Nucleophilic Additions of 2-Furyllithium to Carbonyl Derivatives of L-Serine. Formal Synthesis of (2R,3R)-β-Hydroxy Aspartic Acid
Abstract
:


Acknowledgement
References and Notes
- Novel Applications of Heterocycles in Synthesis. Tetrahedron Symposia-in-Print, no. 59. Tetrahedron 1996, 52, 3057–3374, 23 original articles. Shipman, M. Contemp. Org. Synth. 1995, 2, 1–18. [CrossRef] Padwa, A. Heterocycles as vehicles for synthesis. In Progress in Heterocyclic Chemistry; Suschitzky, H., Scriven, E.F.V., Eds.; Pergamon: Oxford, 1994; Vol. 4, pp. 36–55. [Google Scholar] Lipshutz, B.H. Chem. Rev. 1986, 86, 795–819. Meyers, A.I. Heterocycles in Organic Synthesis; John Wiley & sons: New York, 1974. [Google Scholar]
- For leading references in the use of heterocycles as synthetic equivalents towards the synthesis of natural products see inter alia: Danishefsky, S.J.; Pearson, W.H.; Segmuller, B.E. J. Am. Chem. Soc. 1985, 107, 1280–1285. [CrossRef] Danishefsky, S.J.; DeNinno, M.P.; Chen, S.-H. J. Am. Chem. Soc. 1988, 110, 3229–3940. Martin, S.F.; Zinke, P.W. J. Org. Chem. 1991, 56, 6600–6606. [CrossRef] Dondoni, A.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Chem. Commun. 1995, 2127–2128. Dondoni, A.; Merino, P. J. Org. Chem. 1991, 56, 5294–5301. [CrossRef] Dondoni, A.; Marra, A.; Merino, P. J. Am. Chem. Soc. 1994, 116, 3324–3336. [CrossRef] Martin, S.F.; Chen, H.-J.; Lynch, V.M. J. Org. Chem. 1995, 60, 276–278. [CrossRef] Vogel, P. Bull. Soc. Chim. Belg. 1990, 99, 395–439. [CrossRef]
- Casiraghi, G.; Rassu, G. Synthesis 1995, 607–626. Casiraghi, G.; Zanardi, F.; Rassu, G.; Spanu, P. Chem. Rev. 1995, 95, 1677–1717. Piancatelli, G.; D’Auria, M.; D’Onofrio, F. Synthesis 1994, 867–889. See also refs. 2a-d.
- Fan, W.-Q.; Katritzky, A.R. 1,2,3-Triazoles. In Comprehensive Heterocyclic Chemistry, 2nd edition; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon, 1996; vol. 4, chapter 4.01; pp. 1–64. [Google Scholar] and references cited therein.
- Dondoni, A.; Merino, P. Thiazoles. In Comprehensive Heterocyclic Chemistry, 2nd edition; Katritzky, A.R., Rees, C. W., Scriven, E.F.V., Eds.; Pergamon, 1996; vol. 3, chapter 3.06; pp. 373–474. [Google Scholar] and references cited therein.
- Heaney, H.; Ahn, J.S. Furans and their Benzo Derivatives: Reactivity. In Comprehensive Heterocyclic Chemistry, 2nd edition; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Pergamon, 1996; vol. 2, chapter 2.06; pp. 279–350. [Google Scholar] and references cited therein.
- Lukevits, E.; Pudova, O.A. Chem. Heterocycl. Comp. 1995, 31, 377–431. [CrossRef]
- For additions to aldehydes see: Pikul, S.; Raczko, J.; Ankner, K.; Jurczack, J. J. Am. Chem. Soc. 1987, 109, 3981–3987. Schzecner, B.; Achmatowicz, O. J. Carbohydrate Chem. 1992, 11, 401–406. Poss, M.A.; Reid, J.A. Tetrahedron Lett. 1992, 33, 1411–1414. Raczcko, J.; Golebiowski, A.; Krajewski, J.W.; Gluzinski, P.; Jurczack, J. Tetrahedron Lett. 1990, 31, 3797–3800. Tsubuki, M.; Kanai, K.; Keino, K.; Kakinuma, N.; Honda, T. J. Org. Chem 1992, 57, 2930–2934. [CrossRef] Soai, K.; Kawase, Y. J. Chem. Soc. Perkin Trans. 1 1990, 3214–3215. [CrossRef] Mukaiyama, T.; Suzuki, K.; Yamada, T.; Tabusa, F. Tetrahedron 1990, 46, 265–276. Class, Y.J.; DeShong, P. Tetrahedron Lett. 1995, 36, 7631–7634. Sczehner, B.; Achmatowicz, O.; Galdecki, Z.; Fruzinski, A. Tetrahedron 1994, 50, 7611–7624. Martin, S.F.; Chen, H.-J.; Yang, C.-P. J. Org. Chem. 1993, 58, 2867–2873. [CrossRef] For additions to ketones see: Georgiadis, M.P.; Tsekouras, A.; Kotretsou, S.I.; Haroutounian, S.A.; Polissiou, M.G. Synthesis 1991, 929–932. Georgiadis, M.P.; Haroutounian, S.A.; Apostolopouls, C.D. Synthesis 1991, 379–381. Georgiadis, M.P.; Couladouros, E.A. J. Org. Chem. 1986, 51, 2725–2727. Dinesh, C.U.; Kumar, P.; Reddy, R.S.; Pandey, B.; Puranik, V.G. Tetrahedron: Asymm. 1995, 6, 2961–2970.
- Wang, H.; Yan, S.G.; Hu, X.Q.; Guo, H.F. Huaxue Xuebao 1993, 51, 393–398. Stolze, D.A.; Perron-Sierra, T.; Heeg, M.J.; Albizati, K.F. Tetrahdron Lett. 1991, 32, 4081–4084. Dondoni, A.; Marra, A.; Scherrmann, M.-C. Tetrahedron Lett. 1993, 34, 7323–7326. Yamazaki, T.; Mizutani, K.; Kitazume, T. J. Org. Chem. 1993, 58, 4346–4359. [CrossRef] Albrigh, J.D.; Howell, C.F.; Sum, F.W. Heterocycles 1993, 35, 737–754.
- Nahm, S.; Weinreb, S.M. Tetrahedron Lett. 1981, 22, 3815–3818.
- Parkes, K.E.B.; Richardson, S.K. Ketones: Dialkyl ketones. In Comprehenseive Organic Functional Group Transformations; Katritzky, A.R., Meth-Cohn, O., Rees, C.W., Eds.; Pergamon, 1995; vol.3, p. 131. [Google Scholar]
- Ageno, G.; Banfi, L.; Cascio, G.; Guanti, G.; Manghisi, E.; Riva, R.; Rocca, V. Tetrahedron 1995, 51, 8121–8134.
- Garner, P.; Park, J.M. Org. Synth 1991, 70, 18–28. McKillop, A.; Taylor, R.J.K.; Watson, R.J.; Lewis, N. Synthesis 1994, 31–33.
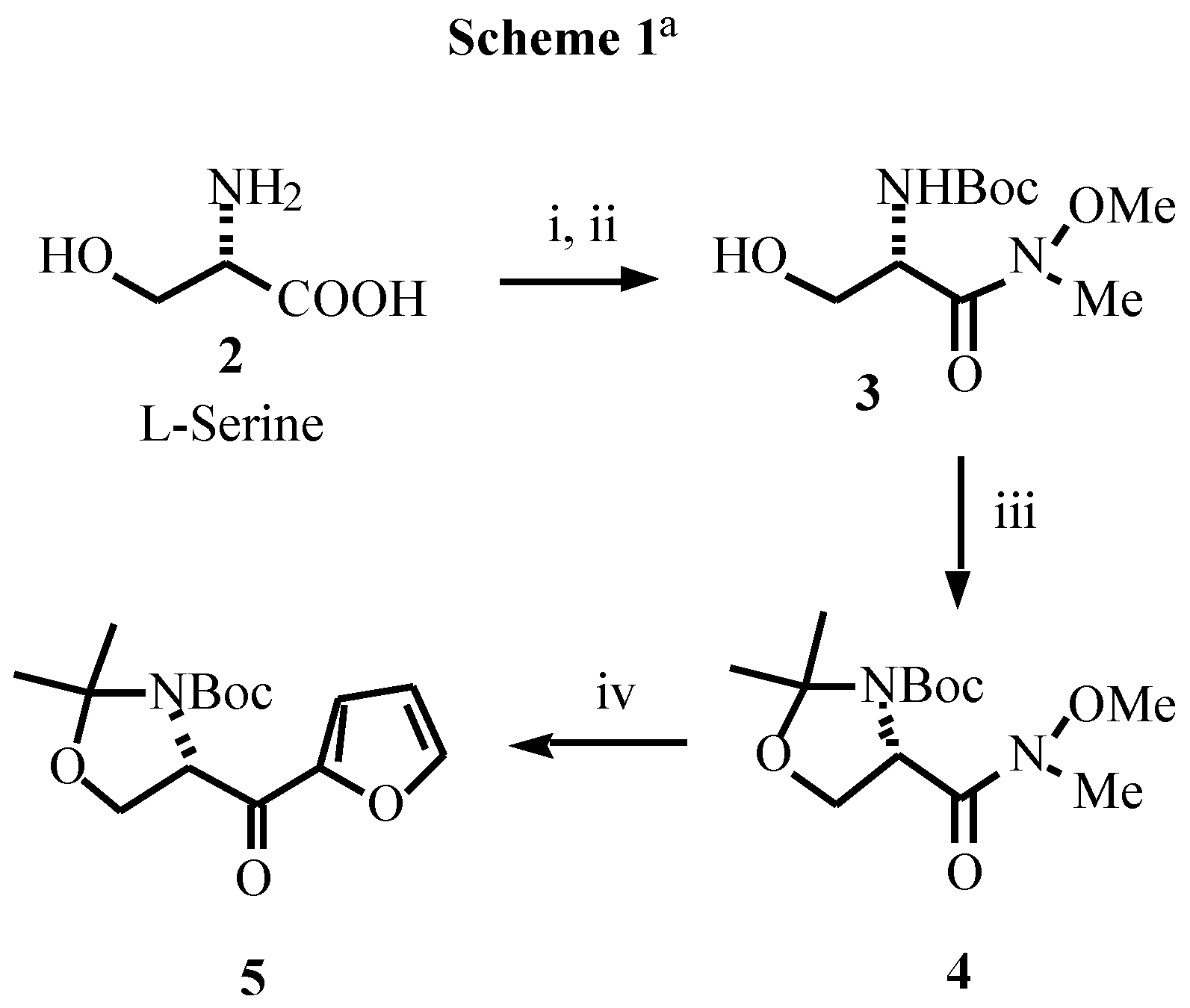
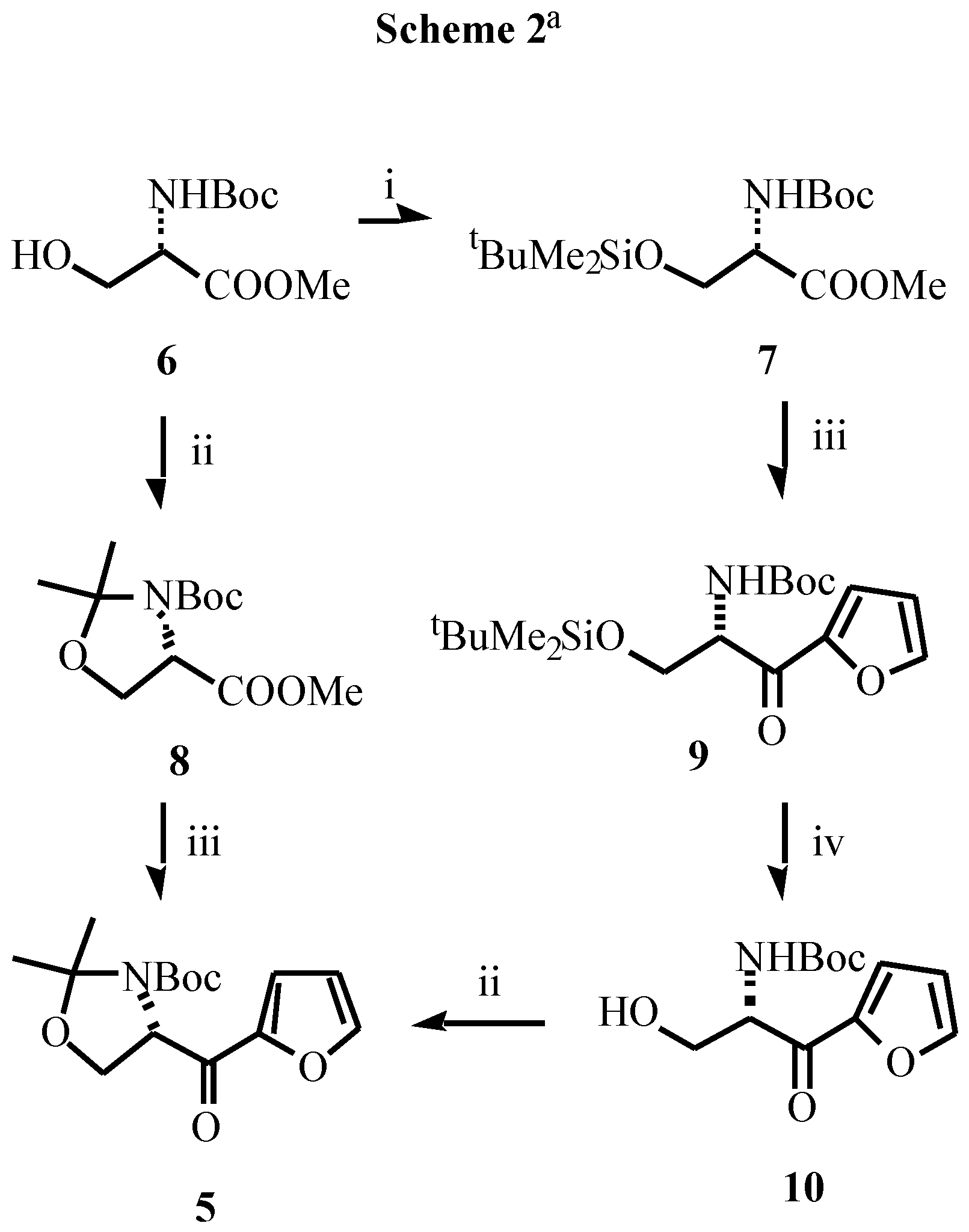
- Typical experimental procedure: To a cold (-80 °C) stirred solution of butyllithium (2.63 mL, 4.2 mmol of 1.6M solution in hexanes) in THF (10 mL), was added, dropwise, a solution of furan (0.272 g, 0.29 mL, 4 mmol) in the same solvent (10 mL). After the solution had been stirred at -80 °C for 5 min and at 0 °C for 2 h, the resulting mixture was cooled to -80 °C and a solution of the corresponding ester (3.86 mmol of 8 or 1.93 mmol of 9) in THF (15 mL) was added slowly. The mixture was allowed to warm to -40 °C, stirred at this temperature for 4 h, and saturated aqueous NaHCO3 (10 mL) was then added. The mixture was allowed to warm to room temperature over 15 min, diethyl ether was added (10 mL) and the layers were separated. The aqueous layer was extracted with diethyl ether (2 x 15 mL). The combined organic extracts were washed with brine (10 mL), dried over MgSO4 and concentrated. The residue was purified by column chromatography to give the pure products. Data for 5: white solid; mp 122-123 °C; [α]D -32.9 (c 0.52, CHCl3); IR (νC=O) 1695, 1678 cm-1; 1H NMR (CDCl3, 55 °C) δ1.28 (s, 3H), 1.57 (s, 3H), 1.71 (s, 9H), 3.95 (dd, 1H, J = 8.8, 3.4 Hz), 4.26 (dd, 1H, J = 8.8, 7.3 Hz), 5.15 (m, 1H), 6.5 (bs, 1H), 7.23 (m, 1H), 7.57 (m, 1H). Data for 9: oil; [α]D -4.9 (c 0.67, CHCl3); IR (νC=O) 1690, 1672 cm-1; 1H NMR (CDCl3) δ-0.10 (s, 3H), -0.08 (s, 3H), 0.75 (s, 9H), 1.42 (s, 9H), 3.90 (dd, 1H, J = 10.1, 4.7 Hz), 4.05 (dd, 1H, J = 10.1, 3.6 Hz), 5.05 (ddd, 1H, J = 10.1, 8.2, 3.6 Hz), 5.55 (bd, 1H, J = 8.2 Hz), 6.52 (dd, 1H, J = 3.5, 1.5 Hz), 7.28 (dd, 1H, J = 3.5, 1.0 Hz), 7.05 (dd, 1H, J = 1.5, 1.0 Hz).
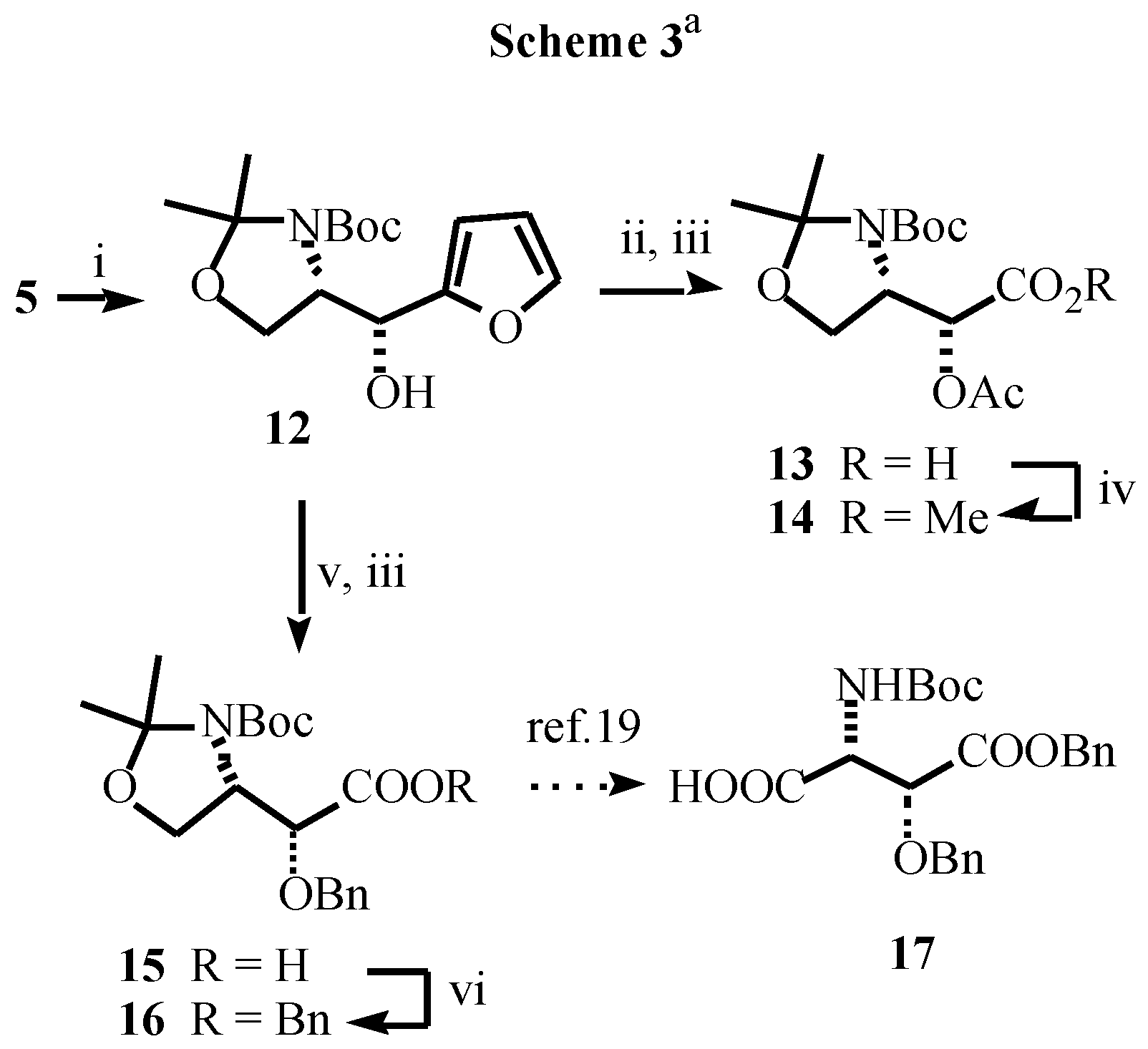
- For the reduction of α-amino ketones see: Dondoni, A.; Merino, P.; Perrone, D. Tetrahedron 1993, 49, 2939–2956. Dondoni, A.; Perrone, D. Synthesis 1993, 1162–1176. Dondoni, A.; Perrone, D.; Merino, P. Chem. Commun. 1991, 1313–1316. For the reduction of a-alkoxy ketones see: Dondoni, A.; Orduna, J.; Merino, P. Synthesis 1992, 201–208.
- Data for 12: sticky oil; [α]D -5.7 (c 0.40, CHCl3); 1H NMR (CDCl3+D2O, 55 °C) δ1.48 (s, 3H), 1.51 (s, 9H), 1.53 (s, 3H), 3.75 (bt, 1H, J = 5.4 Hz), 3.85 (dd, 1H, J = 9.5, 6.1 Hz), 4.35 (bt, 1H, J = 6.8 Hz), 4.78 (d, 1H, J = 8.8 Hz), 6.29 (d, 1H, J = 3.0 Hz), 6.31 (dd, 1H, J = 3.0, 1.6 Hz), 7.38 (d, 1H, J = 1.6 Hz)
- Cherest, M.; Felkin, H.; Prudent, N. Tetrahedron Lett. 1968, 2199–2202. Anh, N.T. Top. Curr. Chem. 1980, 88, 145–162. Wu, Y.-D.; Houk, K.N. J. Am. Chem. Soc. 1987, 109, 908–910. [CrossRef]
- Data for 14: oil; [α]D -65.3 (c 0.81, CHCl3), Lit. [15b]: [α]D -64.2 (c 0.60, CHCl3) ; 1H NMR (DMSO-d6, 115 °C) δ1.62 (s, 3H), 1.65 (s, 9H), 1.69 (s, 3H), 2.10 (s, 3H), 3.66 (s, 3H), 3.95 (m, 2H), 4.20 (m, 1H), 5.21 (bd, 1H, J = 5.2 Hz).
- Wagner, R.; Tilley, J.W. J. Org. Chem. 1990, 55, 6289–6291. [CrossRef]
- The importance of β-hydroxy aspartic acids is welldocumented in the literature. See: Fernandez-Megia, E.; Paz, M.M.; Sardina, F.J. J. Org. Chem. 1994, 59, 7643–7652. [CrossRef] Palomo, C.; Cabre, F.; Ontoria, J.M. Tetrahedron Lett. 1992, 33, 4819–4822. Sardina, F.J.; Paz, M.M.; Fernandez-Megía, E.; deBoer, R.; Alvarez, M.P. Tetrahedron Lett. 1992, 33, 4637–4640. Hansson, T.G.; Kihlberg, J.O. J. Org. Chem. 1986, 51, 4490–4492. [CrossRef]
- Sample Availability: Available from the authors.
© 1998 MDPI. All rights reserved
Share and Cite
Merino, P.; Franco, S.; Merchan, F.L.; Tejero, T. Nucleophilic Additions of 2-Furyllithium to Carbonyl Derivatives of L-Serine. Formal Synthesis of (2R,3R)-β-Hydroxy Aspartic Acid. Molecules 1998, 3, 26-30. https://doi.org/10.3390/30100026
Merino P, Franco S, Merchan FL, Tejero T. Nucleophilic Additions of 2-Furyllithium to Carbonyl Derivatives of L-Serine. Formal Synthesis of (2R,3R)-β-Hydroxy Aspartic Acid. Molecules. 1998; 3(1):26-30. https://doi.org/10.3390/30100026
Chicago/Turabian StyleMerino, P., S. Franco, F. L. Merchan, and T. Tejero. 1998. "Nucleophilic Additions of 2-Furyllithium to Carbonyl Derivatives of L-Serine. Formal Synthesis of (2R,3R)-β-Hydroxy Aspartic Acid" Molecules 3, no. 1: 26-30. https://doi.org/10.3390/30100026



