, S.; Li, C.; Wu, K.; Qi, D.; Wang, S. Effect of Water Activity on Conidia Germination in Aspergillus flavus. Microorganisms 2022, 10, 1744. [Google Scholar] [CrossRef]
- Racchi, I.; Scaramuzza, N.; Hidalgo, A.; Berni, E. Combined effect of water activity and pH on the growth of food-related ascospore-forming molds. Ann. Microbiol. 2020, 70, 69. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Hsieh, Y.H. Foodborne Pathogens in Milk and Dairy Products: Genetic Characterization and Rapid Diagnostic Approach for Food Safety of Public Health Importance. In Dairy in Human Health and Disease across the Lifespan, 1st ed.; Watson, R.R., Collier, R.J., Preedy, V.R., Eds.; Springer: Berlin, Germany, 2017; pp. 127–143. [Google Scholar]
- Perin, L.M.; Pereira, J.G.; Bersot, L.S.; Nero, L.A. The microbiology of raw milk. In Raw Milk, Balance between Hazards and Benefits, 1st ed.; Nero, L.A., Carvalho, A.F., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–64. [Google Scholar]
- Giannuzzi, L.; Contreras, E.; Zaritzky, N. Modeling the aerobic growth and decline of Staphylococcus aureus as affected by pH and potassium sorbate concentration. J. Food Prot. 1999, 62, 356–362. [Google Scholar] [CrossRef]
- Nessianpour, E.; Khodanazary, A.; Hosseini, S.M. Shelf life of Saurida tumbil during storage at refrigeration condition as affected by gelatin-based edible coatings incorporated with propolis extract. Int. J. Food Prop. 2019, 22, 1749–1759. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef]
- Yangılar, F.; Oğuzhan Yıldız, P. Casein/natamycin edible films efficiency for controlling mould growth and on microbiological, chemical and sensory properties during the ripening of Kashar cheese. J. Sci. Food Agric. 2016, 96, 2328–2336. [Google Scholar] [CrossRef]
- Selover, B.; Johnson, J.; Waite-Cusic, J.G. Population dynamics of coliforms in a commercial Cheddar cheese production facility. J. Dairy Sci. 2021, 104, 7480–7488. [Google Scholar] [CrossRef]
- Moatsou, G.; Moschopoulou, E.; Beka, A.; Tsermoula, P.; Pratsis, D. Effect of natamycin-containing coating on the evolution of biochemical and microbiological parameters during the ripening and storage of ovine hard-Gruyère-type cheese. Int. Dairy J. 2015, 50, 1–8. [Google Scholar] [CrossRef]
- Metz, M.; Sheehan, J.; Feng, P.C. Use of indicator bacteria for monitoring sanitary quality of raw milk cheeses–A literature review. Food Microbiol. 2020, 85, 103283. [Google Scholar] [CrossRef]
- Ekici, G.; Dümen, E. Escherichia coli and food safety. In The Universe of Escherichia coli, 1st ed.; Erjavec, M.S., Ed.; IntechOpen: London, UK, 2019; pp. 85–100. [Google Scholar]
- Chung, H.J.; Bang, W.; Drake, M.A. Stress response of Escherichia coli. Compr. Rev. Food Sci. Food Saf. 2006, 5, 52–64. [Google Scholar] [CrossRef]
- Cosciani-Cunico, E.; Dalzini, E.; D’Amico, S.; Sfameni, C.; Bertasi, B.; Losio, M.N.; Giacometti, F.; Daminelli, P. Behaviour of Escherichia coli O157: H7 during the manufacture and ripening of an Italian traditional raw goat milk cheese. Ital. J. Food Saf. 2014, 3, 2243. [Google Scholar] [CrossRef]
- Pasquali, F.; Valero, A.; Possas, A.; Lucchi, A.; Crippa, C.; Gambi, L.; Manfreda, G.; De Cesare, A. Occurrence of foodborne pathogens in Italian soft artisanal cheeses displaying different intra-and inter-batch variability of physicochemical and microbiological parameters. Front. Microbiol. 2022, 13, 959648. [Google Scholar] [CrossRef]
- Tornadijo, M.; García, M.; Fresno, J.; Carballo, J. Study of Enterobacteriaceae during the manufacture and ripening of San Simón cheese. Food Microbiol. 2001, 18, 499–509. [Google Scholar] [CrossRef]
- Hervert, C.J.; Alles, A.S.; Martin, N.H.; Boor, K.J.; Wiedmann, M. Evaluation of different methods to detect microbial hygiene indicators relevant in the dairy industry. J. Dairy Sci. 2016, 99, 7033–7042. [Google Scholar] [CrossRef]
- Petruzzi, L.; Rosaria Corbo, M.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Bevilacqua, A. Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods 2020, 9, 559. [Google Scholar] [CrossRef]
- Watkinson, P.; Coker, C.; Crawford, R.; Dodds, C.; Johnston, K.; McKenna, A.; White, N. Effect of cheese pH and ripening time on model cheese textural properties and proteolysis. Int. Dairy J. 2001, 11, 455–464. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evid.-Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Amara, C.B.; Oulahal, N.; Gharsallaoui, A.; Joly, C.; Tongdeesoontorn, W.; Rawdkuen, S.; Degraeve, P. Gelatin Films with Nisin and Catechin for Minced Pork Preservation. Food Pack. Shelf Life 2018, 18, 173–183. [Google Scholar]
- Ibrahim, M.E.E.D.; Alqurashi, R.M. Anti-fungal and antioxidant properties of propolis (bee glue) extracts. Int. J. Food Microbiol. 2022, 361, 109463. [Google Scholar] [CrossRef]
- Cardinali, F.; Ferrocino, I.; Milanović, V.; Belleggia, L.; Corvaglia, M.R.; Garofalo, C.; Foligni, R.; Mannozzi, C.; Mozzon, M.; Cocolin, L.; et al. Microbial communities and volatile profile of Queijo de Azeitão PDO cheese, a traditional Mediterranean thistle-curdled cheese from Portugal. Food Res. Int. 2021, 147, 110537. [Google Scholar] [CrossRef]
- Araújo-Rodrigues, H.; Tavaria, F.K.; dos Santos, M.T.P.; Alvarenga, N.; Pintado, M.M. A review on microbiological and technological aspects of Serpa PDO cheese: An ovine raw milk cheese. Int. Dairy J. 2020, 100, 104561. [Google Scholar] [CrossRef]
- Fogeiro, É.; Barracosa, P.; Oliveira, J.; Wessel, D.F. Influence of cardoon flower (Cynara cardunculus L.) and flock lactation stage in PDO Serra da Estrela Cheese. Foods 2020, 9, 386. [Google Scholar] [CrossRef]
- Chirife, J.; Fontana, A.J., Jr. Introduction: Historical highlights of water activity research. In Water Activity in Foods: Fundamentals and Applications, 2nd ed.; Barbosa-Cánovas, G.V., Fontana, A.J., Jr., Schmidt, S.J., Labuza, T.P., Eds.; Wiley-Blackwell: Oxford, UK, 2020; pp. 1–11. [Google Scholar]
- Basch, C.Y.; Jagus, R.J.; Flores, S.K. Physical and Antimicrobial Properties of Tapioca Starch-HPMC Edible Films Incorporated with Nisin and/or Potassium Sorbate. Food Bioprocess Technol. 2013, 6, 2419–2428. [Google Scholar] [CrossRef]
- Soleimani-Rambod, A.; Zomorodi, S.; Naghizadeh Raeisi, S.; Khosrowshahi Asl, A.; Shahidi, S.A. The effect of xanthan gum and flaxseed mucilage as edible coatings in cheddar cheese during ripening. Coatings 2018, 8, 80. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E.; Fávaro Perez, M.A.; Ramos Souza Felipe, F.; Padula, M. Application of β-Cyclodextrin/2-Nonanone Inclusion Complex as Active Agent to Design of Antimicrobial Packaging Films for Control of Botrytis Cinerea. Food Bioprocess Technol. 2017, 10, 1585–1594. [Google Scholar] [CrossRef]
- Khodayari, M.; Basti, A.A.; Khanjari, A.; Misaghi, A.; Kamkar, A.; Shotorbani, P.M.; Hamedi, H. Effect of Poly(Lactic Acid) Films Incorporated with Different Concentrations of Tanacetum balsamita Essential Oil, Propolis Ethanolic Extract and Cellulose Nanocrystals on Shelf Life Extension of Vacuum-Packed Cooked Sausages. Food Packag. Shelf Life 2019, 19, 200–209. [Google Scholar] [CrossRef]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic compounds diminish antibiotic resistance of Staphylococcus aureus clinical strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Xue, J.; Davidson, P.M.; Zhong, Q. Thymol nanoemulsified by whey protein-maltodextrin conjugates: The enhanced emulsifying capacity and antilisterial properties in milk by propylene glycol. J. Agric. Food Chem. 2013, 61, 12720–12726. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Paula, V.B.; Estevinho, L.M.; Dias, L.G. Quantification of three phenolic classes and total phenolic content of propolis extracts using a single UV-vis spectrum. J. Agric. Res. 2017, 56, 569–580. [Google Scholar] [CrossRef]
- Paula, V.B.; Estevinho, L.M.; Cardoso, S.M.; Dias, L.G. Comparative Methods to Evaluate the Antioxidant Capacity of Propolis: An Attempt to Explain the Differences. Molecules 2023, 28, 4847. [Google Scholar] [CrossRef]
- Srivastava, A.; Akoh, C.C.; Yi, W.; Fischer, J.; Krewer, G. Effect of storage conditions on the biological activity of phenolic compounds of blueberry extract packed in glass bottles. J. Agric. Food Chem. 2007, 55, 2705–2713. [Google Scholar] [CrossRef]
- Del Caro, A.; Piga, A.; Pinna, I.; Fenu, P.M.; Agabbio, M. Effect of drying conditions and storage period on polyphenolic content, antioxidant capacity, and ascorbic acid of prunes. J. Agric. Food Chem. 2004, 52, 4780–4784. [Google Scholar] [CrossRef]
- Rzepecka-Stojko, A.; Stec, M.; Kurzeja, E.; Gawrońska, E.; Pawłowska-Góral, K. The Effect of Storage of Bee Pollen Extracts on Polyphenol Content. Pol. J. Environ. Stud. 2012, 21, 1007–1011. [Google Scholar]
- Moldovan, B.; Popa, A.; David, L. Effects of storage temperature on the total phenolic content of Cornelian Cherry (Cornus mas L.) fruits extracts. J. Appl. Bot. Food Qual. 2016, 89, 208–211. [Google Scholar]
- Miyagusuku-Cruzado, G.; García-Cano, I.; Rocha-Mendoza, D.; Jiménez-Flores, R.; Giusti, M.M. Monitoring hydroxycinnamic acid decarboxylation by lactic acid bacteria using high-throughput UV-Vis spectroscopy. Molecules 2020, 25, 3142. [Google Scholar] [CrossRef]
- López de Felipe, F. Revised Aspects into the Molecular Bases of Hydroxycinnamic Acid Metabolism in Lactobacilli. Antioxidants 2023, 12, 1294. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- ISO 4833-1:2013(en); Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 21527-2:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less Than or Equal to 0.95. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 152147:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 °C. International Organization for Standardization: Geneva, Switzerland, 1998.
- AOAC Official Method 2005.03 Detection and Confirmed Quantitation of Coliforms and E. coli in Foods. SimPlate Coliform and E. coli Color Indicator. In Official Methods of Analysis of AOAC International; AOAC Publications: New York, NY, USA, 2023.
- ISO 6888-1:1999/Amd 1:2003; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Technique Using Baird-Parker Agar Medium. International Organization for Standardization: Geneva, Switzerland, 2003.
- AOAC 989.13, Motile Salmonella in all foods. Immunodiffusion (1-2 TEST) method. In Official Methods of Analysis of AOAC International; AOAC Publications: New York, NY, USA, 2023.
- ISO/DIS 15213-1(en); Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Clostridium spp.—Part 1: Enumeration of Sulfite-Reducing Clostridium spp. by Colony-Count Technique. International Organization for Standardization: Geneva, Switzerland, 2023.
- ISO 11290-2:1998/Amd. 1:2004(E); Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes—Part 2: Enumeration Method. Amendment 1: Modification of the Enumeration Medium. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 11290-1:1996/Amd. 1:2004(E); Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes—Part 1: Detection Method. Amendment 1: Modification of the Isolation Media and the Haemolysis Test, and Inclusion of Precision Data. International Organization for Standardization: Geneva, Switzerland, 1996.
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Anderson, M.J. Permutational Multivariate Analysis of Variance (PERMANOVA). In Wiley StatsRef: Statistics Reference Online; Balakrishnan, N., Colton, T., Everitt, B., Piegorsch, W., Ruggeri, F., Teugels, J.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–15. [Google Scholar]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. _vegan: Community Ecology Package_. R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 May 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 May 2023).
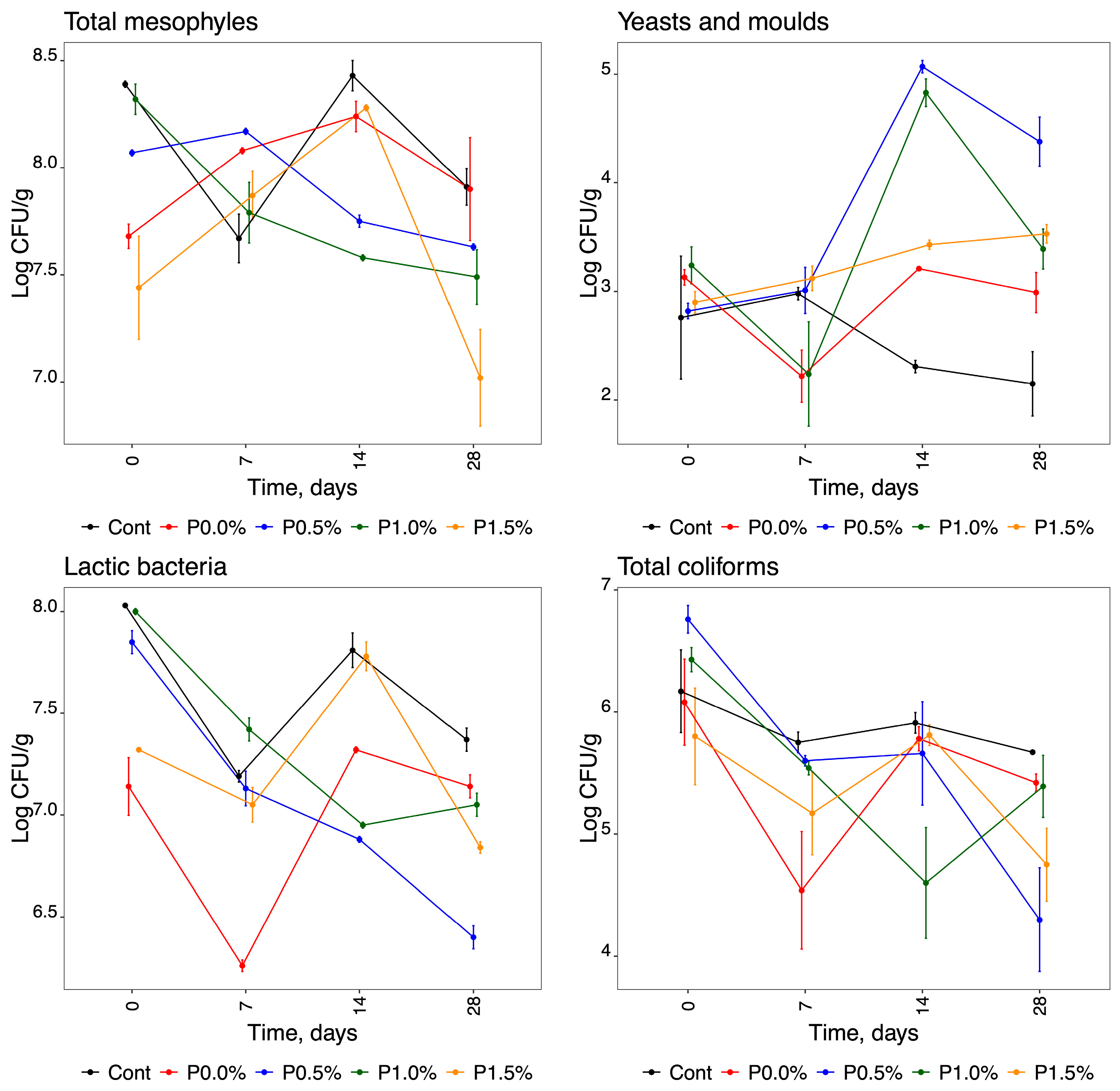


| Microorganisms | Parameter | Days: | 0 | 7 | 14 | 28 |
|---|---|---|---|---|---|---|
| Total mesophiles * | Control | 8.39 ± 0.01 ab | 7.67 ± 0.08 efgh | 8.43 ± 0.05 a | 7.91 ± 0.06 cde | |
| P0.0% | 7.68 ± 0.04 efgh | 8.08 ± 0.01 bcd | 8.24 ± 0.05 ab | 7.90 ± 0.17 cdef | ||
| P0.5% | 8.07 ± 0.01 bcd | 8.17 ± 0.01 abc | 7.75 ± 0.02 efgh | 7.63 ± 0.01 efgh | ||
| P1.0% | 8.32 ± 0.05 ab | 7.79 ± 0.10 defg | 7.58 ± 0.01 fgh | 7.49 ± 0.09 gh | ||
| P1.5% | 7.44 ± 0.17 h | 7.87 ± 0.08 cdef | 8.28 ± 0.01 ab | 7.02 ± 0.16 i | ||
| Yeasts and moulds * | Control | 2.76 ± 0.40 efgh | 2.98 ± 0.04 cde | 2.31 ± 0.04 fgh | 2.15 ± 0.21 h | |
| P0.0% | 3.13 ± 0.05 cde | 2.22 ± 0.17 gh | 3.21 ± 0.01 cde | 2.99 ± 0.13 cde | ||
| P0.5% | 2.82 ± 0.05 defg | 3.01 ± 0.15 cde | 5.07 ± 0.04 a | 4.38 ± 0.16 b | ||
| P1.0% | 3.24 ± 0.12 cde | 2.24 ± 0.34 gh | 4.83 ± 0.09 ab | 3.39 ± 0.13 cd | ||
| P1.5% | 2.90 ± 0.07 def | 3.12 ± 0.08 cde | 3.43 ± 0.03 cd | 3.53 ± 0.06 c | ||
| Lactic bacteria * | Control | 8.03 ± 0.00 a | 7.19 ± 0.02 ef | 7.81 ± 0.06 c | 7.37 ± 0.04 d | |
| P0.0% | 7.14 ± 0.10 f | 6.26 ± 0.02 i | 7.32 ± 0.01 de | 7.14 ± 0.04 f | ||
| P0.5% | 7.85 ± 0.04 bc | 7.13 ± 0.06 f | 6.88 ± 0.01 gh | 6.40 ± 0.04 i | ||
| P1.0% | 8.00 ± 0.01 ab | 7.42 ± 0.04 d | 6.95 ± 0.01 gh | 7.05 ± 0.04 fg | ||
| P1.5% | 7.32 ± 0.00 de | 7.05 ± 0.06 fg | 7.78 ± 0.05 c | 6.84 ± 0.02 h | ||
| Total coliforms * | Control | 6.17 ± 0.24 abc | 5.75 ± 0.06 abcd | 5.91 ± 0.06 abc | 5.67 ± 0.01 abcde | |
| P0.0% | 6.08 ± 0.25 abc | 4.54 ± 0.34 fg | 5.78 ± 0.07 abcd | 5.42 ± 0.05 bcdef | ||
| P0.5% | 6.76 ± 0.08 a | 5.60 ± 0.03 bcdef | 5.66 ± 0.30 abcde | 2.15 ± 3.04 g | ||
| P1.0% | 6.43 ± 0.07 ab | 5.54 ± 0.04 bcdef | 2.30 ± 3.25 efg | 5.39 ± 0.18 bcdefg | ||
| P1.5% | 5.80 ± 0.28 abcd | 5.17 ± 0.24 cdefg | 5.81 ± 0.06 abcd | 4.75 ± 0.21 defg | ||
| Escherichia coli * | Control | 4.05 ± 0.21 a | 2.65 ± 0.49 abcde | 3.29 ± 0.06 abcd | 1.30 ± 1.84 bcde | |
| P0.0% | 0.00 ± 0.00 f | 0.95 ± 1.34 de | 2.98 ± 0.44 abcd | 2.45 ± 0.21 cde | ||
| P0.5% | 3.84 ± 0.34 abc | 3.97 ± 0.05 ab | 2.08 ± 0.11 de | 0.00 ± 0.00 f | ||
| P1.0% | 3.75 ± 0.21 abc | 2.84 ± 0.08 abcde | 1.99 ± 0.30 de | 2.30 ± 0.00 de | ||
| P1.5% | 4.01 ± 0.57 ab | 1.45 ± 0.21 e | 3.19 ± 0.11 abcd | 0.00 ± 0.00 f | ||
| Enterobacteriaceae * | Control | 3.38 ± 0.05 bcd | 2.80 ± 0.28 cd | 3.26 ± 0.67 bcd | 0.00 ± 0.00 e | |
| P0.0% | 2.30 ± 0.00 d | 3.32 ± 0.23 bcd | 2.45 ± 0.21 d | 0.00 ± 0.00 e | ||
| P0.5% | 4.67 ± 0.29 a | 4.02 ± 0.29 ab | 0.00 ± 0.00 e | 0.00 ± 0.00 e | ||
| P1.0% | 2.69 ± 0.13 cd | 4.07 ± 0.02 ab | 0.00 ± 0.00 e | 0.00 ± 0.00 e | ||
| P1.5% | 3.79 ± 0.08 abc | 3.45 ± 0.05 bcd | 0.00 ± 0.00 e | 0.00 ± 0.00 e |
| Variables | Model | Interaction Term | Shapiro–Wilk Test | Levene Test | ||
|---|---|---|---|---|---|---|
| R2 | RSE | p-Value | p-Value | p-Value | p-Value | |
| Total mesophiles | 0.975 | 0.080 | <0.001 | <0.001 | 0.345 | <0.001 |
| Yeast and moulds | 0.981 | 0.154 | <0.001 | <0.001 | 0.598 | <0.001 |
| Latic bacteria | 0.996 | 0.041 | <0.001 | <0.001 | 0.899 | <0.001 |
| Total coliforms | 0.767 | 0.790 | 0.007 | <0.001 | <0.001 | <0.001 |
| E. coli | 0.938 | 0.379 | <0.001 | <0.001 | 0.1 | <0.001 |
| Enterobacteriaceae | 0.987 | 0.203 | <0.001 | <0.001 | <0.001 | <0.001 |
| Measurement | Parameter | Days: | 0 | 7 | 14 | 28 |
|---|---|---|---|---|---|---|
| pH * | Control | 5.55 ± 0.03 gh | 5.70 ± 0.03 de | 6.01 ± 0.04 a | 5.33 ± 0.04 i | |
| P0.0% | 5.89 ± 0.02 b | 5.44 ± 0.05 h | 5.75 ± 0.05 cd | 5.71 ± 0.03 cd | ||
| P0.5% | 5.86 ± 0.01 b | 5.58 ± 0.02 fg | 5.81 ± 0.01 bc | 6.05 ± 0.05 a | ||
| P1.0% | 5.74 ± 0.03 cd | 5.60 ± 0.02 efg | 5.55 ± 0.03 gh | 5.65 ± 0.04 defg | ||
| P1.5% | 5.81 ± 0.04 bc | 5.66 ± 0.05 def | 5.44 ± 0.04 h | 5.75 ± 0.03 cd | ||
| aw * | Control | 0.92 ± 0.008 a | 0.84 ± 0.004 bcd | 0.86 ± 0.005 bc | 0.66 ± 0.03 e | |
| P0.0% | 0.93 ± 0.008 a | 0.84 ± 0.005 bcd | 0.81 ± 0.02 d | 0.66 ± 0.007 e | ||
| P0.5% | 0.90 ± 0.004 a | 0.83 ± 0.007 cd | 0.85 ± 0.002 bc | 0.67 ± 0.008 e | ||
| P1.0% | 0.92 ± 0.004 a | 0.86 ± 0.003 b | 0.85 ± 0.006 bc | 0.62 ± 0.02 e | ||
| P1.5% | 0.91 ± 0.004 a | 0.84 ± 0.007 bcd | 0.84 ± 0.008 bcd | 0.64 ± 0.001 e | ||
| Cheese weight (g) * | Control | 95.27 ± 0.13 d | 82.74 ± 0.10 k | 78.60 ± 0.09 n | 75.32 ± 0.05 p | |
| P0.0% | 104.31 ± 0.11 a | 93.98 ± 0.10 e | 88.77 ± 0.08 g | 84.69 ± 0.06 i | ||
| P0.5% | 98.83 ± 0.15 b | 83.31 ± 0.12 j | 79.16 ± 0.09 m | 76.69 ± 0.07 o | ||
| P1.0% | 96.27 ± 0.14 c | 86.77 ± 0.11 h | 80.97 ± 0.07 l | 76.88 ± 0.03 o | ||
| P1.5% | 91.41 ± 0.12 f | 82.70 ± 0.08 k | 76.82 ± 0.07 o | 72.96 ± 0.04 q |
| Compounds | Parameter | Days: | 7 | 28 |
|---|---|---|---|---|
| HBA class (mg GA/L) | P1.25% | 11.67 ± 1.39 e | 13.85 ± 1.39 e | |
| P2.50% | 16.29 ± 1.45 de | 16.71 ± 1.43 de | ||
| P5.00% | 23.04 ± 1.65 c | 21.92 ± 1.58 cd | ||
| P7.50% | 39.12 ± 2.03 b | 37.68 ± 1.94 b | ||
| P10.00% | 92.18 ± 3.30 a | 89.33 ± 3.08 a | ||
| HCA class (mg FA/L) * | P1.25% | 9.97 ± 1.02 gh | 9.53 ± 1.01 h | |
| P2.50% | 12.00 ± 1.03 g | 9.98 ± 1.02 gh | ||
| P5.00% | 20.40 ± 1.10 e | 17.40 ± 1.07 f | ||
| P7.50% | 35.26 ± 1.22 c | 30.45 ± 1.18 d | ||
| P10.00% | 80.14 ± 1.60 a | 70.28 ± 1.53 b | ||
| FLAV class (mg Q/L) * | P1.25% | 3.79 ± 0.03 j | 3.53 ± 0.03 i | |
| P2.50% | 4.64 ± 0.02 g | 4.13 ± 0.03 h | ||
| P5.00% | 7.75 ± 0.02 e | 6.28 ± 0.02 f | ||
| P7.50% | 13.34 ± 0.02 c | 11.94 ± 0.02 d | ||
| P10.00% | 29.83 ± 0.02 a | 27.46 ± 0.02 b | ||
| TPCs (mg TPC/L) | P1.25% | 28.28 ± 4.18 j | 31.51 ± 4.31 i | |
| P2.50% | 34.90 ± 4.39 g | 34.56 ± 4.40 h | ||
| P5.00% | 69.70 ± 5.54 e | 64.88 ± 5.40 f | ||
| P7.50% | 108.62 ± 6.90 c | 101.55 ± 6.67 d | ||
| P10.00% | 251.36 ± 12.60 a | 236.88 ± 11.62 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paula, V.B.; Dias, L.G.; Estevinho, L.M. Microbiological and Physicochemical Evaluation of Hydroxypropyl Methylcellulose (HPMC) and Propolis Film Coatings for Cheese Preservation. Molecules 2024, 29, 1941. https://doi.org/10.3390/molecules29091941
Paula VB, Dias LG, Estevinho LM. Microbiological and Physicochemical Evaluation of Hydroxypropyl Methylcellulose (HPMC) and Propolis Film Coatings for Cheese Preservation. Molecules. 2024; 29(9):1941. https://doi.org/10.3390/molecules29091941
Chicago/Turabian StylePaula, Vanessa B., Luís G. Dias, and Letícia M. Estevinho. 2024. "Microbiological and Physicochemical Evaluation of Hydroxypropyl Methylcellulose (HPMC) and Propolis Film Coatings for Cheese Preservation" Molecules 29, no. 9: 1941. https://doi.org/10.3390/molecules29091941