NMR spectra were recorded with a Varian GEMINI-200 spectrometer operating at 200 MHz for 1H and 50 MHz for 13C. Coupling constants J are expressed in hertz [Hz]. Two-dimensional HETCOR GHMBC spectra were recorded with a Varian INOVA-500 spectrometer (500 MHz / 125 MHz). The assignments of the chemical shifts accompanied by asterisks*) may be exchanged. 13C NMR spectra were assigned on the basis of literature data and theoretical calculations using the ACD/Labs program. 13C NMR of purine N-oxides 11a-e are discussed in Chapter 2.2.2. IR spectra were measured with a Perkin Elmer 1600 FTIR spectrometer and mass spectra with an AMD 604 (AMD Intectra GmbH, Germany) spectrometer (electron impact and LSIMS methods); m/z values are given as a % of relative intensity. Melting points are uncorrected. TLC analysis was performed on aluminum foil plates precoated with silica gel 60F 254 Merck. Silica gel 200-300 mesh (Merck AG) was used for column chromatography.
N-Benzyl- and
N-methyl-4-nitroimidazoles (
1a,
b) were prepared from commercial 4-nitroimidazole (Fluka AG) by alkylation with CH
3I and PhCH
2Br in
t-BuOK/DMSO system ([1
H]-products were formed selectively and the yields were higher as compared to those described earlier in the literature [
18,
19]; see procedures below). The starting Schiff bases
13a-
h were prepared from imidazolecarbaldehydes according to typical procedures [
20,
21], optimized in our laboratory.
para-Cyanoaniline was obtained by catalytic reduction of
para-cyanonitrobenzene (10% Pd/C, 40 psi, 2 h, in EtOH); quantitatively, mp 90-91°C (EtOH), lit. mp 86°C [
22]. Other aniline derivatives used are commercially available.
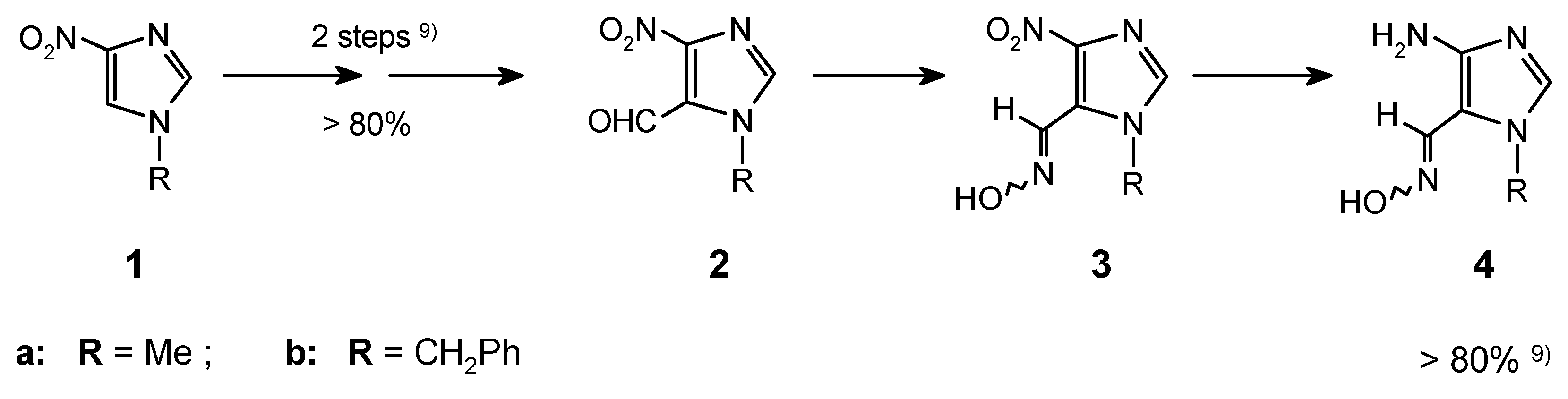
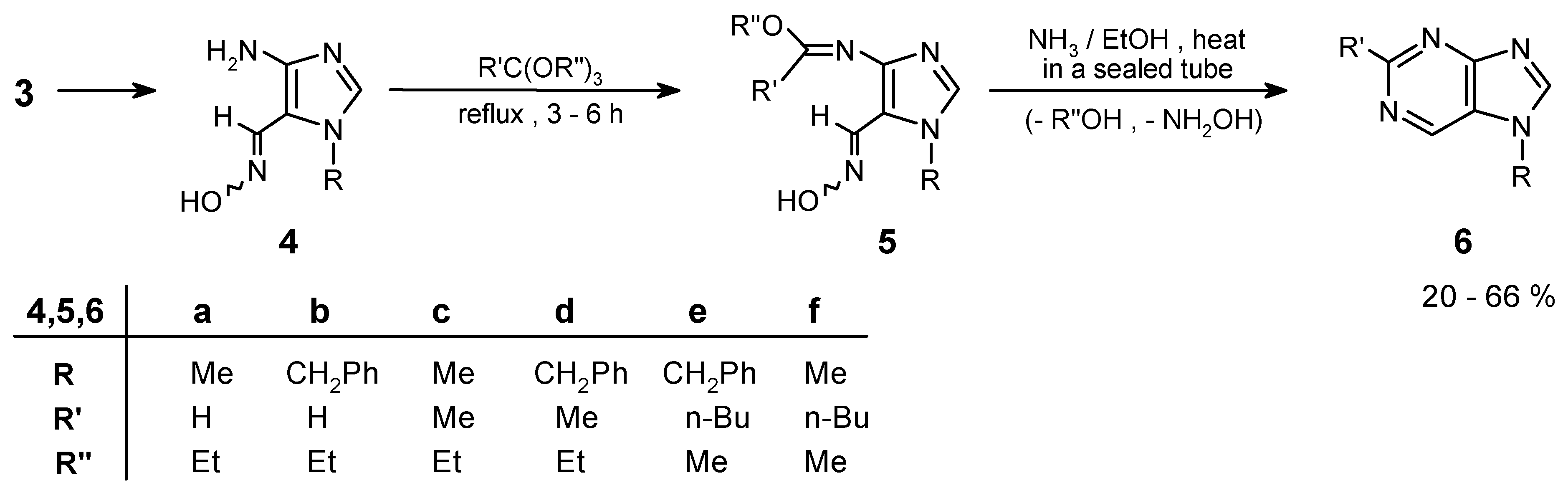
Preparation of Purines 6a-f. General Procedure
The 4-nitro-5-imidazole carbaldehyde oxime derivative (
3a,
3b; 0.5 mmol) [
9] was dissolved in ethanol (5 mL) and it was hydrogenated in Parr Apparatus using a catalytic amount of 10% Pd/C (
ca 20 mg) at 40 psi during 4 h. The catalyst was filtered off, washed with alcohol and the filtrate after evaporation to dryness gave crude aminooximes
4a,
4b quantitatively.
The above aminooximes were suspended in the appropriate orthoester (HC(OEt)3, MeC(OEt)3, n-BuC(OMe)3, 5 mL) and the reaction mixture was heated in reflux until completion (1.5-4 h; except for 5e: 6 h). The excess of the orthoester was removed under reduced pressure and the residue (crude imidates 5a-f) was dissolved in ethanol saturated with ammonia (4 mL). The reaction mixture was heated in a sealed tube at 120-130°C for 2-3 h (except for 6e: 200°C, 6 h). After cooling and evaporation of the solvent the purines were isolated by column chromatography (eluent: from CHCl3 to CHCl3/MeOH - 20:1; except for 6a: from CHCl3 to CHCl3/MeOH - 8:1).
Examples of 1H NMR data for crude imidates 5b-d are given below. Another imidate (5g) is described in the next paragraph.
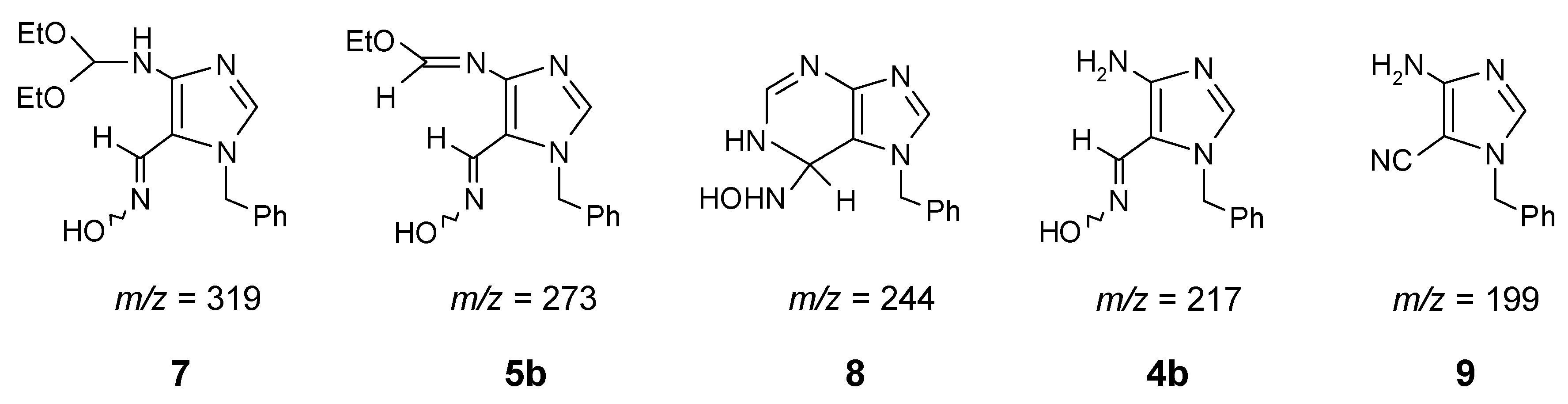
N-[1-Benzyl-5-(hydroxyiminomethyl)-1H-imidazol-4-yl]-formimidic acid ethyl ester (5b)
Crude (E/Z isomers). - 1H NMR (CDCl3, main isomer): δ = 8.49 (s, 1H, (EtO)CH=N), 8.32 (s, 1 H, CH-oxime), 7.37 (s, 1 H, H-2), 7.35-7.12 (m, 5 H, H-Ph), 5.44 (s, 2 H, CH2Ph), 4.30 (q, J = 7.2 Hz, 2 H, CH2CH3), 1.35 (t, J = 7.2 Hz, 3 H, CH2CH3), OH - undetected.
N-[5-(Hydroxyiminomethyl)-1-methyl-1H-imidazol-4-yl]-acetimidic acid ethyl ester (5c)
Crude, ca 80% purity (E/Z isomers). - 1H NMR (CDCl3, main isomer): δ = 8.10 (s, 1H, CH-oxime), 7.29 (s, 1 H, H-2), 4.23 (q, J = 7.1 Hz, 2 H, CH2CH3), 3.78 (s, 3 H, NCH3), 2.10 (s, 3 H, CH3), 1.31 (t, J = 7.1 Hz, 3 H, CH2CH3), OH - undetected. - IR (CHCl3): v = 1664 (C=N imidate), 1621 cm-1 (C=N oxime). - LSIMS (+), m/z (% rel. int.): 211 (M+H, 52).
N-[1-Benzyl-5-(hydroxyiminomethyl)-1H-imidazol-4-yl]-acetimidic acid ethyl ester (5d)
Crude (E/Z isomers). - 1H NMR (CDCl3, main isomer): δ = 8.15 (s, 1H, CH-oxime), 7.42-7.10 (m, 6 H, H-Ph & H-2), 5.46 (s, 2 H, CH2Ph), 4.23 (q, J = 7.1 Hz, 2 H, CH2CH3), 2.15 (s, 3 H, CH3), 1.31 (t, J = 7.1 Hz, 3 H, CH2CH3), OH - undetected.
7-Methyl-7H-purine (6a)
Yield 60%, mp 176-178°C (CHCl
3), lit. [
23a] mp 183-184°C. -
1H NMR (CDCl
3): δ = 9.17 (s, 1 H, H-2), 8.98 (s, 1 H, H-6), 8.20 (s, 1 H, H-8), 4.00 (s, 3 H, NCH
3). - MS,
m/z (% rel. int.): 134 (M
+ ·, 100), 107 (21), 81 (16), 80 (16), 57 (25), 56 (23), 42 (67); HR-MS calcd. for C
6H
8N
4 - 134.0593, found - 134.0593.
7-Benzyl-7H-purine (6b)
Yield 66%, data for this compound - see lit. [
1].
2,7-Dimethyl-7H-purine (6c)
Yield 49%, mp 85°C (CHCl3/MeOH). - 1H NMR (CDCl3): δ = 8.85 (s, 1 H, H-6), 8.13 (s, 1 H, H-8), 3.95 (s, 3 H, NCH3), 2.84 (s, 3 H, CH3). - 13C NMR (CDCl3): δ = 162.8 (C-2), 157.3 (C-4), 148.3 (C-8), 139.4 (C-6), 125.1 (C-5), 32.0 (NCH3), 25.9 (CH3). - MS, m/z (% rel. int.): 148 (M+ ·, 100), 147 (23), 134 (8), 121 (11), 120 (20), 106 (10), 94 (13), 80 (12), 67 (9), 53 (17), 52 (17), 42 (35); HR-MS calcd. for C7H8N4 - 148.0749, found - 148.0744. - LSIMS (+), m/z (% rel. int.): 149 (M+H, 100).
7-Benzyl-2-methyl-7H-purine (6d)
Yield 20%, mp 146-149°C (CHCl3/ether). - 1H NMR (CDCl3): δ = 8.62 (s, 1 H, H-6), 8.23 (s, 1 H, H-8), 7.42-7.18 (m, 5 H, H-Ph), 5.41 (s, 2 H, CH2), 2.83 (s, 3 H, CH3). - 13C NMR (CDCl3): δ = 162.7 (C-2), 161.6 (C-4), 147.8 (C-8), 140.1 (C-6), 133.7, 129.3 (2xC), 128.9, 127.4 (2xC) [C-Ph], 123.0 (C-5), 50.2 (CH2), 25.9 (CH3). - MS, m/z (% rel. int.): 224 (M+ ·, 36), 223 (17), 91 (100), 65 (19); HR-MS calcd. for C13H12N4 - 224.1062, found - 224.1062.
7-Benzyl-2-butyl-7H-purine (6e)
Yield 31%, mp 103-106°C (CHCl3/MeOH). - 1H NMR (CDCl3): δ = 8.67 (s, 1 H, H-6), 8.25 (s, 1 H, H-8), 7.43-7.32 & 7.30-7.15 (2 x m, 5 H, H-Ph), 5.41 (s, 2 H, CH2Ph), 3.08 (t, J = 7.7 Hz, 2 H, CH2C3H7), 1.94-1.30 (m, 4 H, 2 x CH2), 0.93 (t, J = 7.2 Hz, 3 H, CH3). - 13C NMR (CDCl3): δ = 166.2 (C-2), 147.9 (C-8), 139.9 (C-6), 133.7, 129.4 (2xC), 129.1, 127.5 (2xC) [C-Ph], 123.2 (C-5), 50.3 (CH2Ph), 39.1, 31.3, 22.6, 14.0 [C4H9], C-4 - undetected. - MS, m/z (% rel. int.): 266 (M+ ·, 7), 251 (3), 237 (8), 224 (38), 91 (100), 65 (12); HR-MS calcd. for C16H18N4 - 266.1531, found - 266.1539. - LSIMS (+), m/z (% rel. int.): 267 (M+H, 59), 91 (100). - Elemental analysis for C16H18N4 (266.35): calcd. C 72.15, H 6.81, N 21.04; found C 71.61, H 6.42, N 21.16.
2-Butyl-7-methyl-7H-purine (6f)
Yield 35%, semi-crystalline. - 1H NMR (acetone-d6): δ = 9.01 (s, 1 H, H-6), 8.37 (s, 1 H, H-8), 4.04 (s, 3 H, NCH3), 2.97 (t, J = 7.0 Hz, 2 H, CH2C3H7), 1.92-1.20 (m, 4 H, 2 x CH2), 0.87 (t, J = 7.2 Hz, 3 H, CH3). - 13C NMR (CDCl3): δ = 166.2 (C-2), 161.3 (C-4), 148.3 (C-8), 139.4 (C-6), 123.8 (C-5), 39.2, 32.0, 31.4, 22.6, 14.0 [C4H9 & NCH3]. - MS, m/z (% rel. int.): 190 (M+ ·, 6), 175 (12), 162 (7), 161 (33), 149 (10), 148 (100), 147 (8), 42 (9); HR-MS calcd. for C10H14N4 - 190.1218, found - 190.1219.
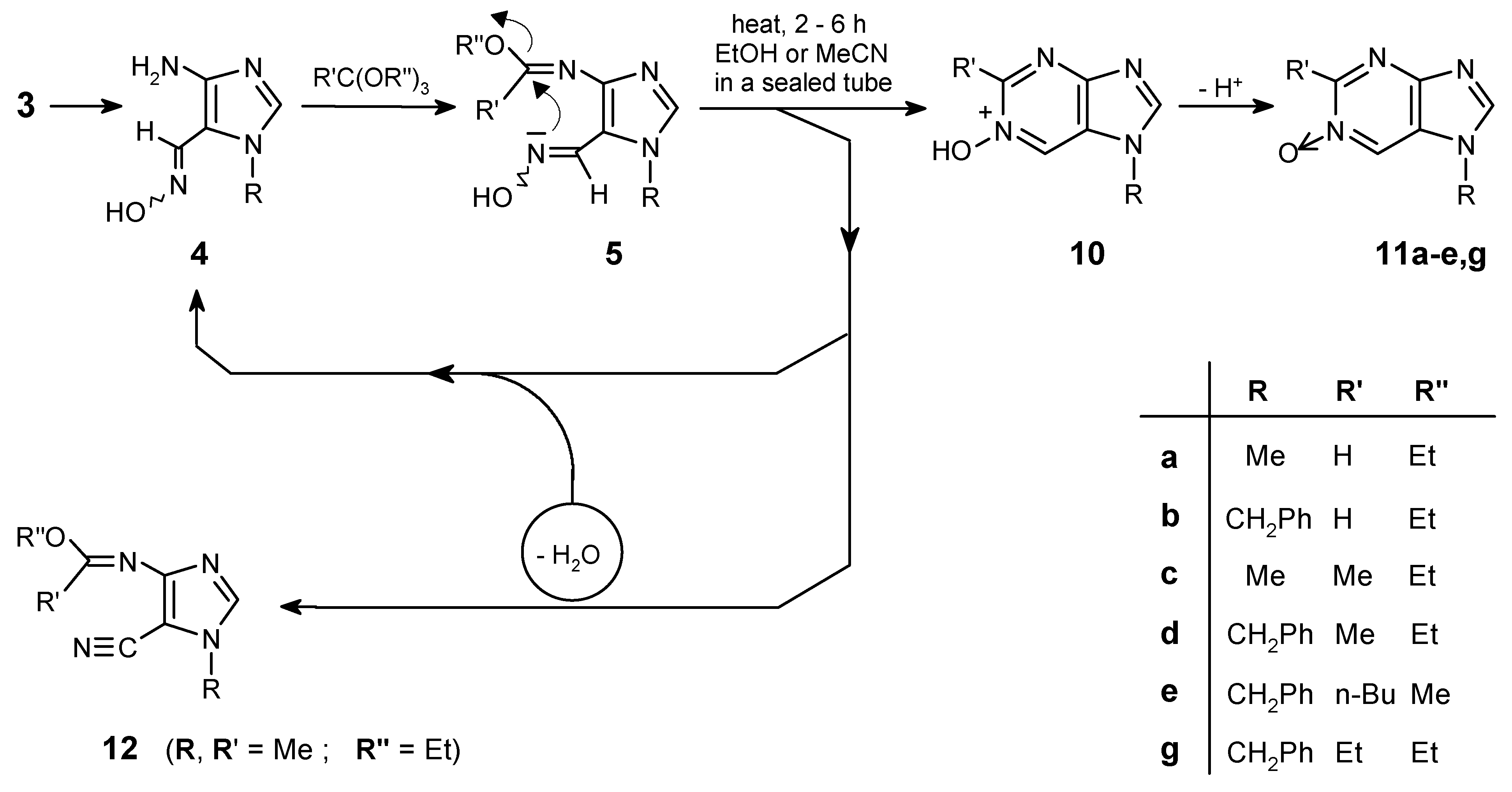
Preparation of Purine mono-N-Oxides (11a-e, g). General Procedure
The intermediates 5a-e,g were prepared according to the procedure used previously for preparation of purines 6a-f.
The appropriate imidate
5a-
e,
g was dissolved in a dry ethanol or dry acetonitrile (4 mL for
ca 50 mg) and the reaction mixture was heated in a sealed tube for 2-4 h at 100-200°C (see
Table 1). After cooling and evaporation of the solvent the residue was purified by chromatography to give the desired purine
N-oxides (eluent: from CHCl
3 to CHCl
3/MeOH - 20:1; except for
11a: from CHCl
3 to CHCl
3/MeOH - 5:1). Yields are given in
Table 1,
13C NMR spectra - in
Table 2.
As the transformation of 5g to N-oxide 11g proved somewhat troublesome, an analytical sample of 5g was purified by column chromatography for characterization (the yield was not determined).
N-[1-Benzyl-5-(hydroxyiminomethyl)-1H-imidazol-4-yl]-propionimidic acid ethyl ester (5g)
Thick oil. - 1H NMR (CDCl3): δ = 8.06 (s, 1 H, CH-oxime), 7.37-7.09 (m, 6 H, H-Ph & H-2), 5.40 (s, 2 H, CH2Ph), 4.25 (q, J = 7.1 Hz) & 4.24 (q, J = 7.1 Hz) [2 H, OCH2CH3, E/Z isomers], 2.52 (q, J = 7.6 Hz) & 2.51 (q, J = 7.6 Hz) [2 H, CH2CH3, E/Z isomers], 1.33 (t, J = 7.1 Hz) & 1.32 (t, J = 7.1 Hz) [3 H, OCH2CH3, E/Z isomers], 1.134 (t, J = 7.6 Hz) & 1.127 (t, J = 7.6 Hz) [3 H, CH2CH3, E/Z isomers], OH - undetected. - MS, m/z (% rel. int.): 300 (M+ ·, 31), 284 (6), 283 (27), 272 (2), 271 (2), 255 (9), 239 (7), 238 (9), 237 (12), 216 (7), 199 (9), 198 (6), 197 (5), 135 (6), 121 (8), 106 (5), 92 (10), 91 (100), 65 (9), 57 (10); HR-MS calcd. for C16H20N4O2 - 300.1586, found - 300.1586.
7-Methyl-7H-purine 1-oxide (11a)
Semi-crystalline. - 1H NMR (CDCl3/DMSO-d6 - 5:1): δ = 8.96 (s, 2 H, H-2 & H-6), 8.32 (s, 1 H, H-8), 3.93 (s, 3 H, NCH3). - MS, m/z (% rel. int.): 150 (M+ ·, <1), 135 (9), 134 (100), 107 (10), 106 (5), 80 (4), 79 (3), 66 (2), 53 (2), 52 (2), 42 (10); HR-MS calcd. for C6H6N4O - 150.0542, found - 150.0537.
7-Benzyl-7H-purine 1-oxide (11b)
Mp 223-224°C (subl.). - 1H NMR (CDCl3): δ = 8.91 (d, J = 1.8 Hz, 1 H, H-2), 8.43 (d, J = 1.8 Hz, 1 H, H-6), 8.28 (s, 1 H, H-8), 7.45-7.20 (m, 5 H, H-Ph), 5.34 (s, 2 H, CH2). - MS, m/z (% rel. int.): 226 (M+ ·, 12), 210 (38), 209 (13), 91 (100), 65 (12); HR-MS calcd. for C12H10N4O - 226.0855, found - 226.0854. - Elemental analysis for C12H10N4O x 1/2H2O (235.24): calcd. C 61.27, H 4.71, N 23.82; found C 62.34, H 4.69, N 22.41.
2,7-Dimethyl-7H-purine 1-oxide (11c)
Mp 190-192°(CHCl3/MeOH). - 1H NMR (CDCl3): δ = 8.85 (s, 1 H, H-6), 8.12 (s, 1 H, H-8), 3.95 (s, 3 H, NCH3), 2.85 (s, 3 H, CH3). - MS, m/z (% rel. int.): 164 (M+ ·, 2), 163 (5), 162 (4), 148 (100), 147 (23), 134 (35), 121 (10), 120 (16), 107 (9), 106 (10), 94 (8), 80 (10), 67 (4), 53 (8), 52 (7), 42 (24); HR-MS calcd. for C7H8N4O - 164.0698, found - 164.0696.
7-Benzyl-2-methyl-7H-purine 1-oxide (11d)
7-Benzyl-2-butyl-7H-purine 1-oxide (11e)
Mp 225-226°C (CHCl3 ). - 1H NMR (CDCl3): δ = 8.47 (s, 1 H, H-6), 8.22 (s, 1 H, H-8), 7.45-7.15 (m, 5 H, H-Ph), 5.31 (s, 2 H, CH2Ph), 3.15 (t, J = 7.7 Hz, 2 H, CH2C3H7), 1.96-1.78 & 1.55-1.33 (2 x m, 4 H, 2 x CH2), 0.96 (t, J = 7.2 Hz, 3 H, CH3). - MS, m/z (% rel. int.): 282 (M+ ·, 2), 266 (12), 265 (42), 224 (19), 198 (6), 91 (100), 65 (8); HR-MS calcd. for C16H18N4O - 282.1481, found - 282.1479. - LSIMS (+), m/z (% rel. int.): 283 (M+H, 100), 91 (91).
7-Benzyl-2-ethyl-7H-purine 1-oxide (11g)
Semi-crystalline. - 1H NMR (CDCl3): δ = 8.65 (s, 1 H, H-6), 8.24 (s, 1 H, H-8), 7.50-7.18 (m, 5 H, H-Ph), 5.41 (s, 2 H, CH2Ph), 3.09 (q, J = 7.6 Hz, 2 H, CH2CH3), 1.40 (t, J = 7.6 Hz, 3 H, CH2CH3). - MS, m/z (% rel. int.): 254 (M+ ·, 6), 253 (5), 252 (4), 238 (22), 237 (14), 210 (5), 147 (9), 134 (10), 106 (15), 91 (100), 77 (14), 65 (17), 44 (9), 39 (8); HR-MS calcd. for C14H14N4O - 254.1168, found - 254.1165.
N-(5-Cyano-1-methyl-1H-imidazol-4-yl)-acetimidic acid ethyl ester (12)
This was obtained as a by-product in the reaction leading to purine N-oxide 11c (yield less than 10%); semi-crystalline, unstable. - 1H NMR (CDCl3): δ = 7.36 (s, 1 H, H-2), 4.28 (q, J = 7.1 Hz, 2 H, CH2), 3.73 (s, 3 H, NCH3), 2.09 (s, 3 H, CH3), 1.33 (t, J = 7.1 Hz, 3 H, CH2CH3). - IR (CHCl3): v = 2219 (CN), 1662 cm-1 (C=N). - MS, m/z (% rel. int.): 192 (M+ ·, 39), 177 (3), 164 (11), 151 (11), 150 (24), 149 (51), 148 (15), 147 (19), 135 (14), 122 (100), 107 (18), 95 (6), 67 (14), 43 (63), 42 (18); HR-MS calcd. for C9H12N4O - 192.1011, found - 192.1026. - LSIMS (+), m/z (% rel. int.): 193 (M+H, 100).
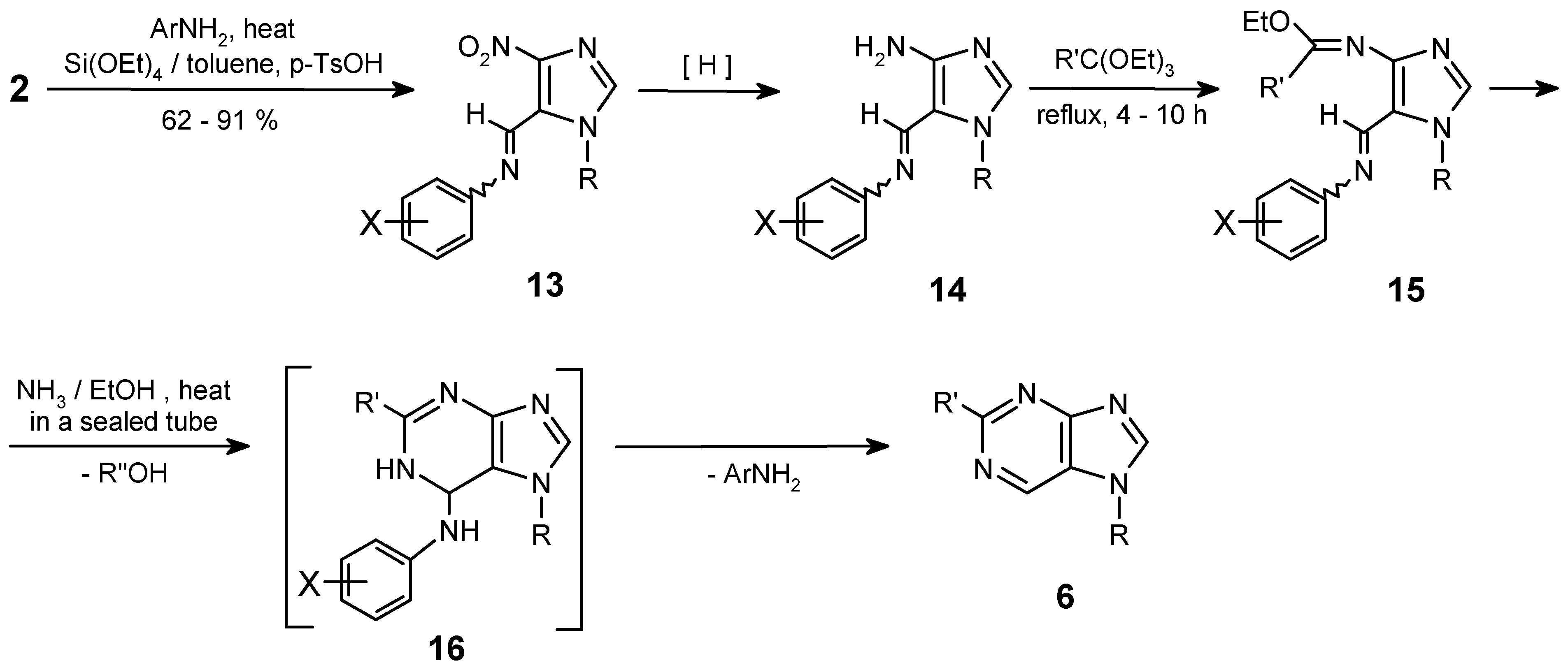
Transformations to Schiff bases
Procedure A. - To a solution of aldehyde (2a, 2b correspondingly; 0.50 mmol) and aniline derivative (0.70 mmol) in toluene (5 mL) a catalytic amount of p-TsOH acid (5 mg, 0.03 mmol) was added and the mixture was heated to reflux for 2.5-3 h. After cooling and evaporation to dryness, crude products were purified by chromatography (CHCl3 as eluent) to give a mixture of E/Z isomers (products 13a, 13b and 13h were obtained).
Procedure B. - Imidazole aldehyde (2a, 2b correspondingly; 1.0 mmol), the proper aniline derivative (1.20 mmol), a catalytic amount of p-TsOH acid (5 mg, 0.03 mmol) and Si(OEt)4 (7.0 g) were dissolved in toluene (30 mL) and the reaction mixture was heated to reflux for 3-20 h until the aldehyde disappeared (TLC monitoring). After cooling, the solvent and most of the Si(OEt)4 was evaporated. The residue was recrystallized from ethanol or purified by chromatography (eluent CHCl3 or CHCl3/MeOH - 20:1) to give pure Schiff bases (mixture of E/Z isomers). Products 13c-g were obtained.
Data for products
13a and
13b were described earlier [
9].
4-{[(E/Z)-1-(1-Methyl-4-nitro-1H-imidazol-5-yl)methylidene]amino}benzonitrile (13c)
Solid, yield 66%. - 1H NMR (CDCl3, main isomer in E/Z mixture): δ = 9.19 (s, 1 H, CH=N), 7.74 (apparent d, J = 8.6 Hz, 2 H, H-Ar), 7.56 (s, 1 H, H-2), 7.31 (apparent d, J = 8.6 Hz, 2 H, H-Ar), 4.15 (s, 3 H, CH3). - MS, m/z (% rel. int.): 255 (M+ ·, 53), 239 (15), 238 (100), 225 (27), 210 (73), 208 (62), 207 (38), 196 (10), 184 (15), 183 (10), 182 (12), 181 (14), 180 (12), 168 (15), 167 (40), 155 (13), 141 (13), 138 (33), 129 (18), 128 (10), 114 (22), 108 (29), 102 (45), 84 (21), 81 (13), 67 (23), 42 (73). - Elemental analysis for C12H9N5O2 (255.24): calcd. C 56.47, H 3.55, N 27.44; found C 56.38, H 3.42, N 27.35.
4-{[(E/Z)-1-(1-Benzyl-4-nitro-1H-imidazol-5-yl)methylidene]amino}benzonitrile (13d)
Solid, yield 62%. - 1H NMR (CDCl3 main isomer in E/Z mixture): δ = 9.12 (s, 1 H, CH=N), 7.70 (apparent d, J = 8.3 Hz, 2 H, H-ArCN), 7.61 (s, 1 H, H-2), 7.42-7.35 (m, 2 H, H-Ar), 7.23-7.13 (m, 5 H, H-Ar), 5.79 (s, 2 H, CH2). - MS, m/z (% rel. int.): 331 (M+ ·, 20), 314 (13), 284 (27), 226 (14), 225 (100), 180 (17), 129 (9), 91 (72), 65 (10). - Elemental analysis for C18H13N5O2 x 1/2H2O (340.34): calcd. C 63.52, H 4.15, N 20.58; found C 63.51, H 4.03, N 19.98.
N-[(E/Z)-1-(1-Benzyl-4-nitro-1H-imidazol-5-yl)methylidene]-N-(2,4,6-tribromophenyl)-amine (13e)
Yield 67%, one isomer only, mp 173-174°C (CHCl3). - 1H NMR (CDCl3): δ = 9.04 (s, 1 H, CH=N), 7.73 (s, 2 H, H-Ar(Br)3), 7.57 (s, 1 H, H-2), 7.43-7.18 (m, 5 H, H-Ph), 5.86 (s, 2 H, CH2). - MS, m/z (% rel. int.): 546 (4), 544 (12), 542 (12), and 540 (4) [isotopic M+ ·], 527 (6), 525 (6), 497 (8), 496 (5), 495 (8), 465 (6), 463 (11), 461 (6), 433 (7), 418 (8), 416 (7), 359 (48), 357 (100), 355 (50), 91 (80), 65 (10). - Elemental analysis for C17H11N4Br3O2 (543.01): calcd. C 37.60, H 2.04, N 10.32; found C 37.69, H 2.18, N 10.34.
N-[(E/Z)-1-(1-Benzyl-4-nitro-1H-imidazol-5-yl)methylidene]-N-tritylamine (13f)
Yield 71%, one isomer only, mp 167-168°C (EtOH). - 1H NMR (CDCl3): δ = 8.59 (s, 1 H, CH=N), 7.52 (s, 1 H, H-2), 7.33-7.24 & 7.07-6.94 (2 x m, 20 H, H-Ph), 5.85 (s, 2 H, CH2). - MS, m/z (% rel. int.): 472 (M+ ·, 0.1), 244 (20), 243 (100), 228 (6), 165 (28), 91 (5). - Elemental anal. for C30H24N4O2 (472.55): calcd. C 76.25, H 5.12, N 11.86; found C 76.07, H 5.11, N 11.75.
N-(4-Bromophenyl)-N-[(E/Z)-1-(1-methyl-4-nitro-1H-imidazol-5-yl)methylidene]-amine (13g)
Solid, yield 90%. - 1H NMR (CDCl3, main isomer in E/Z mixture, >95%): δ = 9.20 (s, 1 H, CH=N), 7.56 (apparent d, J = 7.5 Hz, 2 H, H-Ar), 7.51 (s, 1 H, H-2), 7.16 (apparent d, J = 7.5 Hz, 2 H, H-Ar), 4.14 (s, 3 H, CH3). - MS, m/z (% rel. int.): 310 (99) & 308 (100) [isotopic M+ ·], 293 (74), 291 (74), 265 (18), 264 (10), 263 (54), 262 (20), 261 (38), 260 (14), 239 (12), 237 (18), 236 (13), 235 (9), 234 (8), 229 (27), 222 (42), 220 (40), 212 (31), 199 (26), 198 (27), 184 (67), 182 (16), 169 (10), 157 (27), 155 (28), 138 (11), 108 (13), 84 (15), 81 (10), 76 (16), 75 (14), 67 (18), 42 (34); HR-MS calcd. for C11H9N4BrO2 - 307.9909, found - 307.9908. - Elemental analysis for C11H9N4BrO2 (309.12): calcd. C 42.74, H 2.93; found C 43.14, H 3.15.
N-[(E/Z)-1-(1-Benzyl-4-nitro-1H-imidazol-5-yl)methylidene]-N-(4-bromophenyl)-amine (13h)
Solid, yield 91%. - 1H NMR (CDCl3, main isomer in E/Z mixture): δ = 9.15 (s, 1 H, CH=N), 7.60-7.00 (m, 10 H, H-Ar & H-2), 5.81 (s, 2 H, CH2). - MS, m/z (% rel. int.): 386 (12) & 384 (12) [isotopic M+ ·], 369 (11), 367 (11), 339 (16), 338 (12), 337 (16), 336 (9), 280 (63), 278 (65), 262 (9), 260 (11), 235 (9), 233 (9), 199 (12), 183 (7), 172 (5), 171 (5), 157 (9), 155 (9), 129 (9), 91 (100), 65 (15); HR-MS calcd. for C17H13N4BrO2 - 384.0222, found - 384.0219. - LSIMS (+), m/z (% rel. int.): 387 (47) & 385 (49) [isotopic M+H], 91 (100). - Elemental analysis for C17H13N4BrO2 (385.22): calcd. C 53.01, H 3.40, N 14.54; found C 52.40, H 3.38, N 14.18.
Catalytic Hydrogenation of Schiff Bases 13a-h
The nitrocompound was dissolved in ethanol (5-10 mL for 100 mg) and it was hydrogenated in Parr apparatus using 10-30% of catalyst (10% Pd/C): 13a, 13b, 13g, 13h - 40 psi, 4 h; 13c, 13d - 25 psi, 4 h; 13e - 15 psi, 1 h; 13f - 25 psi, 5 h. The reactions were monitored on TLC (CHCl3/MeOH - 20:1). After the reduction, the catalyst was filtered off, washed with methanol, and the solvent was evaporated to give the crude products 14a-h.
Compounds
14a,
14b were described in the previous paper [
9]. For crude compound
14d MS spectrum was recorded and for compound
14g -
1H NMR and MS spectra are given.
4-{[(E/Z)-1-(4-Amino-1-benzyl-1H-imidazol-5-yl)methylidene]amino}benzonitrile (14d)
Crude, main product in the mixture (ca 90% purity). - MS, m/z (% rel. int.): 301 (M+ ·, 4), 210 (1), 192 (3), 118 (100), 91 (49), 64 (16); HR-MS calcd. for C18H15N5 - 301.1327, found - 301.1331.
N-[(E/Z)-1-(4-Amino-1-methyl-1H-imidazol-5-yl)methylidene]-N-(4-bromophenyl)-amine (14g)
Crude, ca 80% purity. - 1H NMR (DMSO-d6): δ = 8.98 (s, 1 H, CH=N), 8.06 (s, 1 H, H-2), 7.70-7.20 (m, 4 H, H-Ar), 3.70 (s, 3 H, CH3). - MS, m/z (% rel. int.): 280 (5) & 278 (5) [isotopic M+ ·]. - LSIMS (+), m/z (% rel. int.): 281 (91) & 279 (100) [isotopic M+H].
Transformation of Imines 14a-h to Purines
The above imines 14a-h (ca 100 mg) were suspended in the appropriate orthoester (HC(OEt)3, MeC(OEt)3, 5 mL) and the reaction mixture was heated to reflux until the reaction was complete (with HC(OEt)3: 14a, 14b, 14h - 4 h; 14c, 14e, 14f - 10 h; 14d, 14g - 6 h; with MeC(OEt)3: 14g - 4 h, 14h - 6 h).
The excess of the orthoester was removed under reduced pressure and the residue (crude imidates
15a-
j) was dissolved in ethanol saturated with ammonia (5 mL). The reaction mixture was heated in a sealed tube to give the desired purines (conditions for highest yields:
15a,
15b - 130°C, 4 h;
15c - 80°C, 10 h;
15d - 80°C, 4 h;
15e,
15f - 110°C, 6 h;
15g,
15i,
15j - 130°C, 3 h;
15h -140°C, 4 h. The products were isolated as it was described in synthesis
via oximes. Yields - see
Table 3.
N-(7-Benzyl-6,7-dihydro-1H-purin-6-yl)-N-(4-methylphenyl)-amine (16b)
The formation of this compound was observed occasionally (from 13b) with the yields less than 5-10%; attempts to isolate and purify this intermediate gave the sample of ca 80% purity, still contaminated with other by-products. - 1H NMR (acetone-d6, diagnostic signals): δ = 8.30 (s, broad, 1 H, NH), 8.28 (s, 1 H, H-2*)), 8.10 (s, 1 H, H-8*)), 7.52 & 7.12 (AA’XX’, H-Tol), 7.40-7.18 (m, H-Ph & CH*)), 5.68 (s, 2 H, CH2), 2.41 (s, 3 H, CH3). - MS, m/z (% rel. int.): 317 (M+ ·, 10), 316 (44), 315 (24), 225 (4), 91 (100), 65 (26), 59 (24), 57 (27), 55 (26), 43 (51).
N-(4-Cyanophenyl)-formamide (17)
Mp 186-190°C (CHCl
3), lit. [
24] mp 189-190°C. -
1H NMR (CDCl
3): δ = 8.45 (s, 1 H, CHO), 7.80- 7.60 (AA’XX’, 4 H, H-Ar), NH - undetected. - MS,
m/z (% rel. int.): 147 (M+1, 7), 146 (M
+ ·, 63), 119 (12), 118 (100), 117 (7), 91 (51), 90 (13), 64 (12), 63 (9).
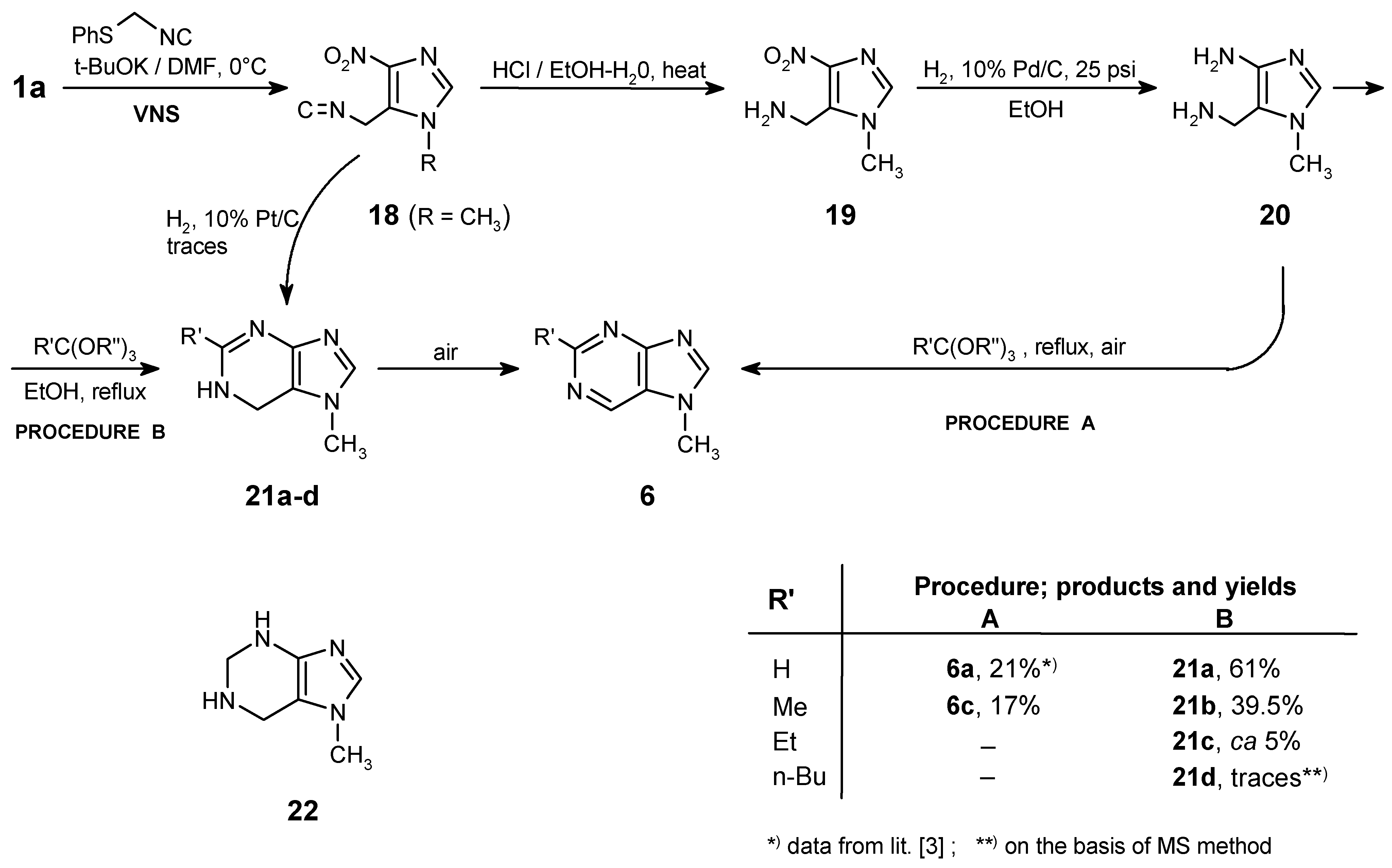
5-Isocyanomethyl-1-methyl-4-nitro-1H-imidazole (18)
To a stirred solution of t-BuOK (616 mg, 5.50 mmol) in anhydrous DMF (4 mL), cooled to 0°C, a solution of 1-methyl-4-nitro-1H-imidazole 1a (254 mg, 2.00 mmol) and phenylthiomethylisocyanide (333 mg, 2.23 mmol) in DMF (3 mL) was added dropwise via syringe - keeping the temperature at ca 0-3°C. After additional 0.5 h of stirring at this temperature the mixture was poured into crushed dry ice (ca 5 g) followed by slow addition of ethyl acetate (40 mL). The mixture was washed with cold brine (3 x 15 mL) and the organic layer was dried with anhydrous Na2SO4. After evaporation of the solvent the product was isolated by column chromatography using at the beginning CCl4, then CHCl3 as eluent. Yield 285 mg (86%); mp 68-70°C (CHCl3). - 1H NMR (CDCl3): δ = 7.50 (s, 1 H, H-2), 5.19 (s, 2 H, CH2), 3.87 (s, 3 H, NCH3). - MS, m/z (% rel. int.): 166 (M+ ·, 37), 140 (5), 134 (5), 120 (10), 119 (32), 109 (12), 95 (19), 94 (30), 93 (27), 83 (16), 81 (30), 68 (16), 67 (40), 66 (15), 55 (14), 54 (26), 53 (12), 52 (15), 42 (100); HR-MS calcd. for C6H6N4O2 - 166.0491, found - 166.0490.
Hydrolysis of Isocyanide 18
The isocyanide 18 (1.0 mmol) was dissolved in aqueous 80% ethanol (5 mL) and a four drops of concd. HCl were added. The mixture was refluxed for 3 h, evaporated to dryness and the crude product (as hydrochloride, quantitative yield) was recrystallized from methanol.
(1-Methyl-4-nitro-1H-imidazol-5-yl)methylamine (19)
As hydrochloride, mp 230-245°C (MeOH, decomp.). - 1H NMR (DMSO-d6): δ = 8.54 (s, broad, 3 H, NH3 +), 7.92 (s, 1 H, H-2), 4.34 (s, 2 H, CH2), 3.85 (s, 3 H, NCH3). - MS, m/z (% rel. int.): 157 (MH+, 4), 156 (1), 155 (3), 153 (3), 139 (49), 138 (58), 121 (31), 120 (17), 111 (8), 110 (12), 109 (37), 108 (79), 94 (28), 93 (15), 83 (29), 82 (28), 81 (23), 69 (14), 68 (19), 67 (24), 58 (12), 56 (12), 55 (17), 54 (24), 42 (100), 38 (30) & 36 (94) [isotopic HCl+ ·]; HR-MS calcd. for C5H7N4O (M-OH) - 139.0620, found - 139.0621. - LSIMS (+), m/z (% rel. int.): 157 (MH+, 100).
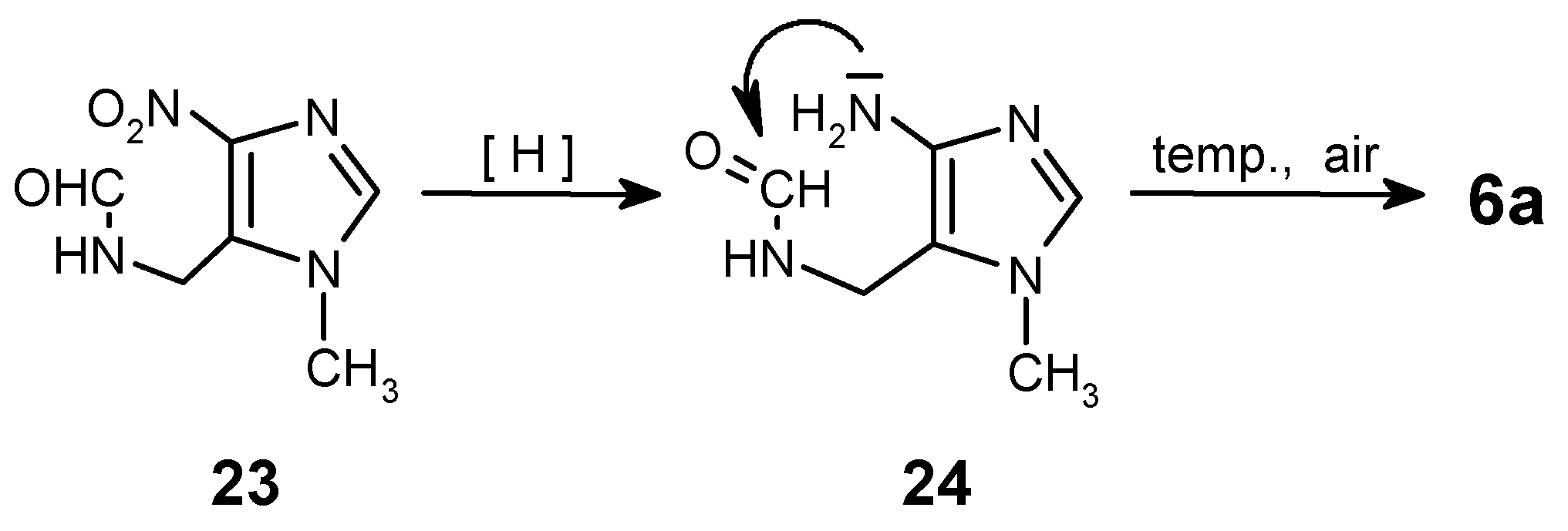
N-[(1-Methyl-4-nitro-1H-imidazol-5-yl)methyl]-formamide (23)
This side-product (small amounts, less than 5%; usually obtained due to a partial hydrolysis of isocyano group in 18) was isolated by column chromatography for characterization (eluent: CHCl3/MeOH - 20:1); ca 90% purity. - 1H NMR (CDCl3): δ = 8.18 (s, 1 H, CHO), 7.34 (s, 1 H, H-2), 6.78 (s, broad, 1 H, NH), 4.71 (d, J = 6.6 Hz, 2 H, CH2), 3.92 (s, 3 H, NCH3). - MS, m/z (% rel. int.): 184 (M+ ·, 5), 167 (6), 138 (100), 121 (21), 120 (65), 111 (51), 110 (16), 109 (33), 108 (53), 94 (17), 93 (17), 83 (23), 82 (22), 81 (16), 67 (17), 54 (12), 42 (57); HR-MS calcd. for C6H8N4O3 - 184.0596, found - 184.0571. - LSIMS (+), m/z (% rel. int.): 185 (M+H, 100).
(4-Amino-1-methyl-1H-imidazol-5-yl)methylamine (20)
This was obtained from hydrochloride of
19 by catalytic hydrogenation in Parr Apparatus according to known procedure (10% Pd/C, 25 psi, 4 h, in EtOH) [
2,
3,
9]. Hydrochloride of
19 was neutralized by alcoholic solution of ammonia before hydrogenation. After the reduction the crude residue was used directly for the next steps.
For crude intermediate 20 (as hydrochloride) MS spectrum was recorded.
- MS, m/z (% rel. int.): 127 (MH+, 15), 110 (12), 109 (13), 108 (18), 94 (7), 81 (9), 68 (13), 58 (16), 42 (56), 38 (37) & 36 (100) [isotopic HCl+ ·].
Cyclocondensation of Diamine 20 with Orthoesters
Procedure A: The crude 5-aminomethyl-1-methyl-1H-imidazole-4-yl amine (20; ca 0.3 mmol) was suspended in the appropriate orthoester (HC(OEt)3, MeC(OEt)3, 3 mL) and the reaction mixture was heated to reflux for 6 h. Under these conditions the intermediates 21a, 21b underwent spontaneous oxidation to 6a, 6c. The excess of the orthoester was removed under reduced pressure and the products (6a, 6c) were isolated by column chromatography (eluent: from CHCl3 to CHCl3/MeOH - 20:1).
Procedure B: The crude diamine (
20;
ca 0.3 mmol) and the appropriate orthoester (HC(OEt)
3, MeC(OEt)
3, EtC(OEt)
3, n-BuC(OMe)
3; 2 mL) were dissolved in ethanol (10 mL) and the mixture was heated to reflux for 6 h. The ethanol and orthoester were evaporated and the products (
21a-
d) were isolated by preparative TLC (eluent: CHCl
3/MeOH - 10:1). The yields are given in Table on
Scheme 5.
7-Methyl-6,7-dihydro-1H-purine (21a)
Unstable, decomposed slowly yielding 6a. - 1H NMR (CDCl3/DMSO-d6 - 2:1): δ = 8.08 (s, 1 H, H-2), 7.54 (s, 1 H, H-8), 4.77 (s, 2 H, CH2), 3.72 (s, 3 H, NCH3), NH - undetected. - MS, m/z (% rel. int.): 136 (M+ ·, 5), 135 (6), 134 (46), 122 (14), 107 (14), 69 (24), 44 (84), 42 (100); HR-MS calcd. for C6H8N4 - 136.0749, found - 136.0742. - LSIMS (+), m/z (% rel. int.): 137 (M+H, 26).
2,7-Dimethyl-6,7-dihydro-1H-purine (21b)
Unstable, decomposed slowly yielding 6c. - 1H NMR (CDCl3): δ = 8.13 (s, 1 H, H-8), 5.33 (s, broad, 1 H, NH), 4.00 (s, 2 H, CH2), 3.96 (s, 3 H, NCH3), 2.87 (s, 3 H, CH3). - MS, m/z (% rel. int.): 150 (M+ ·, 3), 149 (24), 148 (100), 147 (24), 134 (59), 120 (16), 107 (13), 106 (11), 80 (11), 59 (21), 57 (25), 42 (33); HR-MS calcd. for C7H10N4 - 150.0905, found - 150.0954; calcd. for C7H9N4 (M-H) - 149.0827, found -149.0841.
2-Ethyl-7-methyl-6,7-dihydro-1H-purine (21c)
This was identified by MS analysis only, unstable. - MS, m/z (% rel. int.): 164 (M+ ·, 5), 163 (14), 162 (78), 161 (100), 134 (48), 110 (43), 107 (17), 80 (14), 56 (28), 42 (59); HR-MS calcd. for C8H11N4 (M-H) - 163.0984, found - 163.0984.
2-Butyl-7-methyl-6,7-dihydro-1H-purine (21d)
This was not isolated; the structure was proposed on the basis of MS (the molecular ion was detected while the post-reaction mixture was analyzed); m/z (% rel. int.) = 192 (M+ ·, 9), 191 (M-H, 38).

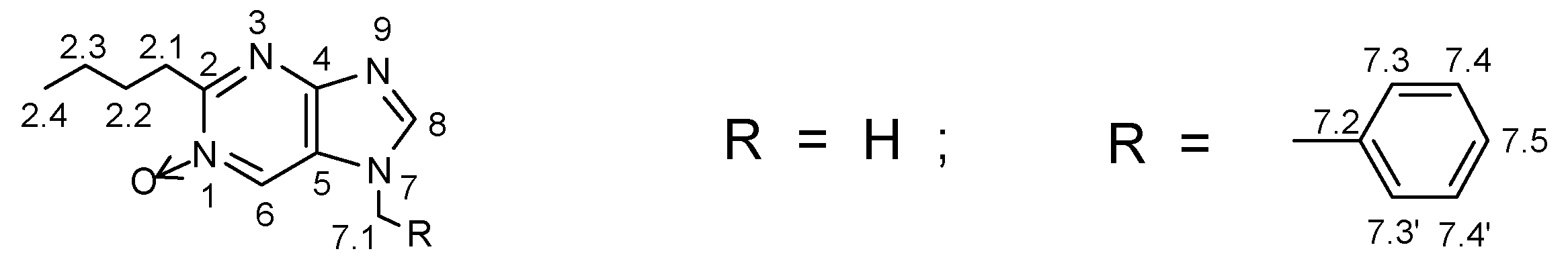
 11a
11a 11c
11c 11b
11b 11d
11d 11e
11e



