Substitution Effects on Reactivity of N-Acyl-2-amino-2-desoxyglucopyranoses. Quantum Chemical Study
Abstract
:Introduction
Results and Discussion

| HOMO | LUMO | E | Heat of Formation kcal/mol | Binding Energy kcal/mol | Total Energy kcal/mol | |
|---|---|---|---|---|---|---|
| 1 | -10.531 | 2.639 | 13.170 | -221.67 | -2335.13 | -63900.96 |
| 2 | -10.345 | 0.888 | 11.233 | -253.48 | -2872.48 | -77875.57 |
| 3 | -10.332 | 0.878 | 11.210 | -286.43 | -3180.53 | -81128.65 |
| 4 | -10.109 | -1.084 | 9.024 | -288.57 | -4938.22 | -125656.11 |
| 5 | -10.128 | -1.105 | 9.023 | -301.06 | -5225.79 | -128810.07 |
| 6 | -10.188 | -1.171 | 9.017 | -307.95 | -5507.78 | -132403.79 |
| 7 | -10.102 | -1.076 | 9.026 | -300.99 | -5775.92 | -136477.83 |
| 8 | -10.173 | -1.150 | 9.022 | -304.79 | -6054.81 | -140084.72 |
| 2-Aminodesoxyglucopyranose skeleton | C-α(O) | O(Cα) | N-phthalimide | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-1 | C-2 | C-3 | C-4 | C-5 | O-1 | O-2(H) | H-1 | N-1 | N-1′ | C-2′ | O-1′ | |||
| 1 | 0.305 | 0.016 | 0.129 | 0.096 | 0.137 | -0.379 | -0.324 | 0.018 | -0.249 | |||||
| 2 | 0.289 | 0.110 | 0.151 | 0.073 | 0.110 | -0.371 | -0.330 | 0.027 | -0.378 | 0.343 | -0.328 | |||
| 3 | 0.289 | 0.108 | 0.151 | 0.073 | 0.110 | -0.371 | -0.328 | 0.028 | -0.378 | 0.353 | -0.323 | |||
| 4 | 0.269 | 0.073 | 0.119 | 0.090 | 0.104 | -0.375 | -0.343 | 0.031 | -0.380 | 0.350 | -0.318 | -0.452 | 0.413 | -0.305 |
| 5 | 0.272 | 0.076 | 0.120 | 0.088 | 0.135 | -0.376 | -0.340 | 0.030 | -0.376 | 0.341 | -0.313 | -0.454 | 0.403 | -0.301 |
| 6 | 0.289 | 0.108 | 0.152 | 0.074 | 0.110 | -0.372 | -0.330 | 0.025 | -0.378 | 0.354 | -0.329 | -0.451 | 0.402 | -0.301 |
| 7 | 0.290 | 0.109 | 0.152 | 0.074 | 0.109 | -0.330 | -0.330 | 0.027 | -0.381 | 0.353 | -0.322 | -0.452 | 0.402 | -0.306 |
| 8 | 0.289 | 0.111 | 0.151 | 0.074 | 0.110 | -0.371 | -0,330 | 0.025 | -0.380 | 0.355 | -0.329 | 0.450 | 0.402 | -0.303 |
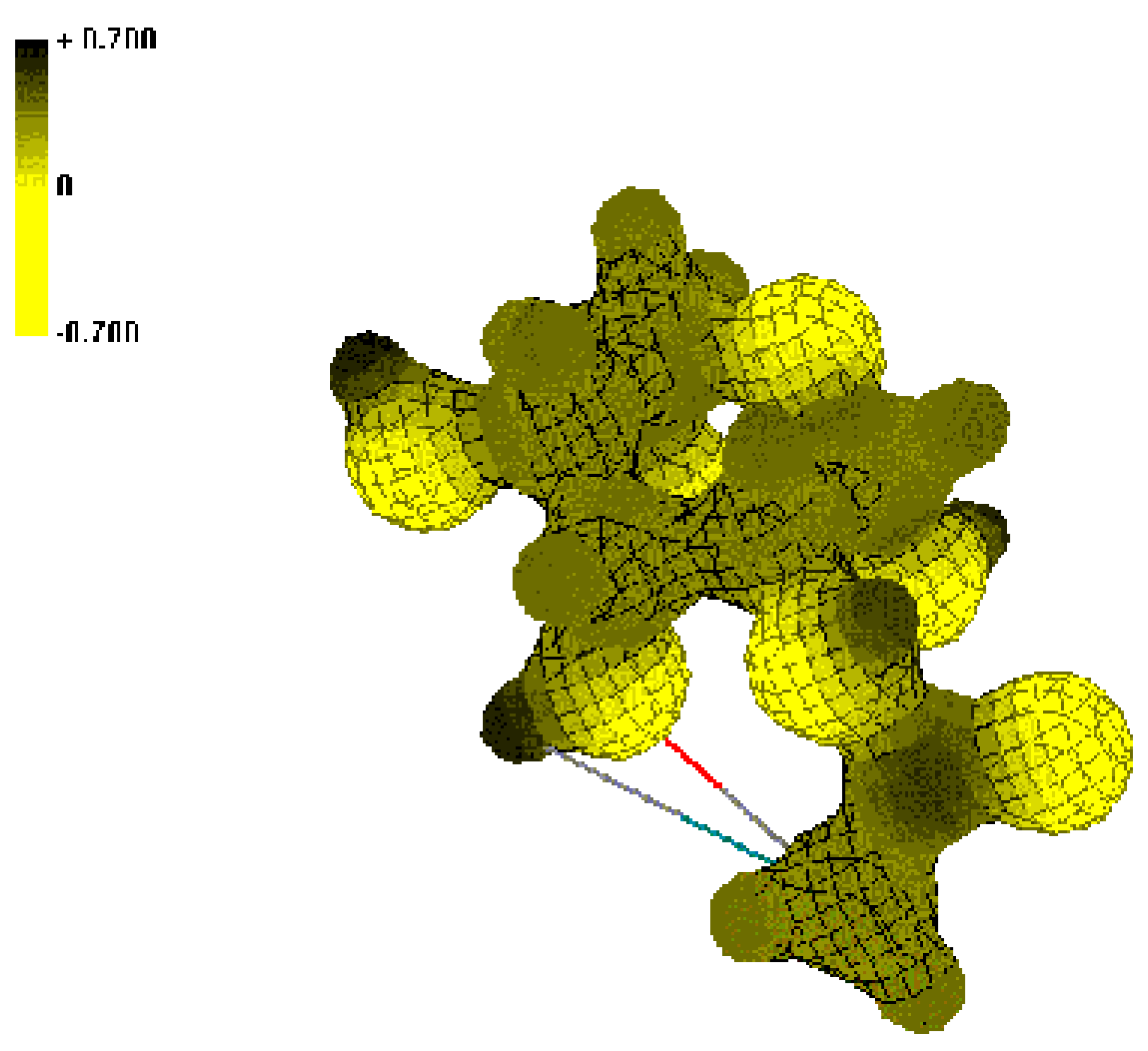
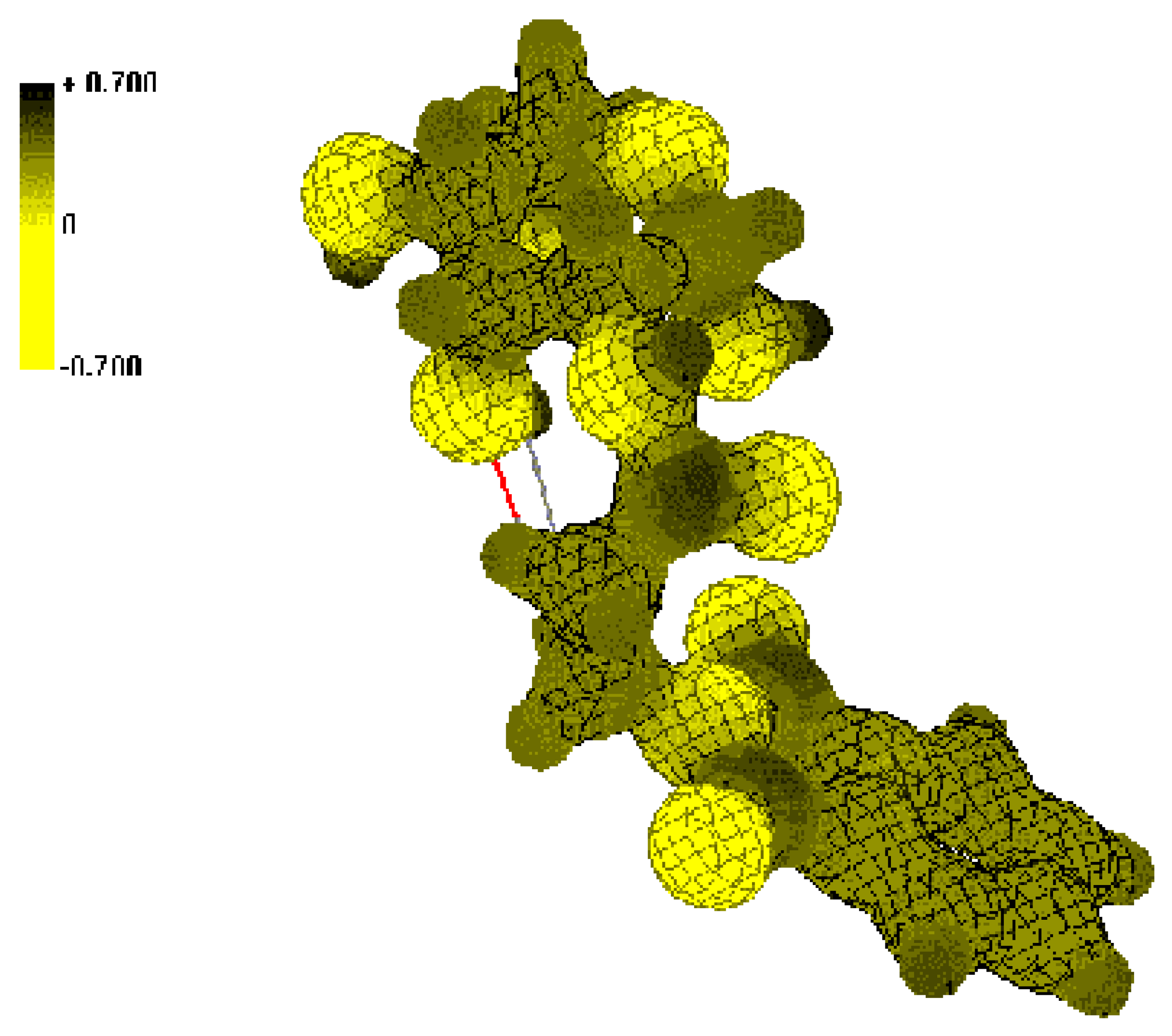
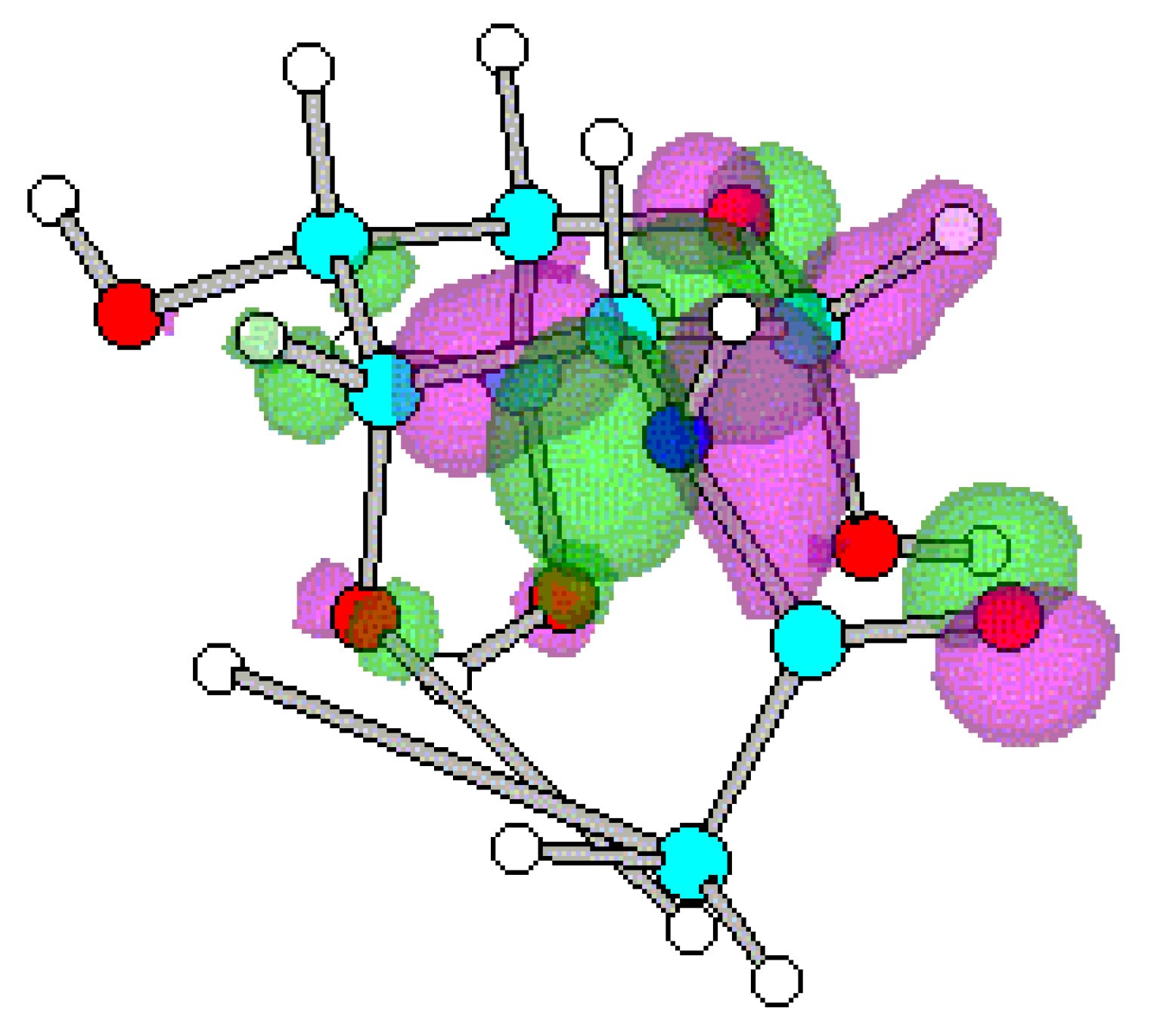
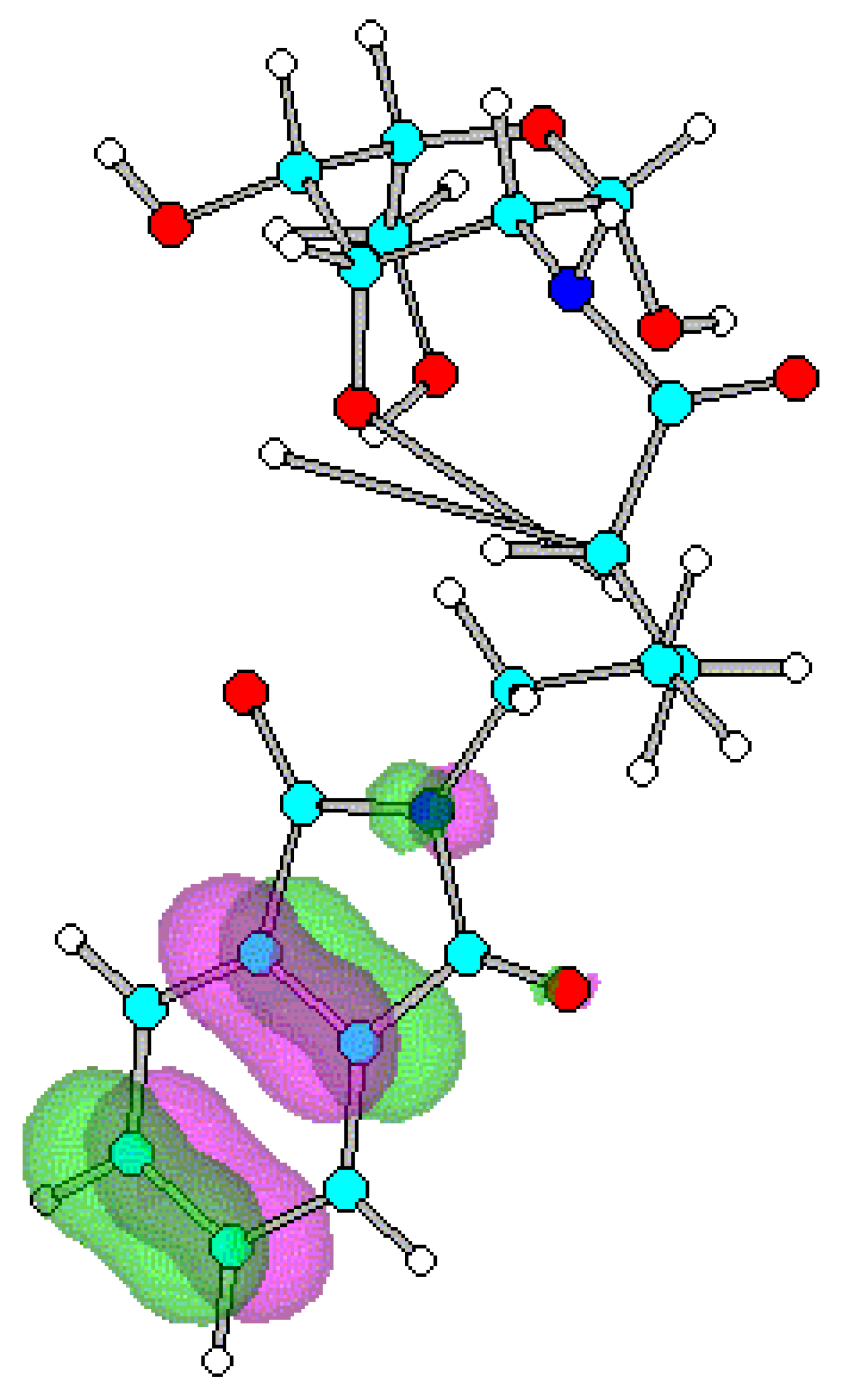
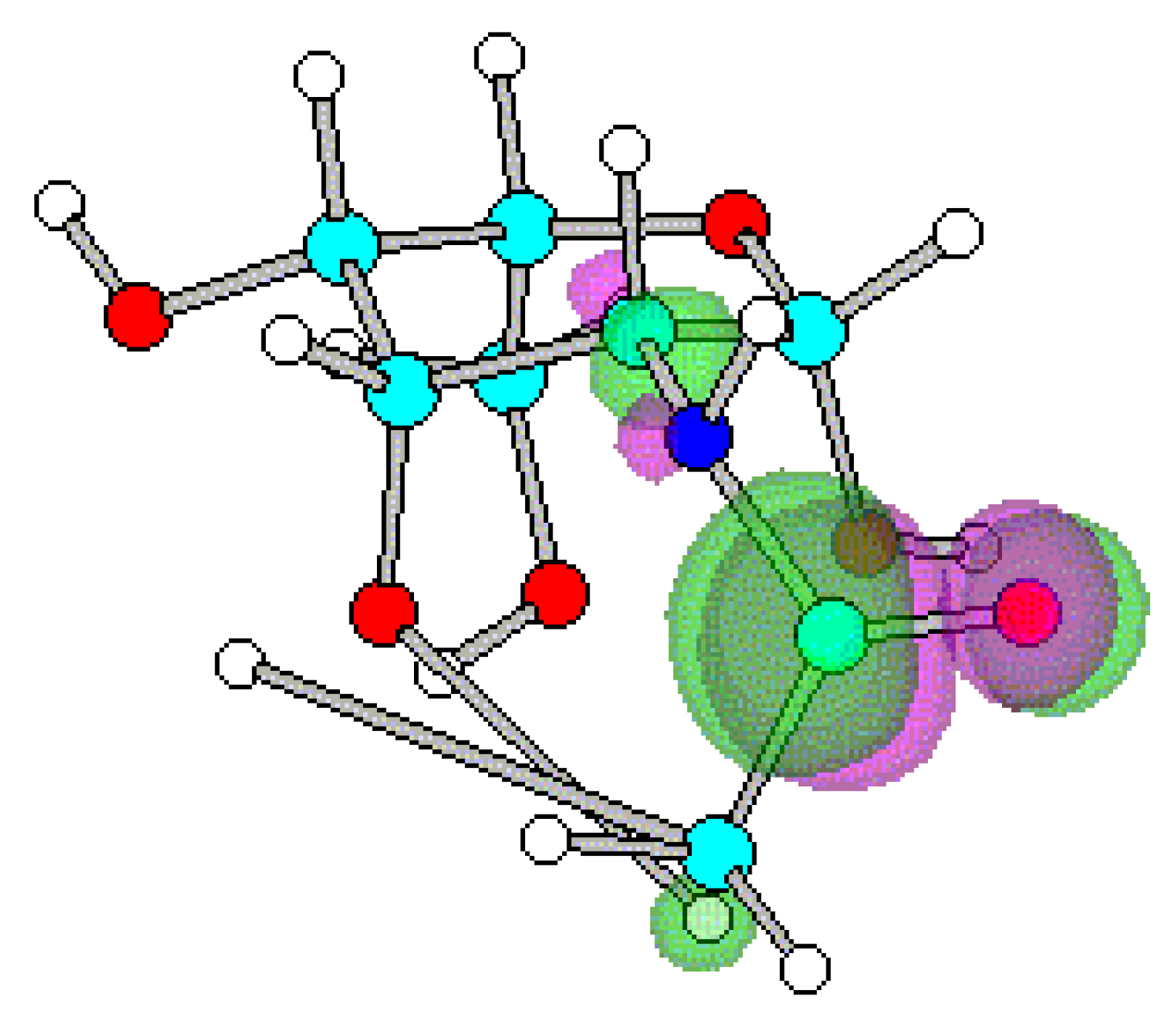
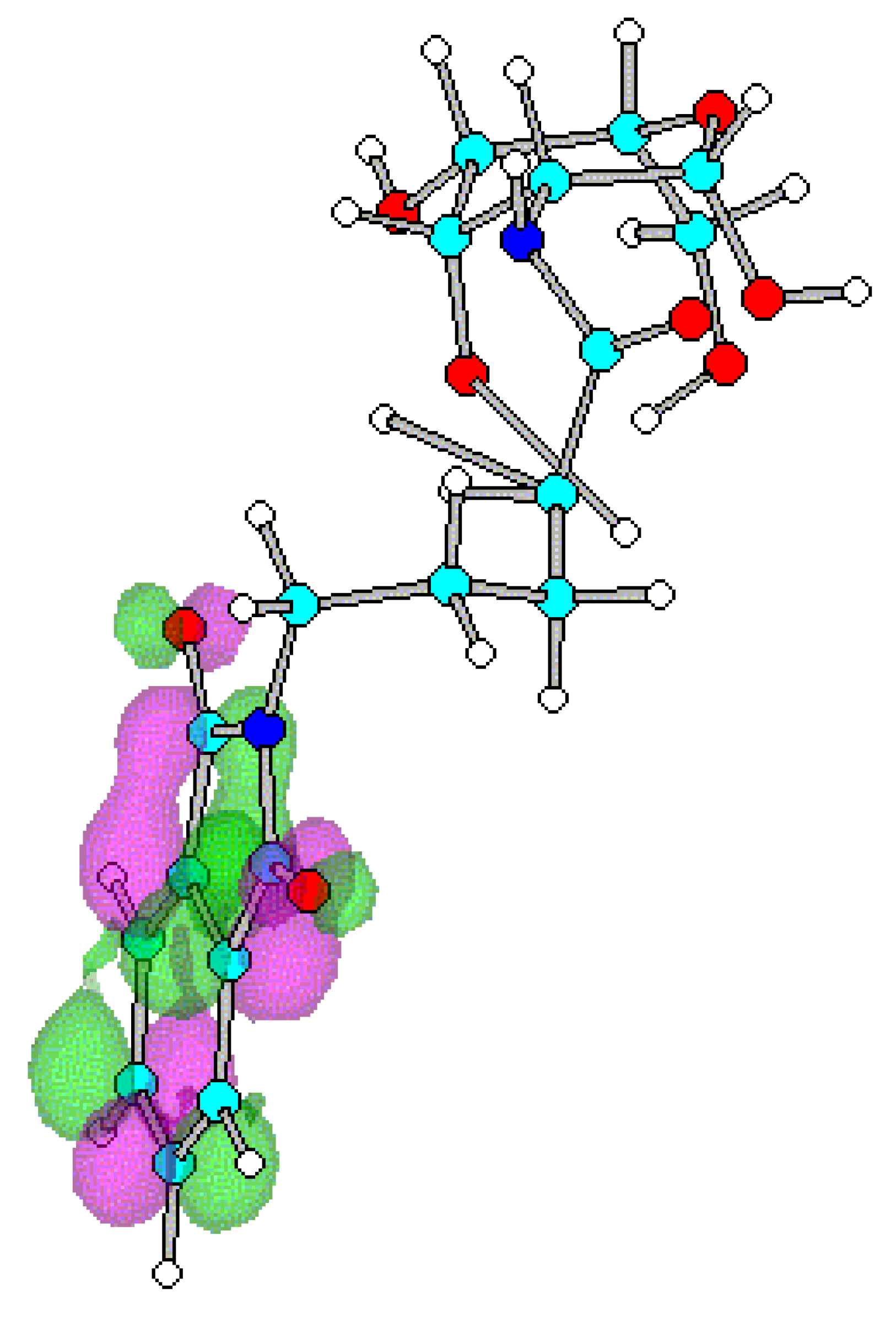
Conclusions
Acknowledgments
References and Notes
- Op Den Kamp, J. A. F.; Bonsen, P. P. M.; Van Deenen, L. L. M. Structural Investigation on Glucosaminyl Phosphatidyl-glicerol from Bacillus Megaterium. Biochim Biophys. Acta 1969, 176, 298–305. [Google Scholar] [CrossRef]
- Redlich, H.; Roy, W. Synthesen einiger β-Desosaminglycoside. Liebigs Ann. Chem. 1981, 7, 1215–1222. [Google Scholar] [CrossRef]
- Tang, P. W.; Williams, J.M. Further Studies of the Hydrazinolysis of 2-acetamido-1-N-acyl-2-desoxy-D-glucopyranosylamines. Carbohydr. Res. 1983, 121, 89–97. [Google Scholar] [CrossRef]
- Juodviršis, A.; Butenas, S.; Astrauskas, V. Derivatives of 2-N-(ω-phthalimidealkanoyl)amino-desoxy-1,3,4,6-tetra-O-acetyl-D-glucopyranoses Bearing Antiviral Activity. A. s. 1139148 (SU). 1984. [Google Scholar]
- Butenas, S. Synthesis and Investigation of N-acylated 2-amino-2-desoxyglucopyranoses and their Derivatives. Summary of doctoral thesis, Kaunas Politechnical Institute, Kaunas, 1985. [Google Scholar]
- Tang, P. W.; Williams, J.M. Degradation During the Hydrazinolysis of 2-acetamido-1-N-acyl-2-deoxy-b-D-glucopyranosylamines. Carbohydr.Res. 1983, 113, 13–15. [Google Scholar]
- Hyperchem 5.0, Hypercube, Inc., 1115 NW 4th Street, Gainesville, Florida 32601, USA.
- Sawicka, D.; Houk, K. Aromaticity and Antiaromaticity in Small Ring Transition States, Assessed by NICS Values and Energetics. J. Mol. Model. 2000, 6, 158–165. [Google Scholar] [CrossRef]
- Sella, A.; Basch, H.; Hoz, S. Reactivity of Strained Compounds: Is Ground State Destabilization the Major Cause for Rate Enhancement. J. Am. Chem. Soc. 1996, 118, 416. [Google Scholar] [CrossRef]
- Jager, R.; Debowski, M.; Manners, I.; Vancso, G. J. Study of the Molecular Geometry, Electronic Structure, and Thermal Stability of Phosphazene and Heterophosphasene Rings with ab Initio Molecular Orbital Calculations. Inorg. Chem. 1999, 38, 1153–1159. [Google Scholar] [CrossRef]
- Harrison, R.; Fletcher, H. G. Synthesis with Partially Benzylated Sugars. IV. A Route to Some 1-O-Acyl-2-acylamido-2-deoxy-D-glucopyranoses and -D-galactopyranoses. J. Org. Chem. 1965, 30, 2317–2321. [Google Scholar] [CrossRef] [PubMed]
- Jeanloz, R.W. 3,6-Di-O-methyl-D-glucosamide Hydrochloride (2-Amino-2-deoxy-3,6-di-O-methyl-D-glucose Hydrochloride). J. Org. Chem. 1961, 26, 905–908. [Google Scholar] [CrossRef]
- Sample Availability: Samples not available.
© 2000 by MDPI (http://www.mdpi.org).
Share and Cite
Vektariene, A.; Juodviršis, A.; Vektaris, G. Substitution Effects on Reactivity of N-Acyl-2-amino-2-desoxyglucopyranoses. Quantum Chemical Study. Molecules 2000, 5, 1399-1407. https://doi.org/10.3390/51201399
Vektariene A, Juodviršis A, Vektaris G. Substitution Effects on Reactivity of N-Acyl-2-amino-2-desoxyglucopyranoses. Quantum Chemical Study. Molecules. 2000; 5(12):1399-1407. https://doi.org/10.3390/51201399
Chicago/Turabian StyleVektariene, Aušra, Arvydas Juodviršis, and Gytis Vektaris. 2000. "Substitution Effects on Reactivity of N-Acyl-2-amino-2-desoxyglucopyranoses. Quantum Chemical Study" Molecules 5, no. 12: 1399-1407. https://doi.org/10.3390/51201399
APA StyleVektariene, A., Juodviršis, A., & Vektaris, G. (2000). Substitution Effects on Reactivity of N-Acyl-2-amino-2-desoxyglucopyranoses. Quantum Chemical Study. Molecules, 5(12), 1399-1407. https://doi.org/10.3390/51201399




