Supramolecular Bioorganic Chemistry: Nucleic Acids Recognition and Synthetic Vectors for Gene Transfer
Abstract
:1.Introduction
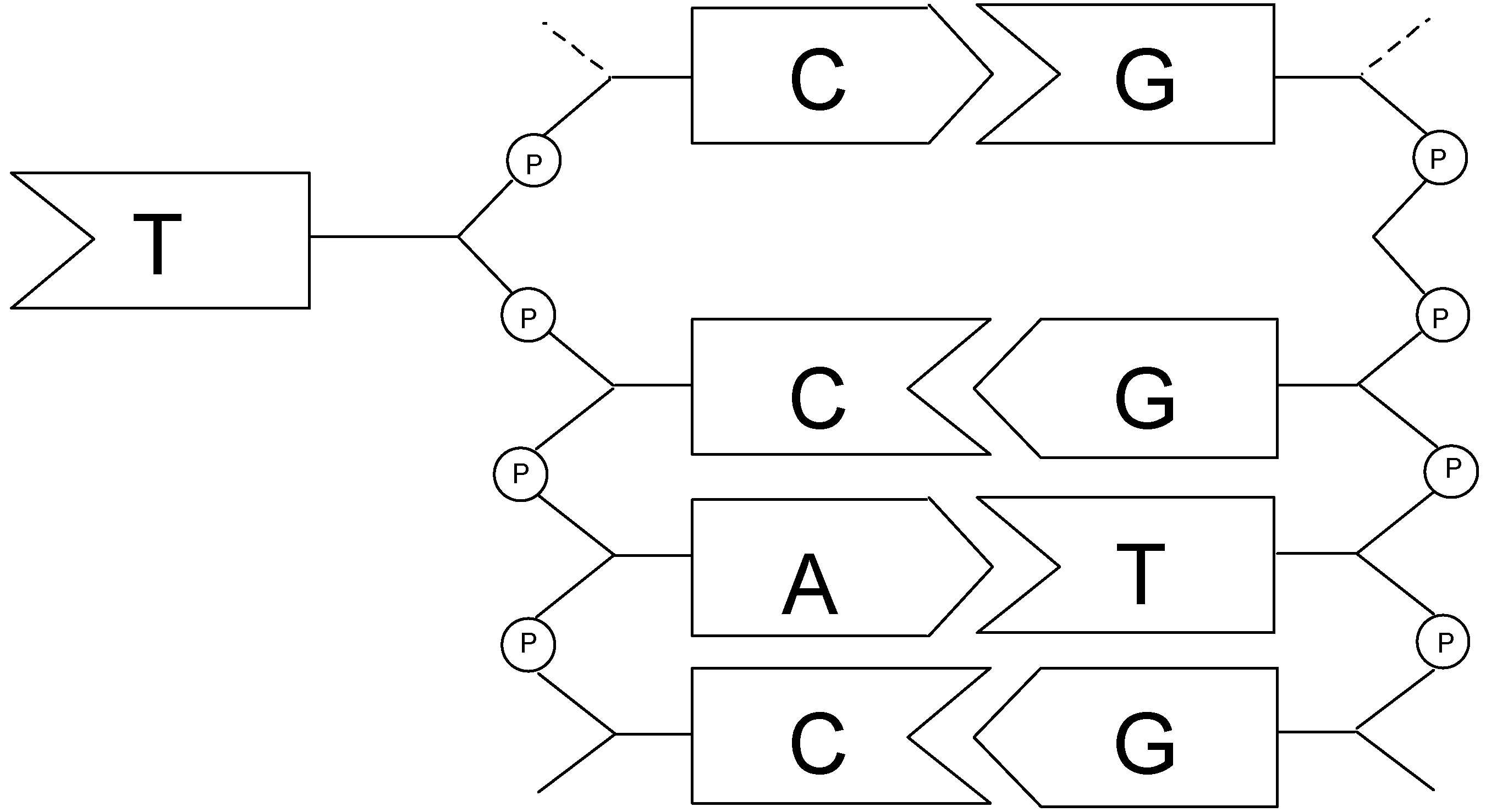
2. Nucleic Acid Recognition
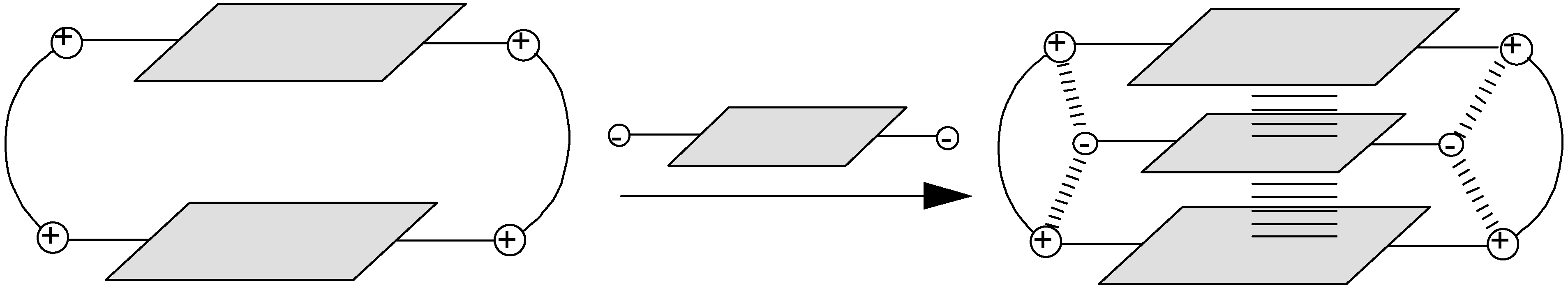
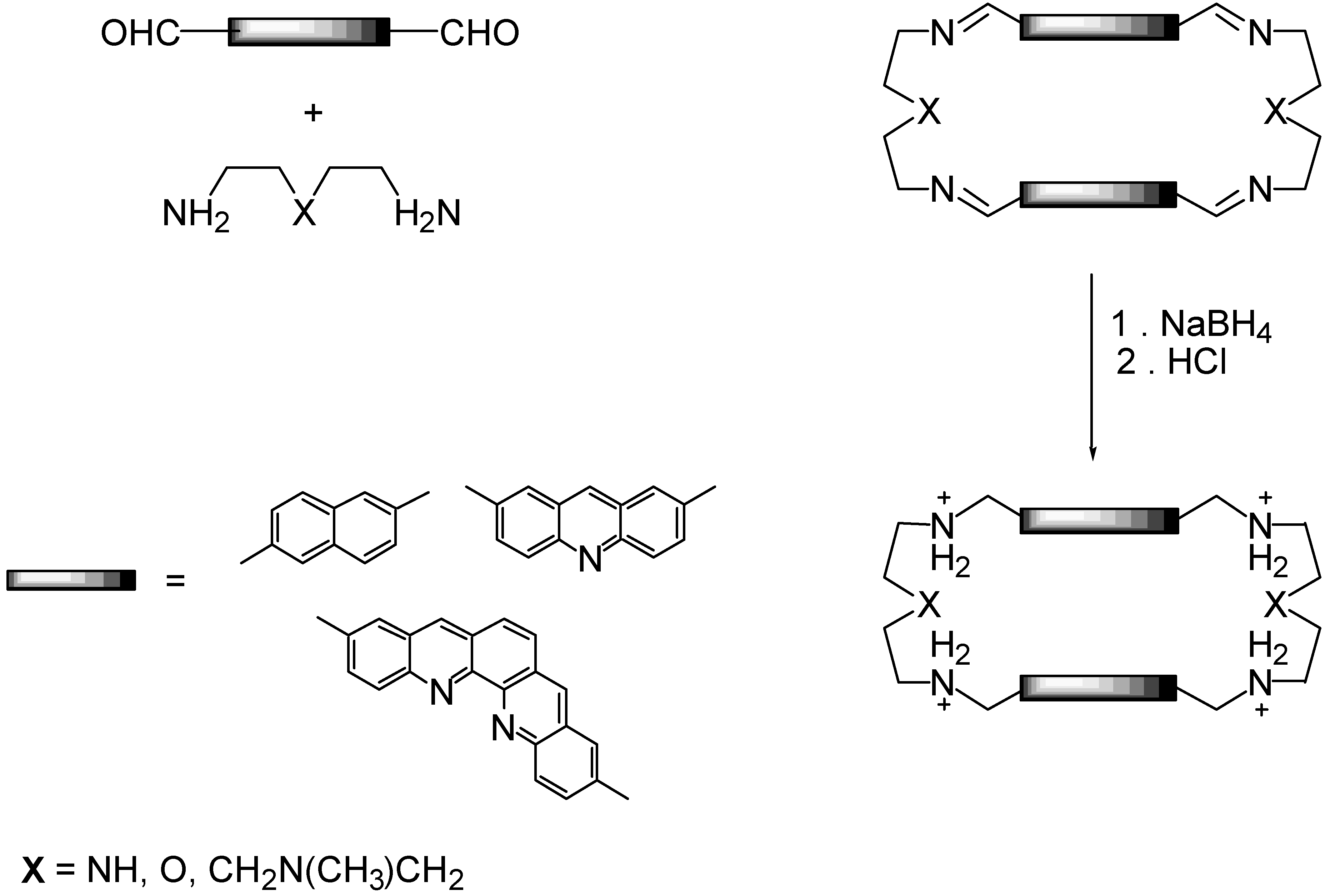
2.1. Design and Preparation of Cyclobisintercaland Receptors
2.2. Binding Properties of Cyclobisintercaland Receptors
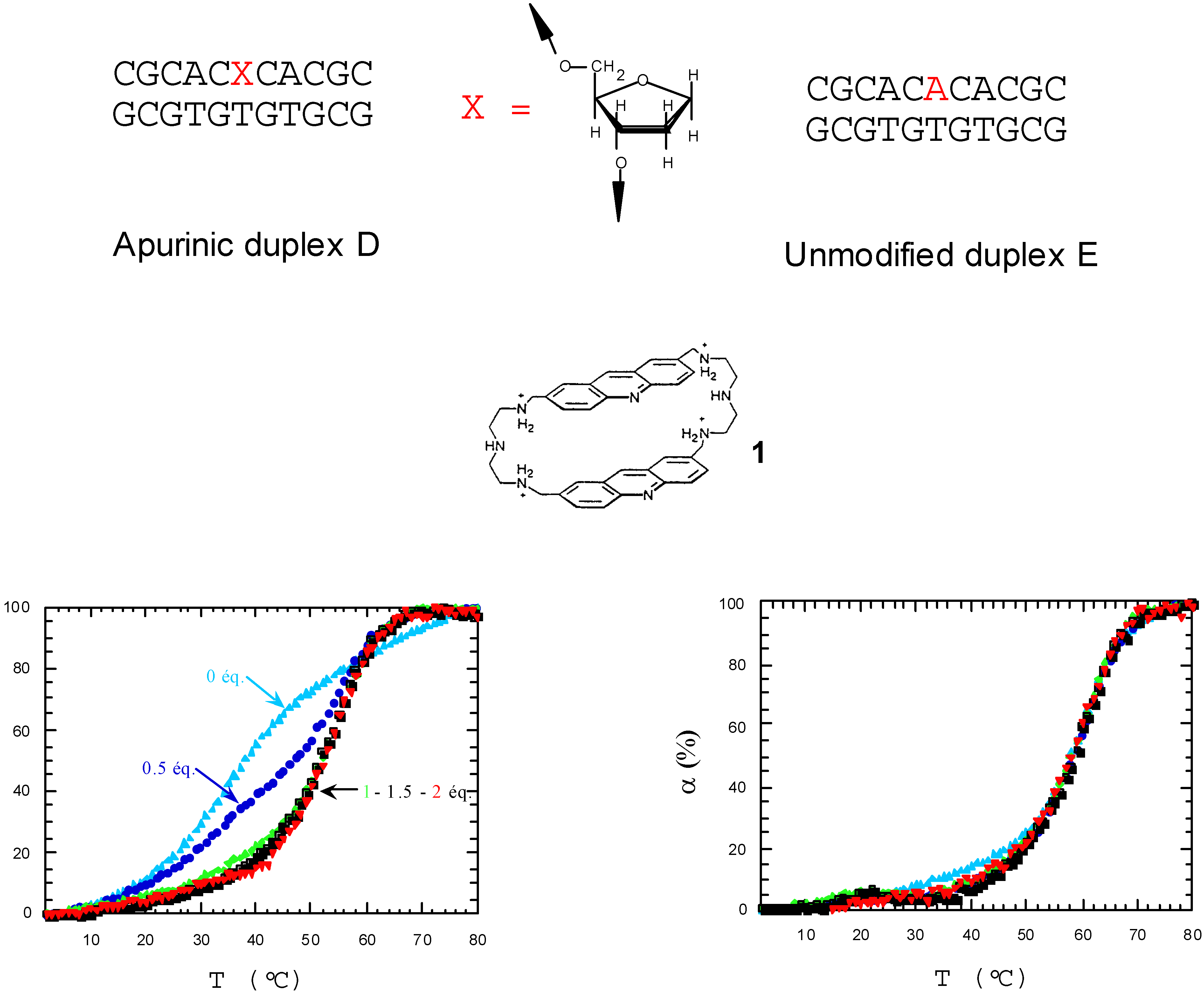
2.3. Discrimination between simple and double-stranded polynucleotides
Conclusion of part I
3. Gene Transfer by Guanidinium-Cholesterol Cationic Lipids
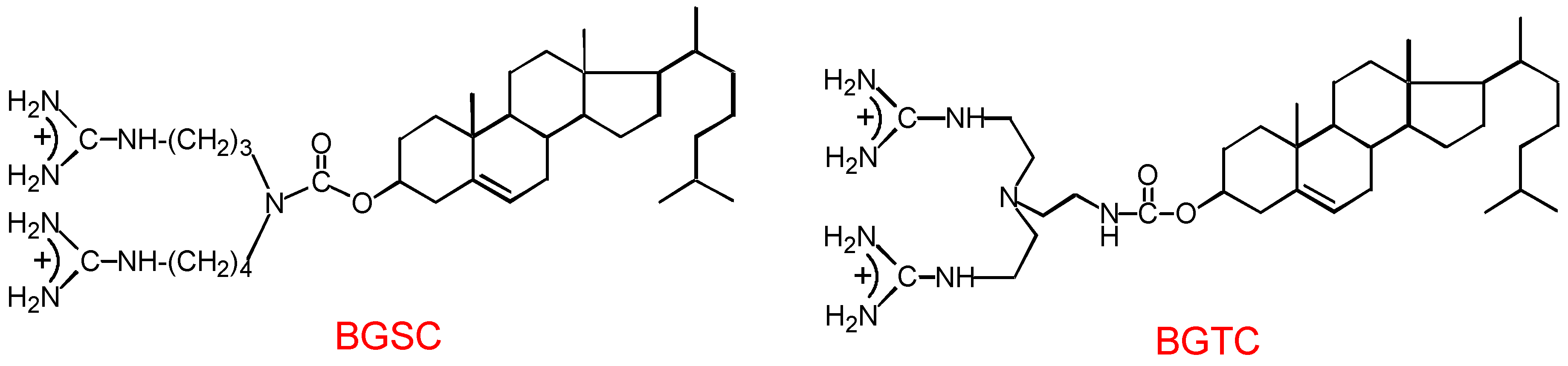
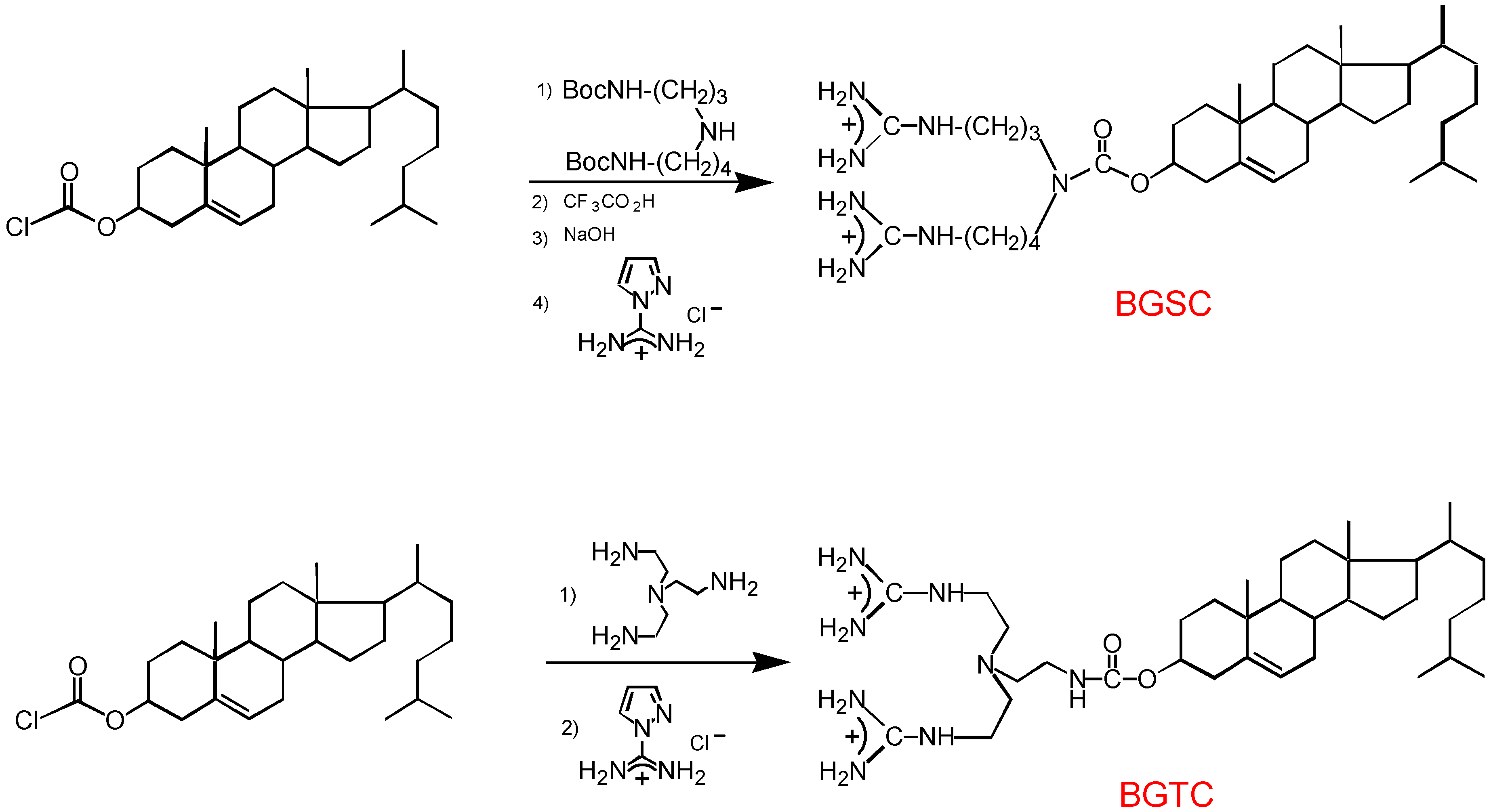
3.1. Design and preparation of new cationic lipids
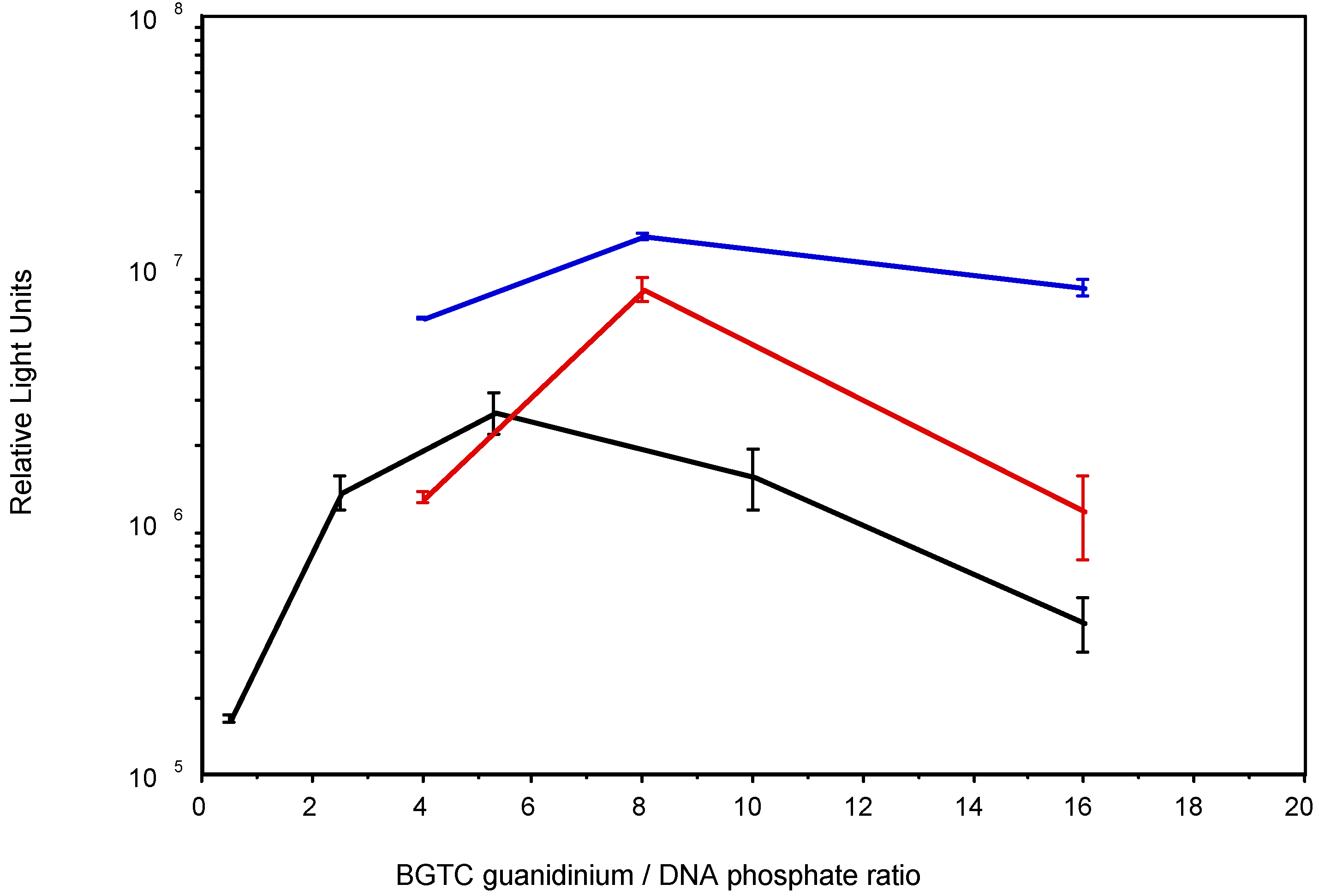
3.2. Transfection activity of BGTC in aqueous solution
3.3. Transfection efficiency of BGSC and BGTC formulated as cationic liposomes with DOPE
3.4. In vivo transfection
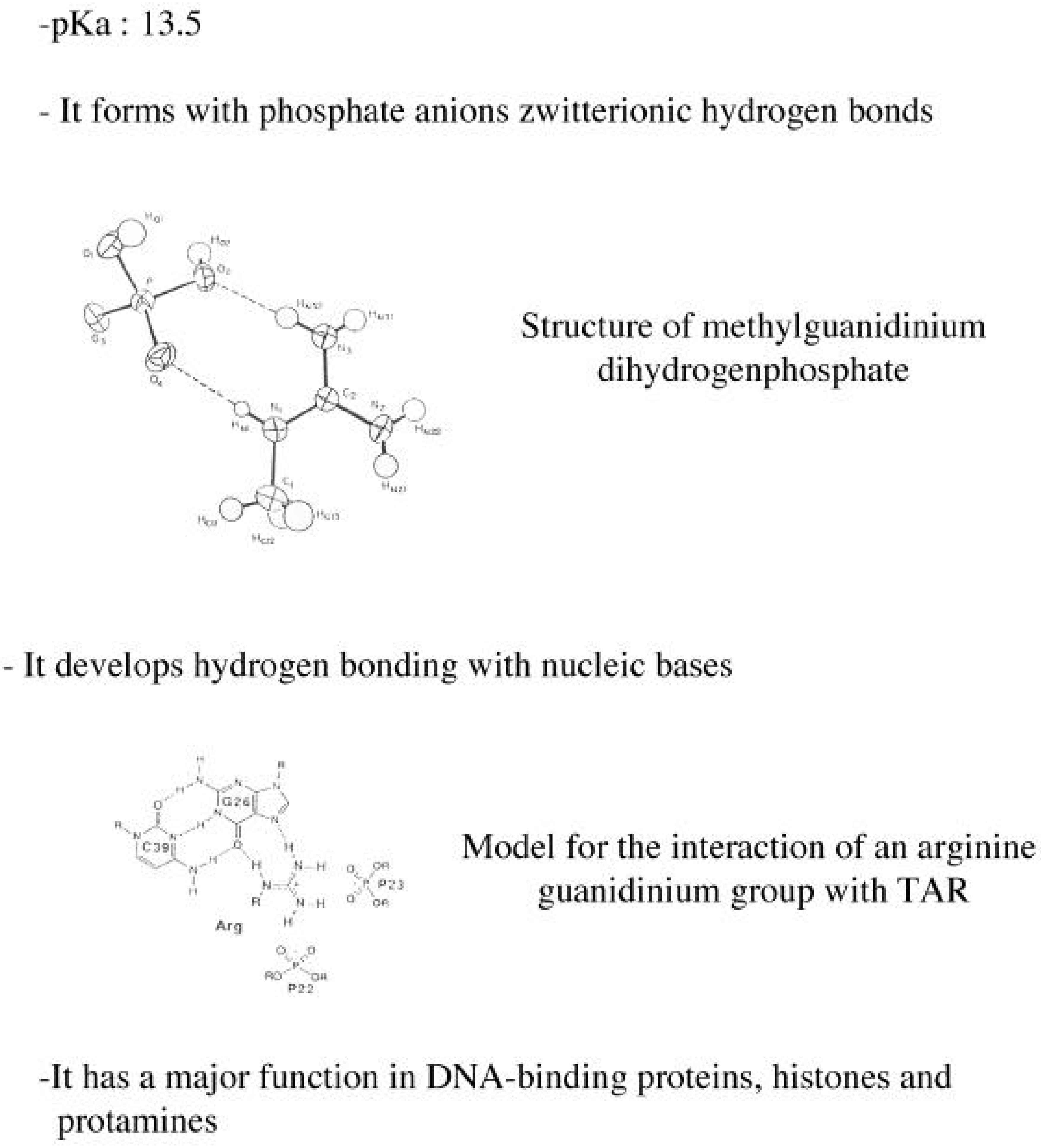
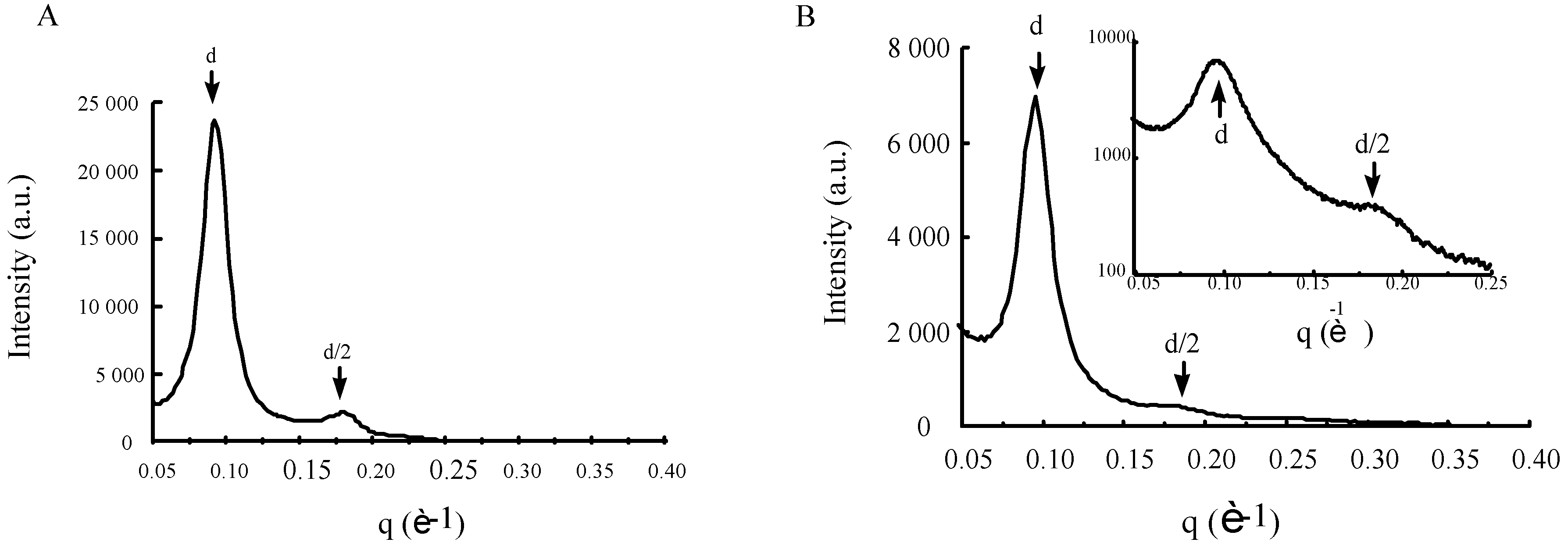
3.5. Structural characteristics of supramolecular assemblies formed by guanidinium-cholesterol reagents
4. Conclusion
Acknowledgments
References and Notes
- Nucleic acids recognition
- Lehn, J.-M.; Schmidt, F.; Vigneron, J.P. Cyclointercalands. - Incorporation of the phenazine group and of metal binding subunits into macrocyclic receptor molecules. Tetrahedron Lett. 1988, 29, 5255. [Google Scholar] [CrossRef]
- Claude, S.; Lehn, J.-M.; Vigneron, J.P. Bicyclo-bis-Intercalands: Synthesis of triply bridged bisintercalands based on acridine subunits. Tetrahedron Lett. 1989, 30, 941. [Google Scholar] [CrossRef]
- Lehn, J.-M.; Schmidt, F.; Vigneron, J.-P. Preparation and reactivity of polyfunctional phenazine derivatives. J. Heterocyclic Chem. 1990, 27, 1633. [Google Scholar] [CrossRef]
- Claude, S.; Lehn, J.-M.; Schmidt, F.; Vigneron, J.-P. Binding of nucleosides, nucleotides and anionic planar substrates by bis-intercaland receptor molecules. J. Chem. Soc. Chem. Commun. 1991, 1182. [Google Scholar]
- Zinic, M.; Cudic, P.; Skaric, V.; Vigneron, J.-P.; Lehn, J.-M. Cyclo-bis-intercaland receptors with phenanthridine subunits. Tetrahedron Lett. 1992, 33, 7417. [Google Scholar] [CrossRef]
- Claude, S.; Lehn, J.-M.; Pérez de Vega, M.-J.; Vigneron, J.-P.; Baudoin, O. Synthèse de bicyclobis-intercalants dérivés de l’acridine. New J. Chem. 1992, 16, 21. [Google Scholar]
- Dhaenens, M.; Lehn, J.-M.; Vigneron, J.-P. Molecular recognition of nucleosides, nucleotides and anionic planar substrates by a water soluble bis-intercaland type receptor molecule. J. Chem. Soc., Perkin Trans 2 1993, 1379. [Google Scholar] [CrossRef]
- Teulade-Fichou, M.-P.; Vigneron, J.-P.; Lehn, J.-M. Molecular recognition of nucleosides and nucleotides by a water soluble cyclo-bis-intercaland type receptor molecule based on acridine subunits. Supramolecular Chemistry 1995, 5, 139. [Google Scholar] [CrossRef]
- Cudic, P.; Zinic, M.; Tomisic, V.; Simeon, V.; Vigneron, J.-P.; Lehn, J.-M. Binding of nucleotides in water by phenanthridinium bis-intercaland receptor molecules. J. Chem. Soc., Chem. Commun. 1995, 1073. [Google Scholar]
- Espinosa, J.F.; Jaime, C.; Lehn, J.-M.; Vigneron, J.-P. Cyclo-bis-intercalands with acridine subunits linked by rigid spacers. Tetrahedron Lett. 1995, 36, 5261. [Google Scholar]
- Lorente, A.; Fernández Saiz, M.; Lehn, J.-M.; Vigneron, J.-P. Cyclo-bis and cyclo-tris-intercalands based on acridine subunits. Tetrahedron Lett. 1995, 36, 8279. [Google Scholar] [CrossRef]
- Cudic, P.; Zinic, M.; Skaric, V.; Kiralj, R.; Kojic-Prodic, B.; Vigneron, J.-P.; Lehn, J.-M. Synthesis of cyclo-bis-intercaland receptor molecules with phenanthridinium units. Croatica Chem. Acta 1996, 69, 569. [Google Scholar]
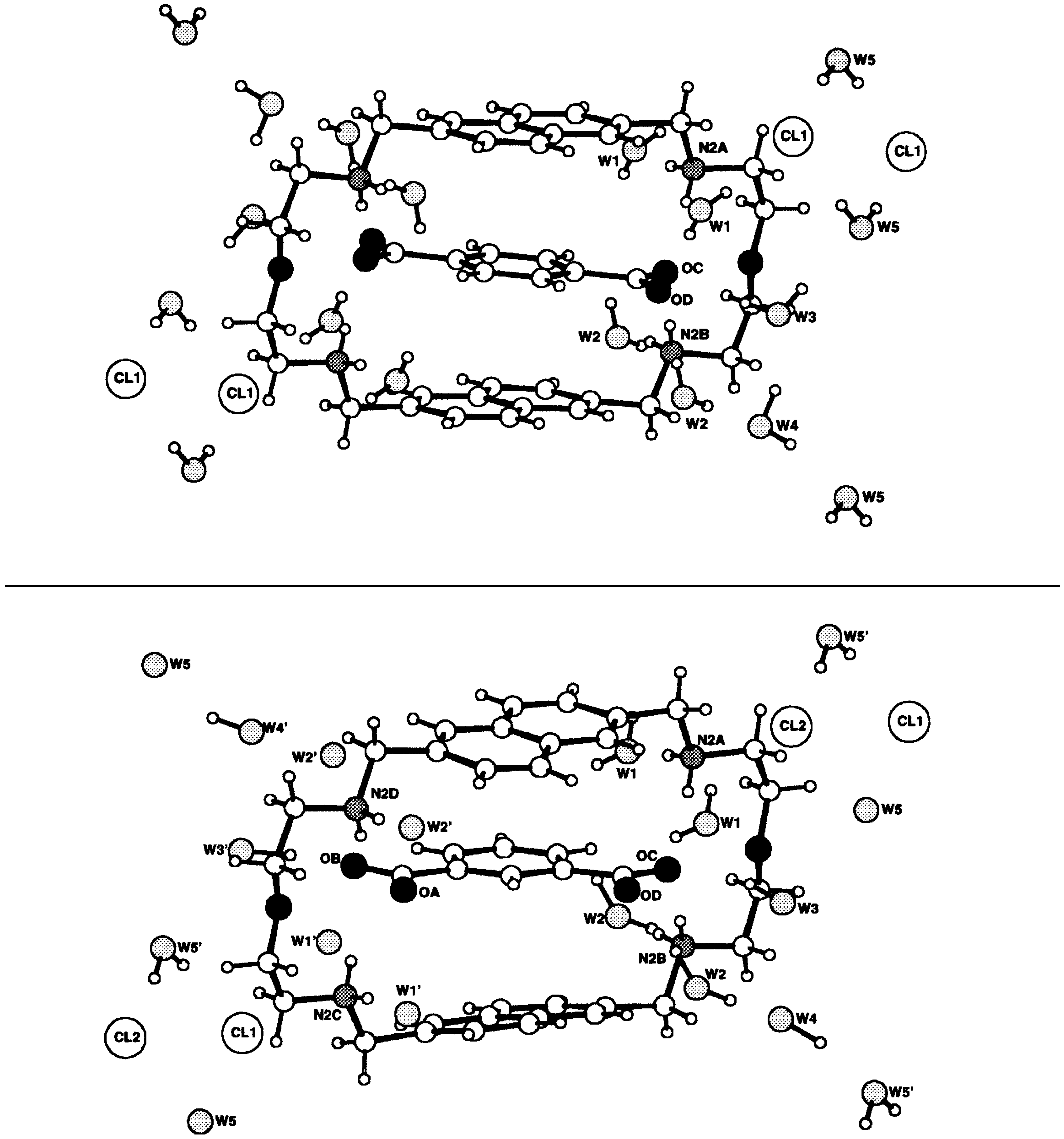
- Paris, T.; Vigneron, J.-P.; Lehn, J.-M.; Cesario, M.; Guilhem, J.; Pascard, C. Molecular Recognition of Anionic Substrates. Crystal structures of the supramolecular inclusion complexes of terephthalate and isophthalate dianions with a bis-intercaland receptor molecule. J. Incl. Phenom. 1999, 33, 191. [Google Scholar] [CrossRef]
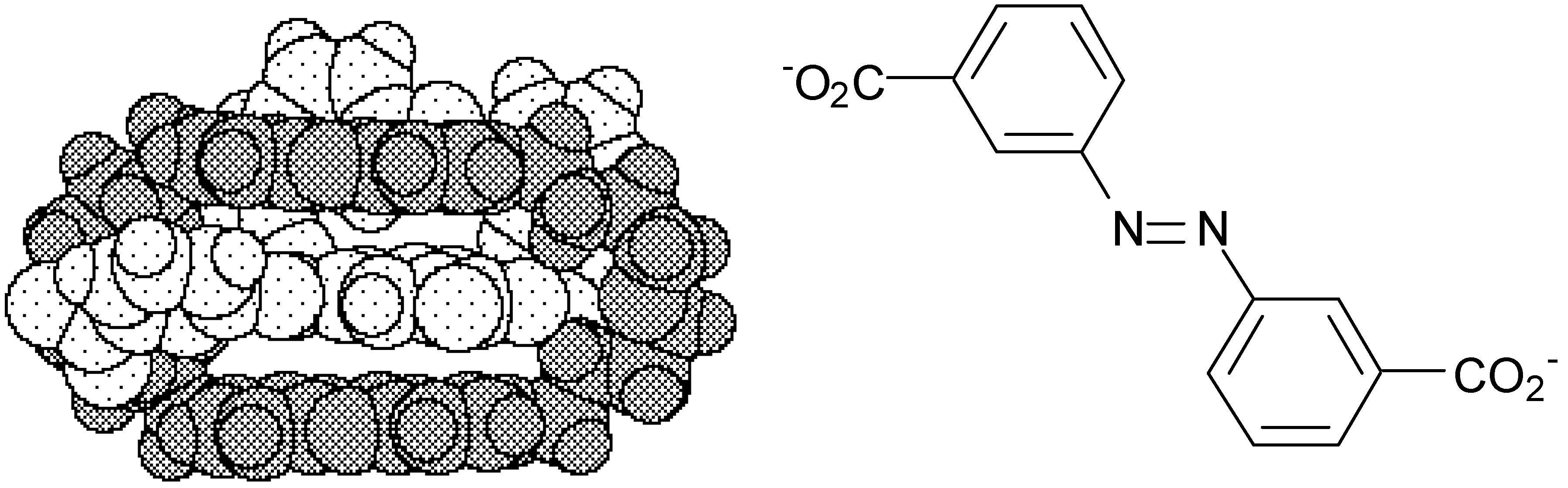
- Cudic, P.; Vigneron, J.-P.; Lehn, J.-M.; Cesario, M.; Prangé, T. Molecular Recognition of Azobenzene Dicarboxylates by Acridine-based Receptor Molecules. Crystal Structure of the Supramolecular Inclusion Complex of trans-3,3’-Azobenzene Dicarboxylate with a Cyclo-bisintercaland Receptor. Eur. J. Org. Chem. 1999, in press. [Google Scholar] [CrossRef]
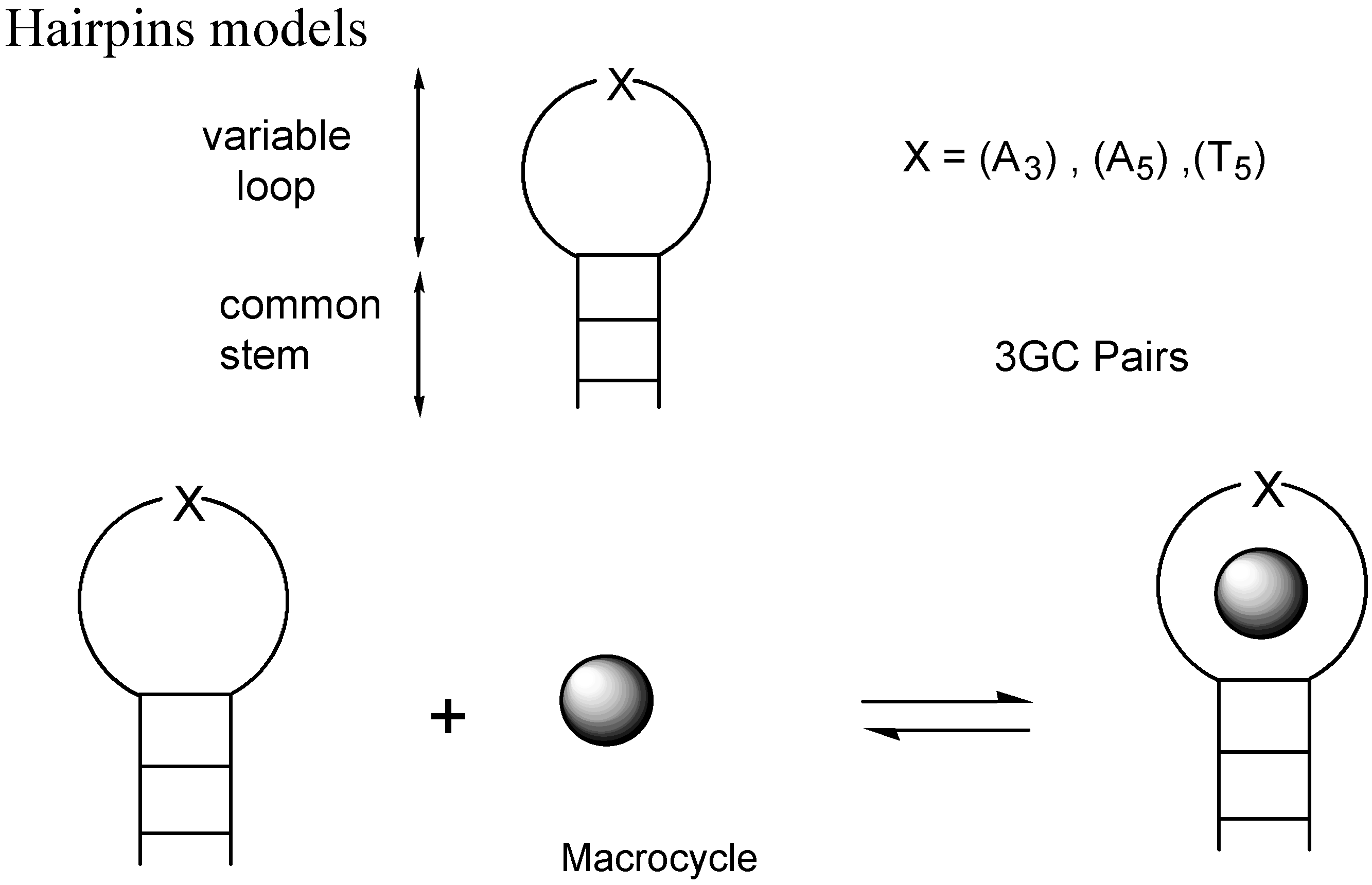
- Slama-Schwok, A.; Teulade-Fichou, M.-P.; Vigneron, J.-P.; Taillandier, E.; Lehn, J.-M. Selective binding of macrocyclic bis-acridine to a DNA hairpin. J. Am. Chem. Soc. 1995, 117, 6822. [Google Scholar] [CrossRef]
- Slama-Schwok, A.; Peronnet, F.; Hantz-Brachete, E.; Taillandier, E.; Teulade-Fichou, M.-P.; Vigneron, J.-P.; Baudoin, O.; Best-Belpomme, M.; Lehn, J.-M. A macrocyclic bis-acridine shifts the equilibrium from duplexes towards DNA hairpins. Nucleic Acids Res. 1997, 25, 2574. [Google Scholar] [CrossRef]
- Blacker, A.J.; Teulade-Fichou, M.-P.; Vigneron, J.-P.; Fauquet, M.; Lehn, J.-M. Selective photocleavage of single stranded DNA plasmids by cyclo-bisintercaland compounds. Bioorg. Med. Chem. Lett. 1998, 8, 601. [Google Scholar] [CrossRef]
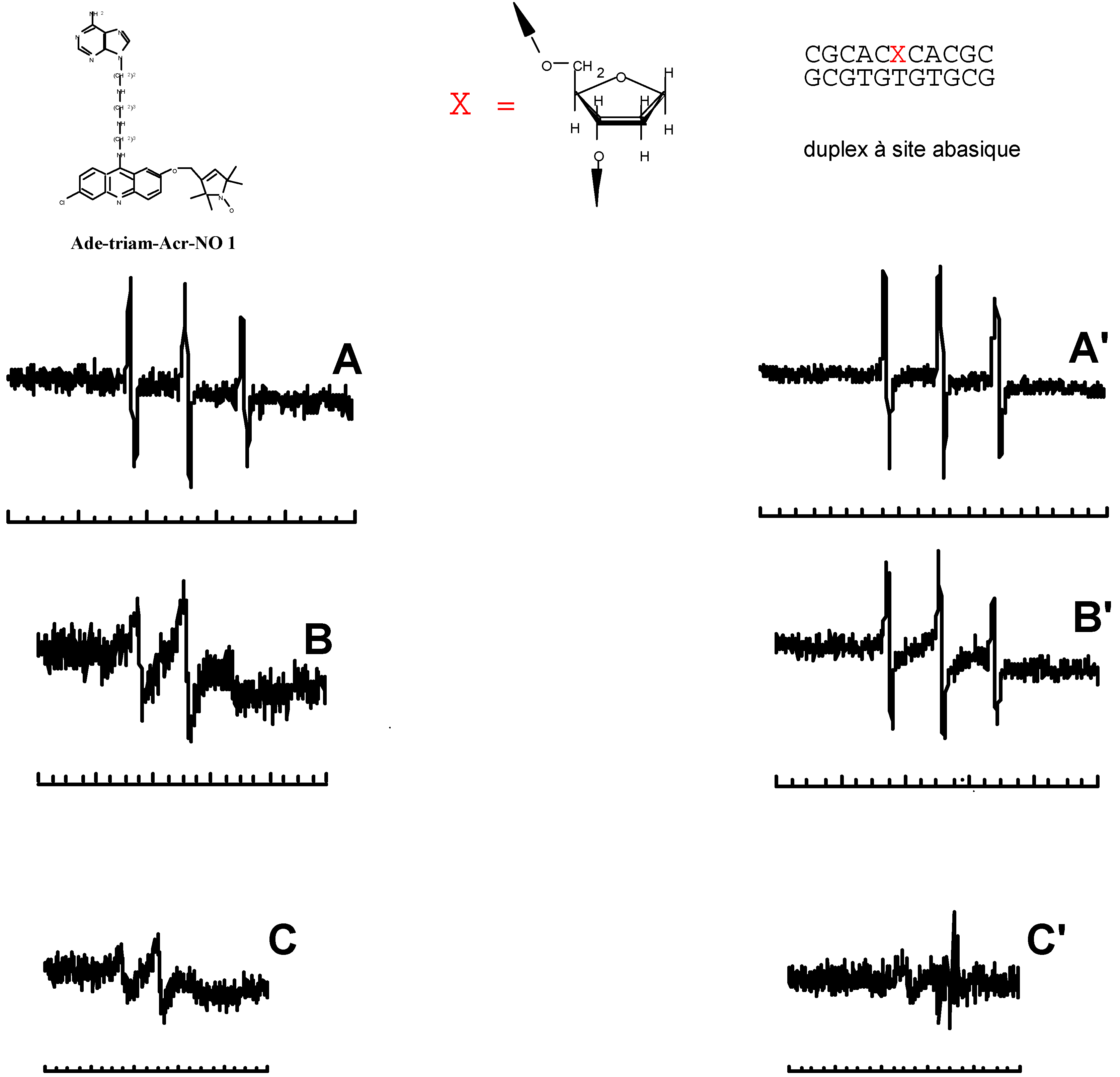
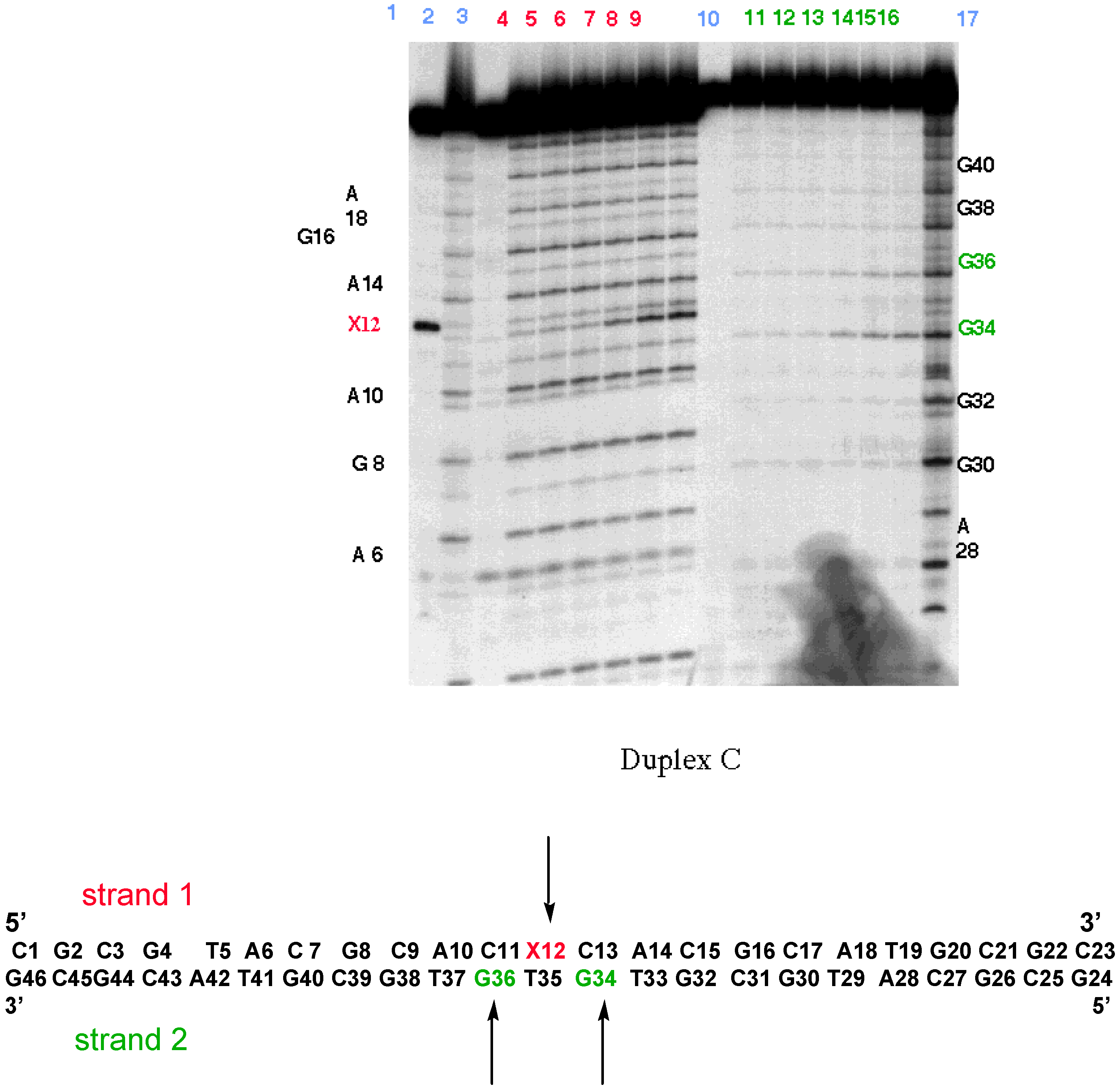
- Berthet, N.; Michon, J.; García, J.; Jourdan, M.; Lhomme, J.; Teulade-Fichou, M.-P.; Vigneron, J.P.; Lehn, J.-M. Specific Recognition and Stabilization of an Abasic Site-Containing DNA Duplex by a Macrocyclic Bis-acridine. Nucleic Acid Res. 1999, in press. [Google Scholar]
- Jourdan, M.; García, J.; Lhomme, J.; Teulade-Fichou, M.-P.; Vigneron, J.-P.; Lehn, J.-M. Threading Bis-intercalation of a Macrocyclic Bisacridine at Abasic Sites in DNA: 1H NMR and Molecular Modeling Study. Biochemistry. (submitted).
- Gene transfer studies
- Vigneron, J.-P.; Oudrhiri, N.; Fauquet, M.; Vergely, L.; Bradley, J.-C.; Basseville, M.; Lehn, P.; Lehn, J.-M. Guanidinium-cholesterol cationic lipids: Efficient vectors for the transfection of eukaryotic cells. Proc. Natl. Acad. Sci. USA 1996, 93, 9682. [Google Scholar] [CrossRef] [PubMed]
- Oudrhiri, N.; Vigneron, J.-P.; Peuchmaur, M.; Leclerc, T.; Lehn, J.-M.; Lehn, P. Gene transfer by guanidinium-cholesterol cationic lipids into airway epithelial cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA 1997, 94, 1651. [Google Scholar] [CrossRef] [PubMed]
- Oudrhiri, N.; Vigneron, J.-P.; Hauchecorne, M.; Toury, R.; Lemoine, A.I.; Peuchmaur, M.; Navarro, J.; Lehn, J.-M.; Lehn, P. Guanidinium cholesterol cationic lipids: novel reagents for gene transfection and perspectives for gene therapy. Biogenic Amines 1998, 14, 537. [Google Scholar]
- Pitard, B.; Oudrhiri, N.; Vigneron, J.-P.; Hauchecorne, M.; Aguerre, O.; Toury, R.; Airiau, M.; Ramasawmy, R.; Scherman, D.; Grouzet, J.; Lehn, J.-M.; Lehn, P. Structural characteristics of supramolecular assemblies formed by guanidinium-cholesterol reagents for gene transfection. Proc. Natl. Acad. Sci. USA 1999, 96, 2621. [Google Scholar] [CrossRef] [PubMed]
- Samples Availability: Available from MDPI.
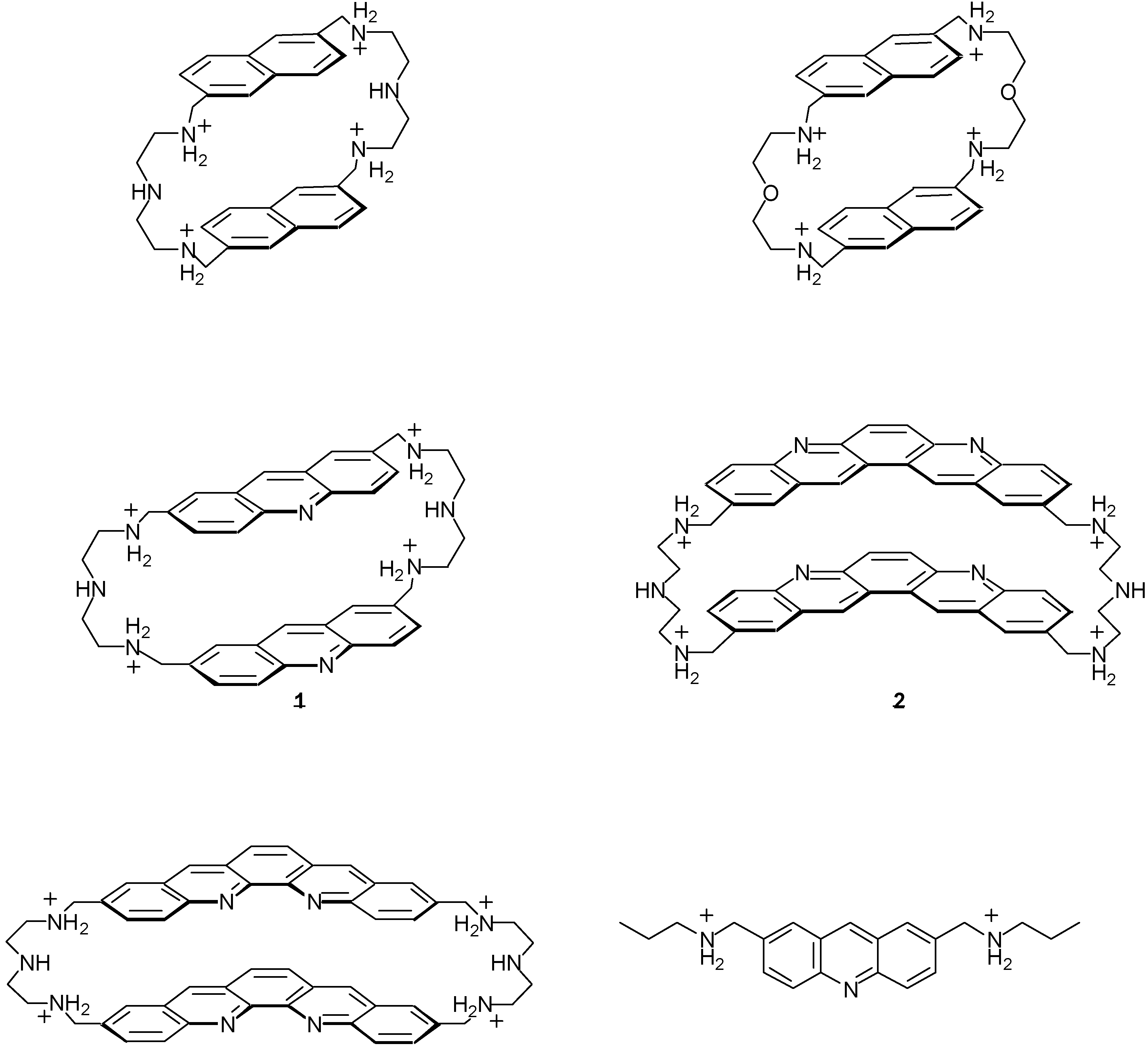


| Species | Cell line | Tissue | RLU/mg of cell protein | ||
|---|---|---|---|---|---|
| BGTC lipid | Calcium Phosphate | Transfectam® | |||
| Human | A549 | Lung carcinoma | 4x105 | 7x103 | 3.1x105 |
| Monkey | COS-7 | SV-40 transformed kidney | 2.1x107 | 3.7x105 | 8.4x106 |
| Dog | MDCK-1 | Kidney | 3x106 | 4.5x105 | 2.2x106 |
| Rat | RIN-m5F | Pancreatic islet cell tumor | 2x107 | 2x105 | 3.3x105 |
| ROS | Osteosarcoma | 4.0x106 | 4.5x105 | 2.3x106 | |
| PC12 | Pheochromocytoma | 3x107 | 1.7x105 | 6x106 | |
| Mouse | AtT-20 | Pituitary tumor | 2x107 | 8x105 | 3x107 |
| Cell lines | RLU/mg of cell protein | ||
|---|---|---|---|
| BGSC liposomes | BGTC liposomes | Lipofectin ® | |
| HeLa | 4.6 x 106 | 7.7 x 106 | 3.3 x 106 |
| A 549 | 6 x 105 | 2 x 105 | 4 x 106 |
| COS-7 | ND | 1.4 x 107 | 9.5 x 106 |
| MDCK-1 | 1 x 106 | 7 x 106 | 1.9 x 106 |
| ROS | ND | 9 x 106 | 6 x 106 |
| NB2 A a | 1.5 x 107 | 1.4 x 107 | ND |
| NIH 3T3 a | 7 x 106 | 1.5 x 106 | ND |
© 1999 by the authors. Reproduction of this article, by any means, is permitted for noncommercial purposes.
Share and Cite
Vigneron, J.-P. Supramolecular Bioorganic Chemistry: Nucleic Acids Recognition and Synthetic Vectors for Gene Transfer. Molecules 1999, 4, 180-203. https://doi.org/10.3390/40700180
Vigneron J-P. Supramolecular Bioorganic Chemistry: Nucleic Acids Recognition and Synthetic Vectors for Gene Transfer. Molecules. 1999; 4(7):180-203. https://doi.org/10.3390/40700180
Chicago/Turabian StyleVigneron, Jean-Pierre. 1999. "Supramolecular Bioorganic Chemistry: Nucleic Acids Recognition and Synthetic Vectors for Gene Transfer" Molecules 4, no. 7: 180-203. https://doi.org/10.3390/40700180
APA StyleVigneron, J.-P. (1999). Supramolecular Bioorganic Chemistry: Nucleic Acids Recognition and Synthetic Vectors for Gene Transfer. Molecules, 4(7), 180-203. https://doi.org/10.3390/40700180




