Synthesis of α-Hydroxyacetosyringone
Abstract
:Introduction
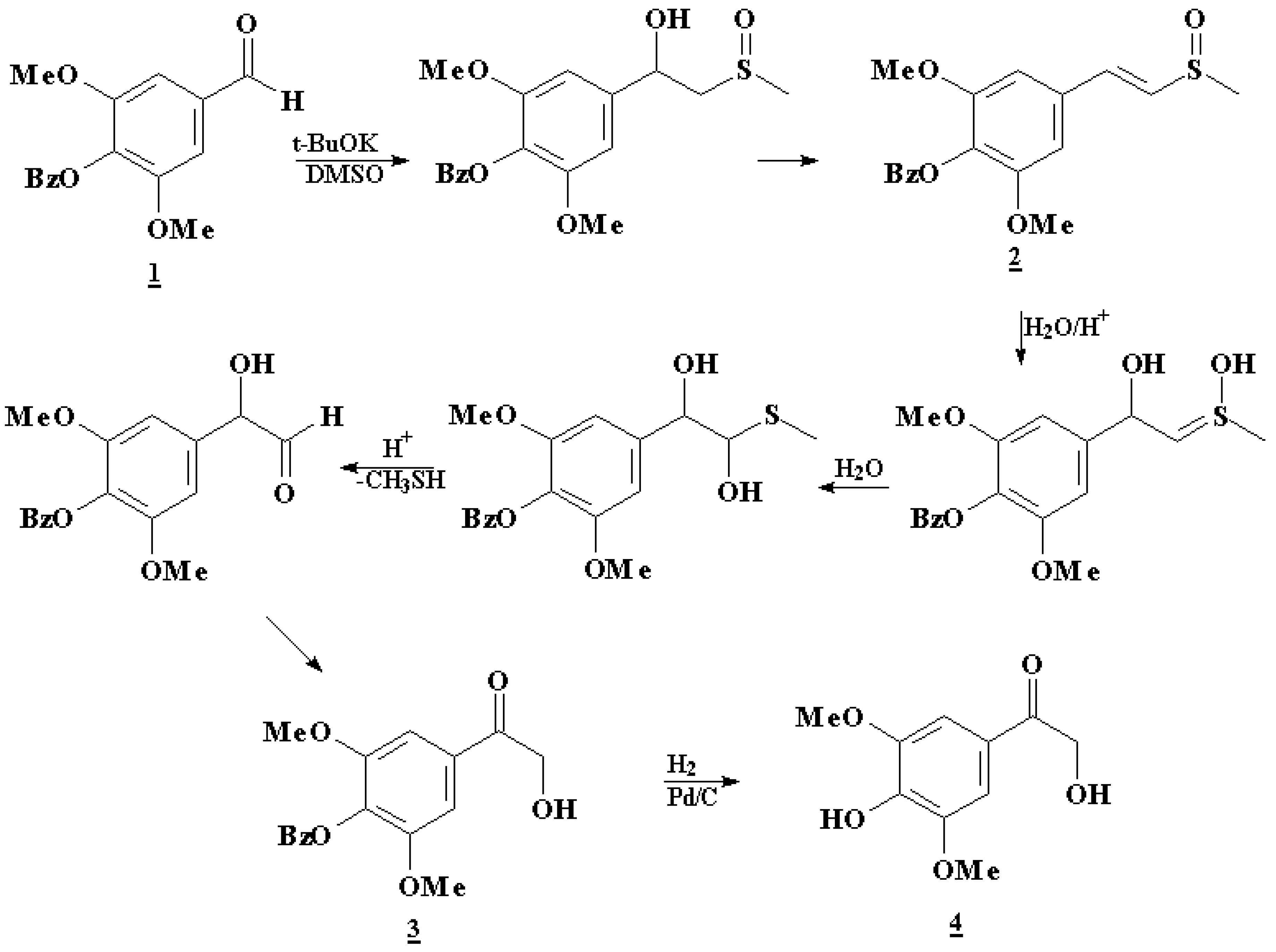
Results and Discussion
Experimental
General
4-Benzyloxysyringaldehyde (1)
3’,5’-Dimethoxy-4’-benzyloxyethenylmethylsulfoxide (2)
4’-O-benzyl-α-hydroxyacetosyringone (3)
α-Hydroxyacetosyringone (4)
Acknowledgments
References
- Echeverri, F.; Torres, F.; Quiñones, W.; Cardona, G.; Archbold, R.; Roldán, J.; Gutierrez, J.; Hassane, E. L. Phytochemistry 1996, 44, 255–256.
- Echeverri, F.; Torres, F.; Quiñones, W.; Cardona, G.; Archbold, R.; Roldán, J.; Brito, I.; Gutierrez, J.; Lahlou, E-H. “Memorias III Simposio Internacional de Química de Productos Naturales y sus Aplicaciones”; Sociedad Chilena de Química-U. de Chile, Santiago y Catolica de Chile: Punta de Tralca (Chile), 1996 December 4-7; pp. 125–126. [Google Scholar]
- Song, Y. N.; Shibuya, M.; Ebizuka, Y.; Sankawa, U. Chem. Pharm. Bull. 1990, 38, 2063–2065.
- Sheikholeslam, S. N.; Weeks, D.B. Plant Mol. Biol. 1987, 8, 291–298. [PubMed]
- Luis, J. G.; San Andres, L. J. Chem. Res. (S) 1999, 220–221. [CrossRef]
- Russell, G. A.; Becker, H. D. J. Am. Chem. Soc. 1963, 85, 3406–10.
- Becker, H. D.; Mikol, G.; Russell, G. J. Am. Chem. Soc. 1963, 85, 3410–3414.
- Russell, G.; Mikol, G. J. Am. Chem. Soc. 1966, 88, 5498–5504.
- Sample Availability: Samples are available from the authors.
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Echeverri, F.; Quiñones, W.; Torres, F.; Duque, M.; Archbold, R. Synthesis of α-Hydroxyacetosyringone. Molecules 2000, 5, 1287-1290. https://doi.org/10.3390/51201287
Echeverri F, Quiñones W, Torres F, Duque M, Archbold R. Synthesis of α-Hydroxyacetosyringone. Molecules. 2000; 5(12):1287-1290. https://doi.org/10.3390/51201287
Chicago/Turabian StyleEcheverri, Fernando, Winston Quiñones, Fernando Torres, Mario Duque, and Rosendo Archbold. 2000. "Synthesis of α-Hydroxyacetosyringone" Molecules 5, no. 12: 1287-1290. https://doi.org/10.3390/51201287
APA StyleEcheverri, F., Quiñones, W., Torres, F., Duque, M., & Archbold, R. (2000). Synthesis of α-Hydroxyacetosyringone. Molecules, 5(12), 1287-1290. https://doi.org/10.3390/51201287



