Abstract
The 1,3-dipolar cycloaddition reaction of N-benzyl-C-(2-furyl)nitrones with electron-rich alkenes gives preferentially trans-substituted 3,5-disubstituted isoxazolidines (endo approach). These experimental results are in good qualitative agreement with the predicted ones by semiempirical (AM1 and PM3) and ab initio (HF/3-21G) methods.
Introduction
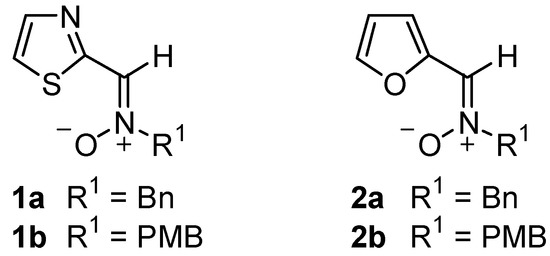
While abundant information on the reactivity of several classes of nitrones exists [1], hetaryl nitrones, i.e. nitrones bearing an heterocyclic ring at the carbon atom, have been the subject of very few investigations [2]. Recent publications from this laboratory have been concerned with the use of C-(2-thiazolyl) and C-(2-furyl)nitrones 1 and 2 as suitable substrates for both nucleophilic additions [3] and 1,3-dipolar cycloadditions [4]. We also provided a theoretical study of the 1,3-dipolar cycloadditions of several hetaryl nitrones with methyl acrylate [5]. Our continued interest in the reactivity of hetaryl nitrones, which has been scarcely explored, led us to investigate the reaction of C-(2-furyl)nitrones 2 with electron-rich alkenes and in this paper we report on these results.
Scheme 1.
Results
Furan-derived nitrones were prepared according to our method [6] from furfural and the corresponding benzyl hydroxylamine. In both cases only the Z-isomer was detected, the configuration being assigned on the basis of NOE experiments (a 10-12% enhancement was observed in the difference spectra upon irradiation of the azomethine proton of the nitrone and the benzylic protons. Further confirmation arose from the recording of the corresponding 1H NMR spectra in deuterochloroform and hexadeuterobenzene. The observed ASIS effect [7] confirmed the Z-configuration of nitrones 2. In addition it was possible to obtain a single crystal of 2a whose X-ray diffraction analysis confirmed a Z-configuration [8].
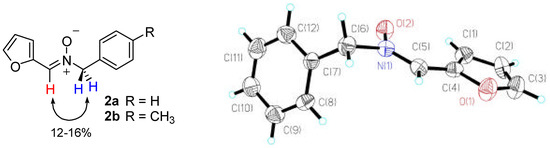
Figure 1.
Z-configuration of N-benzyl-C-(2-furyl)nitrones (left: observed NOE’s; right: ORTEP representation of 2a showing ellipsoids at 50% probability level).
The configurational stability of nitrones 2 was also checked. After refluxing in toluene for a week no changes in their structure were observed and the E-isomer could not be detected in any instance.
Refluxing nitrones 2 either in a solvent or neat with an excess of alkene 3 until TLC indicated disappearance of the nitrone afforded a mixture of isoxazolidines (Scheme 2).
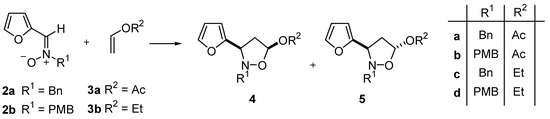
Scheme 2.

Table 1.
Cycloaddition of nitrones 2 with alkenes 3.
| entry | R1 | R2 | reaction conditions | 4 | 5 | yield(%) |
| 8 | Bn | OEt | Toluene / reflux / 10 d | 40 | 60 | 25 |
| 8 | Bn | OEt | neat / reflux / 10 d | 45 | 55 | 56 |
| 9 | Bn | OAc | CHCl3 / reflux / 10 d | 12 | 88 | 36 |
| 9 | Bn | OAc | Toluene / reflux / 10 d | 26 | 74 | 48 |
| 9 | Bn | OAc | neat / reflux / 10 d | 40 | 60 | 58 |
| 9 | PMB | OEt | Toluene / reflux / 13 d | 38 | 62 | 53 |
| 16 | PMB | OEt | neat / reflux / 10 d | 41 | 59 | 48 |
| 17 | PMB | OAc | CHCl3 / reflux / 17 d | 40 | 60 | 16 |
| 18 | PMB | OAc | neat / reflux / 10 d | 35 | 65 | 43 |
Bn: benzyl; PMB: p-methoxybenzyl
The obtained crude mixture was analyzed by NMR to determine the ratio of isomers, and the corresponding adducts were separated by preparative centrifugally accelerated radial thin layer chromatography (see experimental). The isoxazolidines obtained are indicated in Scheme 2 and the corresponding reaction conditions, selectivities and yields in Table 1.
For the purpose of comparison, the cycloaddition of nitrones 2 with disubstituted electron-poor alkenes, e.g. dimethyl fumarate and dimethyl maleate was also studied [9]. The results of this study are illustrated in Scheme 3 and summarized in Table 2.
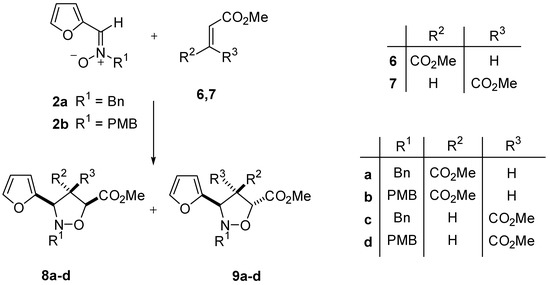
Scheme 3.

Table 2.
Cycloaddition of nitrones 2 with alkenes 6-7.
| entry | R1 | R2 | R3 | reaction conditions | 8 | 9 | yield(%) |
| 1 | Bn | CO2Me | H | CH2Cl2 / reflux / 48 h | 47 | 53 | 91 |
| 2 | Bn | CO2Me | H | Toluene / reflux / 12 h | 61 | 39 | 54 |
| 3 | Bn | H | CO2Me | 1,2-DCE / reflux / 16 h | 81 | 19 | 79 |
| 4 | Bn | H | CO2Me | Toluene / reflux / 12 h | 82 | 18 | 89 |
| 5 | PMB | CO2Me | H | Toluene / reflux / 12 h | 93 | 7 | 86 |
| 6 | PMB | H | CO2Me | Toluene / reflux / 12 h | 85 | 15 | 83 |
Bn: benzyl; PMB: p-methoxybenzyl
The structure and stereochemistry of isoxazolidines 4, 5, 8 and 9 were ascertained by careful examination of the 1H NMR spectra (using, when necessary, homonuclear proton NMR decoupling) and nuclear Overhausser effect (NOE) experiments. Compounds 4 and 5 showed the same 1H NMR trend (doublet-of-doublets) for H-3 and H-5 protons, thus allowing the assignment of the regiochemistry. In addition, the 1H NMR spectra of these compounds exhibited two well-defined doublet-of-doublet-of-doublets thereby confirming the substitution pattern of the oxazolidie ring. (see experimental for proton assignments).
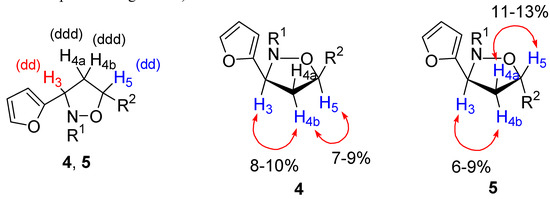
Figure 2.
1H NMR multiplicities of isoxazolidines and selected NOE data for isoxazolidines.
The analysis of vicinal coupling constants J3,4 and J4,5 did not result in an unambiguous configurational assignment of isoxazolidines 4, 5, 8 and 9. We have used nuclear Overhausser effects obtained by difference spectroscopy experiments for establishing the relative stereochemistry of the ring substituents (Figure 2). The irradiation of H-4b in 4 resulted in enhancement of the signals for H-3 and H-5. On the other hand, no effect was detected upon irradiation of H-4a. The same experiment performed on products 5 produced signal enhancements for H-3 when H-4b was irradiated and for H-5 upon irradiation of H-4a. All these effects are in agreement with a cis configuration for compounds 4 and a trans configuration for 5.The configuration of compounds 8 and 9 was also established by NOE experiments. In addition compounds 8 and 9 were transformed into the corresponding pyrrolidin-2-ones (Scheme 4) 10 and 11. NOE experiments were also carried out with these compounds in order to further assess the relative configuration of the substituents.
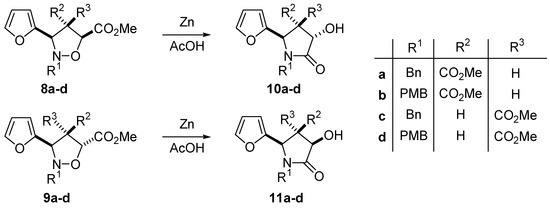
Scheme 4.
Discussion
The stereochemical outcome of the cycloadditions did not appear to be affected by the electron density of the dipolarophile. Both with electron-rich (Table 1) and electron-deficient (Table 2) alkenes similar results were obtained. In the case of vinyl acetate and ethyl vinyl ether the 3,5-regioisomers were obtained as the only adducts. This observed regioselectivity is in agreement with previously reported data for other nitrones [10]. A frontier molecular orbitals treatment showed that the HOMO(nitrone)-LUMO(alkene) interactions dominate the reactions in the case of electron-deficient alkenes and vinyl acetate.
In the case of cycloaddition with ethyl vinyl ether the interactions LUMO(nitrone)-HOMO(alkene) are more favorable [11]. In this case the smallest HOMO-LUMO gap exists for the LUMO of the nitrone and the HOMO of the alkene. The energies and the coefficients of the HOMO and LUMO of nitrones 2 and alkenes 3 calculated at a semiempirical level (MOPAC97, PM3) [12] were shown in Figure 3 (methyl acrylate is shown for comparison). The PM3-calculated FMO energies and the corresponding energy gaps for HOMO-LUMO interactions are given in Table 3 (favorable interactions are shown in blue; unfavorable interactions are shown in red).
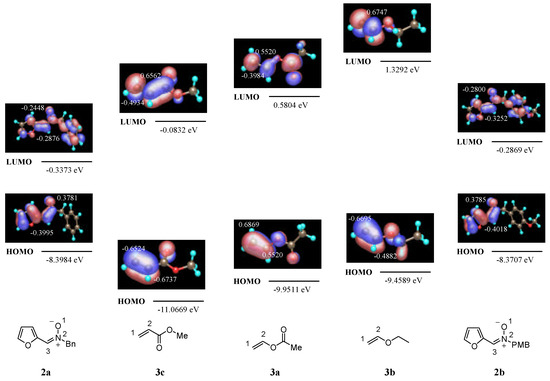
Figure 3.
PM3-calculated FMO energies and atomic contributions for 2 and 3.

Table 3.
PM3-calculated FMO energies and energy gaps for the cycloaddition of 2 with vinyl acetate 3a, ethyl vinyl ether 3b and methyl acrylate 3c.
| HOMO-nitrone | LUMO-nitrone | ||||||||||
| LUMO-alkene | 2a | -8.3984 | 2b | -8.3707 | HOMO-alkene | 2a | -0.3373 | 2b | -0.2869 | ||
| 3a | 0.5804 | 3a | -9.9511 | ||||||||
| 3b | 1.3292 | 3b | -9.4589 | ||||||||
| 3c | -0.0832 | 3c | -11.0669 | ||||||||
These energy gaps are in good qualitative agreement with the observed reactivity. The lowest difference in FMO’s energies was observed for cycloadditions to methyl acrylate. On the other hand values higher than 0.6 eV were found for cycloadditions to electron-rich alkenes 3a and 3b. Therefore, it is not surprising that a lower reactivity was observed for cycloadditions with vinyl acetate and ethyl vinyl ether with respect to those with methyl acrylate [4b]. The observed regioselectivity can be explained by the magnitude of the atomic components in the FMO of interest. Thus, for cycloadditions with methyl acrylate [4b] and vinyl acetate the atom with the calculated larger HOMO coefficient on the nitrone reacts with the atom with the larger LUMO coefficient of the alkene.
To discuss the stereoselectivities observed in the 1,3-dipolar cycloadditions studies it is required a careful evaluation of the different effects which can operate in the transition states leading to two diastereomeric cis and trans cycloadducts. The possibility of interconversion of the E and Z forms of the nitrones, although suggested for several authors [13], can be discarded on the basis of the proven stability of compounds 2 (vide supra). The preferential formation of trans isoxazolidines 5 can be explained by assuming an endo-approach of the dipolarophile to the nitrone. In order to corroborate this hypothesis, we carried out a theoretical study of the cycloaddition reaction by using both semiempirical[14] and ab initio methods[15]. We studied the cycloaddition of nitrones 2 with both vinyl acetate and ethyl vinyl ether [16]. For the purpose of comparison all optimized structures including the reactants, transition states and products were calculated at AM1, PM3 and HF/3-21G levels. The theoretical study included the starting system (nitrone and alkenes), the transition states and the primary cycloadduct for both endo and exo approaches. The more stable conformation was chosen for nitrone and alkenes. A PES exploration of the nitrone was made in order to find the absolute minimum corresponding to the more stable conformation. For vinyl acetate and methyl vinyl ether scis conformations were used (Figure 4). Sum of the calculated energies of nitrone and alkene was considered to be the energy of the reactants. The optimized geometries of the nitrone and alkenes showed the expected bond lengths and angles. In fact, rather similar structural data were obtained when the optimized geometry of the nitrone was compared with its X-ray structure [8].
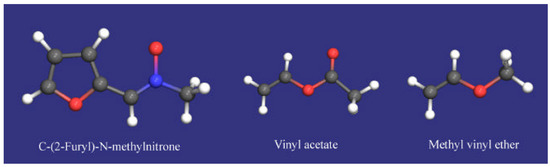
Figure 4.
Optimized (HF/3-21G) structures for nitrone 2 and alkenes 3a and 3b.
The TSs for the endo and exo approaches of vinyl acetate (TSa(endo) and TSa(exo)) and methyl vinyl ether (TSb(endo) and TSb(exo)) to the nitrone were located by the calculation of a reaction path profile starting from optimized geometries of the corresponding isoxazolidines, followed by an optimization of the TS with respect to all structural variables. The TSs were characterized through the calculation of the force constant matrix by ensuring that they had one and only one imaginary harmonic vibrational frequency corresponding to the formation of new bonds.
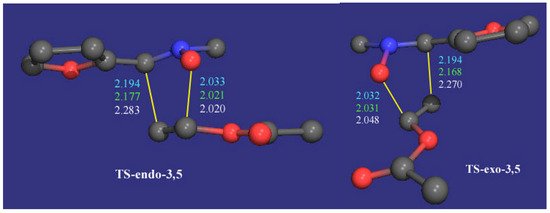
Figure 5.
Transition states for the reaction of 2 with 3a. Bonds distances are given in amstrongs (AM1 in cyan, PM3 in green and HF/3-21G in white). The hydrogen atoms have been omitted for clarity.
By intrinsic reaction coordinate calculation the transition states, the reagents and the corresponding cycloadducts were confirmed to be in the same reaction coordinate. In all cases the ZPE corrections were evaluated by carrying out the corresponding frequency analysis. The optimized transition structures are illustrated in Figure 5 and Figure 6 and the calculated parameters are given in Table 4.
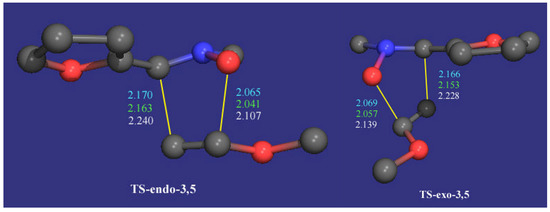
Figure 6.
Transition states for the reaction of 2 with 3b. Bonds distances are given in amstrongs (AM1 in cyan, PM3 in green and HF/3-21G in white). The hydrogen atoms have been omitted for clarity.

Table 4.
Semiempirical and ab initio energies of ground states, transition structures and adducts for cycloadditions of 2 with alkenes 3.a
| structure | AM1(kcal mol-1) | PM3(kcal mol-1) | HF / 3-21G*(Hartrees)c |
| nitrone 2ab | 27.46 | 9.05 | -432.764765 |
| vinyl acetate | -67.86 | -68.54 | -302.880245 |
| methylvinyl ether | -25.69 | -23.73 | -190.785715 |
| TSa(endo) | -19.69 | -26.34 | -735.612270 |
| TSa(exo) | -18.58 | -25.93 | -735.610633 |
| TSb(endo) | 20.68 | 17.59 | -623.496836 |
| TSb(exo) | 22.30 | 18.90 | -623.493286 |
| trans-5a,bb,d | -80.67 | -97.69 | -735.719216 |
| cis-4a,bb,e | -79.14 | -96.48 | -735.717813 |
| trans-5c,db,f | -39.03 | -51.09 | -623.602537 |
| cis-4c,db,g | -37.27 | -50.44 | -623.600680 |
a For TSs potential energy barriers are given in brackets and red colour. ZPVE correction has been evaluated. b For simplicity the N-benzyl group of the nitrone was replaced by a methyl group. c Energy barriers given in kcal mol-1. d from TSa(endo). e from TSa(exo). f from TSb(endo). g from TSb(exo).
As expected for 1,3-dipolar cycloadditions all TSs were asynchronous, the newly created C-O bond being formed to a greater extent than the C-C bond. The differences between the semiempirical and ab initio values of the C-O and C-C forming bonds are about 0.05 . The energies of the TSs depend on the approximation. In Figure 7 and Figure 8 the energy profiles are shown for the cycloaddition of nitrone to both vinyl acetate and methyl vinyl ether (with inclusion of ZPVE), respectively.
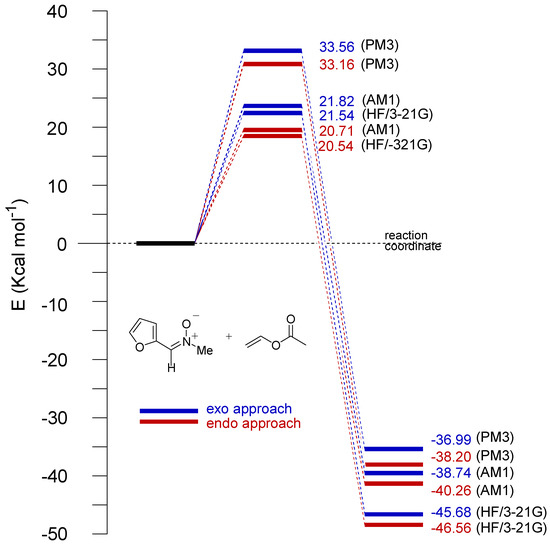
Figure 7.
Reaction profiles for the reaction of 2 with 3a (endo and exo approaches are shown in red and blue, respectively) according to the AM1, PM3, and HF/3-21G procedures.
In all cases, irrespective of the level of calculation, experimental results are qualitatively well reproduced by the calculations, which showed the endo approach (leading to trans adducts) to be the most favorable. Nevertheless, the calculated energy barriers for endo and exo approaches indicate that the reaction should led to the formation of cis/trans mixtures of cycloadducts.
Interestingly, in the case of cycloaddition with vinyl acetate the energy barriers obtained with PM3 are larger than those corresponding to AM1 and HF/3-21G, which, in turn, are rather similar. On the other hand, for the cycloaddition with vinyl methyl ether, rather different values of energy barriers we obtained for AM1 calculations. These latter values were shown to be lower than those obtained from PM3 and HF/3-21G calculations. In this case, however, these two approaches gave rise to similar values.
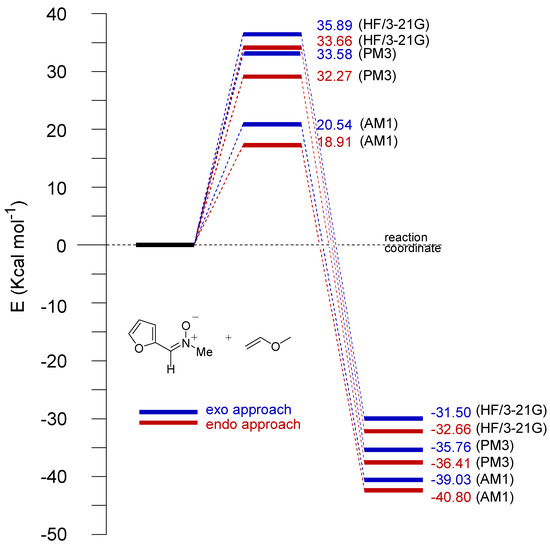
Figure 8.
Reaction profiles for the reaction of 2 with 3b (endo and exo approaches are shown in red and blue, respectively) according to the AM1, PM3, and HF/3-21G procedures.
Conclusion
In conclusion, our studies have revealed that the 1,3-dipolar cycloaddition of N-benzyl-C-(2-furyl) nitrones with electron-rich alkenes gives predominantly trans-3,5-disubstituted isoxazolidines although in lower yields than with electron-deficient alkenes. The regiochemistry of the cycloaddition seems to be controlled by FMO interactions, whereas the stereochemistry of the cycloaddition is mainly dominated by the preference for an endo approach. Such a preference is qualitatively well-reproduced by both semiempirical and ab initio calculations. With these results in hand, we are now expanding this reaction to other hetaryl nitrones and applying it to the synthesis of several nitrogenated compounds.
Experimental
General
The reaction flasks and other glass equipment were heated in an oven at 130°C overnight and assembled in a stream of Ar. All solvents were dried by the usual methods. All reactions were monitored by TLC on silica gel 60 F254; the position of the spots were detected with 254 nm UV light or by spraying with one of the following staining systems: 50% methanolic sulfuric acid, 5% ethanolic phosphomolybdic acid and iodine. Preparative column chromatography was performed on columns of silica gel (60-240 mesh) and with solvents that were distilled prior to use. Preparative centrifugally accelerated radial thin-layer chromatography (PCAR-TLC) was performed with a Chromatotron® Model 7924 T (Harrison Research, Palo Alto, CA, USA); the rotors (1 or 2 mm layer thickness) were coated with silica gel Merck grade type 7749, TLC grade, with binder and fluorescence indicator (Aldrich 34,644-6) and the eluting solvents were delivered by the pump at a flow-rate of 0.5-1.5 mL min-1. Melting points were uncorrected. 1H and 13C NMR spectra were recorded either on a Varian Unity or on a Bruker 300 instrument. Chemical shifts are reported in ppm (δ) relative to CHCl3 (δ = 7.26) in CDCl3. Elemental analyses were performed on a Perkin Elmer 240B microanalyzer.
N-Benzyl-C-(2-furyl)nitrone 2a
To a well stirred solution of furfural (0.96 g, 10 mmol) in dichloromethane (20 ml) were added N-benzylhydroxylamine (1.23 g, 10 mmol) and magnesium sulfate (2.4 g, 20 mmol). The resulting mixture was stirred for 4 h at which time the reaction mixture was filtered and the filtrate evaporated under reduced pressure. The residue was purified by column chromatography (Et2O) to give the nitrone (1.78 g, 88%) as a crystalline solid: mp 99-101°C; Rf (Et2O)= 0.36; 1H NMR (CDCl3): δ 4.99 (s, 2H, NCH2Ph), 6.51 (dd, 1H, J = 1.7, 3.4 Hz, H4’), 7.36-7.44 (m, 6H, ArH and H3’), 7.50 (s, 1H, H1), 7.75 (d, 1H, J = 3.4 Hz, H5’); 13C NMR (CDCl3): δ 69.6, 112.3, 115.4, 125.2, 129.0, 129.1, 129.4, 132.8, 143.7, 146.8.
Anal. Calcd for C12H11NO2 (201.22): C, 71.63; H, 5.51; N, 6.96. Found: C, 71.86; H, 5.38; N, 6.83.
N-(4-Metoxybenzyl)-C-(2-furyl)nitrone 2b
The method described above to prepare 2a was carried out using N-(4-methoxybenzyl)hydroxylamine (1.53 g, 10 mmol) to give, after column chromatography (Et2O), pure 2b (1.64 g, 71%) as a white solid;mp 108-110 °C; Rf (Et2O)= 0.35; 1H NMR (CDCl3): δ 3.80 (s, 3H, OCH3), 4.93 (s, 2H, NCH2Ph), 6.50 (dd, 1H, J = 1.8, 3.5 Hz, H4’), 6.91 (m, 2H, ArH), 6.90 (m, 2H, ArH), 7.34 (d, 1H, J = 1.8 Hz, H3’), 7.43 (s, 1H, H1), 7.74 (d, 1H, J = 3.5 Hz, H5’); 13C NMR (CDCl3): δ: 55.3, 69.0, 112.3, 114.4, 115.3, 124.6, 124.8, 131.1, 143.6, 146.8, 160.2.
Anal Calcd. for C13H13NO3 (231.25): C, 67.52; H, 5.67; N, 6.06. Found: C, 67.36; H, 5.52; N, 6.17.
General Procedure 1,3-Dipolar Cycloaddition of Nitrones 2 with vinyl acetate 3a
To a solution of the corresponding nitrone (1 mmol) in toluene was added vinyl acetate (4.3 g, 50 mmol) and the resulting solution was heated under an inert atmosphere (Ar) at reflux for 10 days at which time the mixture was cooled to ambient temperature and concentrated under reduced pressure. The diastereomeric ratio (d.r. %) of the residue was determined by 1H NMR analysis. The crude material was purified using a Chromatotron® (2 mm layer thickness).
(3S*,5R*)-5-(Acetoxy)-2-benzyl-3-(2-furyl)isoxazolidine 4a
Rf (hexane-Et2O, 1:1) = 0.51; 1H NMR (CDCl3, 55°C): δ 2.08 (s, 3H, CH3), 2.69 (ddd, 1H, J = 2.9, 9.4, 13.6 Hz, H4a), 2.91 (ddd, 1H, J = 6.8, 7.9, 13.6 Hz, H4b), 3.91 and 4.22 (2d, 2H, J = 14.5 Hz, NCH2Ph), 3.94 (dd, 1H, J = 7.9, 9.4 Hz, H3), 6.31-6.36 (m, 2H, H3’ and H4’), 6.37 (dd, 1H, J = 2.9, 6.8 Hz, H5), 7.22-7.36 (m, 5H, ArH), 7.40 (bs, 1H, H5’).
13C NMR (CDCl3, 55°C): δ 21.2, 41.8, 60.1, 62.4, 95.2, 108.8, 110.5, 127.3, 128.1, 129.3, 136.3, 142.8, 150.2, 170.4.
Anal Calcd. for C16H17NO4 (287.31): C, 66.89; H, 5.96; N, 4.88. Found: C, 66.71; H, 5.86; N, 4.84.
(3S*,5S*)-5-(Acetoxy)-2-benzyl-3-(2-furyl)isoxazolidine 5a
Rf (hexane-Et2O, 1:1) = 0.44; 1H NMR (CDCl3, 55°C): δ 2.10 (s, 3H, CH3), 2.63 (dd, 1H, J = 6.6, 13.2 Hz, H4a), 2.88 (ddd, 1H, J = 5.4, 8.5, 13.2 Hz, H4b), 4.07 and 4.15 (2d, 2H, J = 13.9 Hz, NCH2Ph), 4.39 (dd, 1H, J = 6.8, 8.5 Hz, H3), 6.23 (d, 1H, J = 3.2 Hz, H3’), 6.31-6.34 (m, 1H, H4’), 6.44 (d, 1H, J = 5.4 Hz, H5), 7.25-7.40 (m, 6H, ArH and H5’); 13C NMR (CDCl3, 55°C): δ 21.2, 41.4, 60.3, 62.1, 96.9, 108.0, 110.4, 127.4, 128.2, 129.3, 136.4, 142.6, 151.5, 169.8.
Anal Calcd. for C16H17NO4 (287.31): C, 66.89; H, 5.96; N, 4.88. Found C, 67.00; H, 5.73; N, 4.92.
(3S*,5R*)-5-(Acetoxy)-2-(4-methoxybenzyl)benzyl-3-(2-furyl)isoxazolidine 4b
Rf (hexane-Et2O, 2:1) = 0.49; 1H NMR (CDCl3, 55°C): δ 2.09 (s, 3H, CH3), 2.72 (ddd, 1H, J = 2.5, 8.6, 13.5 Hz, H4a), 2.88 (ddd, 1H, J = 6.4, 8.0, 13.5 Hz, H4b), 3.76 (s, 3H, OCH3), 3.90 and 4.25 (2d, 2H, J = 14.1 Hz, NCH2Ph), 3.90 (dd, 1H, J = 8.0, 8.6 Hz, H3), 6.30-6.34 (m, 2H, H3’ and H4’), 6.41 (dd, 1H, J = 2.5, 6.4 Hz, H5), 6.78 (m, 2H, ArH), 7.22 (m, 2H, ArH), 7.39 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C) δ 21.5, 43.6, 55.4, 62.3, 63.5, 98.1, 109.2, 110.4, 113.7, 129.4, 129.9, 142.9, 152.4, 158.9, 170.2.
Anal Calcd. for : C17H19NO5 (317.13): C, 64.34; H, 6.03; N, 4.41. Found C, 64.48; H, 5.96; N, 4.31.
(3S*,5S*)-5-(Acetoxy)-2-(4-methoxybenzyl)-3-(2-furyl)isoxazolidine 5b
Rf (hexane-Et2O, 2:1) = 0.38; 1H NMR (CDCl3, 55°C): δ 2.08 (s, 3H, CH3), 2.55 (ddd, 1H, J = 1.1, 5.9, 13.3 Hz, H4a), 2.90 (ddd, 1H, J = 6.0, 9.1, 13.3 Hz, H4b), 3.76 (s, 3H, OCH3), 4.12 and 4.20 (2d, 2H, J = 14.2 Hz, NCH2Ph), 4.36 (dd, 1H, J = 5.9, 9.1 Hz, H3), 6.21 (d, 1H, J = 3.2 Hz, H3’), 6.30-6.33 (m, 1H, H4’), 6.50 (d, 1H, J = 1.1, 6.0 Hz, H5), 6.78 (m, 2H, ArH), 7.22 (m, 2H, ArH), 7.40 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C): δ 21.3, 40.9, 56.7, 61.0, 61.9, 95.6, 108.9, 109.3, 113.6, 129.2, 129.8, 142.7, 150.9, 160.2, 170.4.
Anal. Calcd. for: C17H19NO5 (317.13): C, 64.34; H, 6.03; N, 4.41. Found C, 64.56; H, 6.17; N, 4.55.
General Procedure 1,3-Dipolar Cycloaddition of Nitrones 2 with ethyl vinyl ether 3b
To a solution of the corresponding nitrone (1 mmol) in toluene was added ethyl vinyl ether (3.6 g, 50.0 mmol) and the resulting solution was heated under an inert atmosphere (Ar) at reflux for 10 days at which time the mixture was cooled to ambient temperature and concentrated under reduced pressure. The diastereomeric ratio (d.r. %) was determined on the residue by 1H NMR analysis. The crude material was purified with the Chromatotron® (2 mm layer thickness).
(3S*,5R*)-5-Ethoxy-3-(2-furyl)-2-benzylisoxazolidine 4c
Rf (hexane-Et2O, 3 : 1)= 0.55; 1H NMR (CDCl3, 55°C): δ 1.22 (t, 3H, J = 7.1 Hz, CH3CH2O), 2.58 (ddd, 1H, J = 1.7, 6.9, 12.7 Hz H4a), 2.70 (ddd, 1H, J = 5.1, 8.2, 12.7 Hz, H4b), 3.47 (q, 2H, J = 7.1 Hz, CH3CH2O), 4.13 (s, 2H, NCH2Ph), 4.39 (t, 1H, J = 7.6 Hz, H3), 5.25 (dd, 1H, J = 1.7, 5.1 Hz, H5), 6.31 (dd, 1H, J = 1.8, 3.3 Hz, H4’), 6.35 (d, 1H, J = 3.3 Hz, H3’), 7.18-7.60 (m, 6H, ArH and H5’). 13C NMR (CDCl3, 55°C): δ 14.9, 41.5, 59.9, 63.1, 63.5, 102.9, 107.0, 110.2, 126.8, 128.0, 128.8, 137.8, 142.0, 150.2.
Anal. Calcd. for C16H19NO3 (273.33): C, 70.31; H, 7.01; N, 5.12. Found C, 70.26; H, 7.22; N, 5.18.
(3S*,5S*)-5-Ethoxy-3-(2-furyl)-2-benzylisoxazolidine 5c
Rf (hexane-Et2O, 3 : 1)= 0.48; 1H NMR (CDCl3, 55°C): δ 1.15 (t, 3H, J = 7.1 Hz, CH3CH2O), 2.57 (ddd, 1H, J = 3.3, 6.5, 13.2 Hz, H4a), 2.76 (ddd, 1H, J = 6.5, 8.1, 13.2 Hz, H4b), 3.71 (q, 2H, J = 7.1 Hz, CH3CH2O), 3.80 and 4.20 (2d, 2H, J = 14.4 Hz, NCH2Ph), 4.30 (t, 1H, J = 7.3 Hz, H3), 5.17 (dd, 1H, J = 3.3, 6.5 Hz, H5), 6.16 (d, 1H, J = 3.3 Hz, H3’), 6.27 (dd, 1H, J = 1.8, 3.3 Hz, H4’), 7.18-7.60 (m, 6H, ArH and H5’); 13C NMR (CDCl3, 55°C): δ 15.0, 42.0, 61.4, 63.0, 63.3, 100.9, 108.3, 110.1, 126.9, 127.8, 128.7, 137.4, 142.4, 151.0.
Anal. Calcd. for C16H19NO3 (273.33): C, 66.89; H, 5.96; N, 4.88. Found C, 66.72; H, 5.93; N, 4.95.
(3S*,5R*)-5-Ethoxy-3-(2-furyl)-2-(4-metoxybenzyl)isoxazolidine 4d
Rf (hexane-Et2O, 7:3) = 0.33; 1H NMR (CDCl3, 55°C): δ 0.90 (t, 3H, J = 6.1 Hz, CH3CH2O), 2.56 (ddd, 1H, J = 1.5, 7.6, 13.4 Hz, H4a), 2.69 (ddd, 1H, J = 5.3, 7.6, 13.4 Hz, H4b), 3.75 and 4.13 (2d, 2H, J = 14.5 Hz, NCH2Ph), 3.86 (s, 3H, OCH3), 4.20 (q, 2H, J = 6.1 Hz, CH3CH2O), 4.38 (t, 1H, J = 7.6 Hz, H3), 5.24 (dd, 1H, J = 1.5, 5.3 Hz, H5), 6.30 (dd, 1H, J = 1.9, 3.1 Hz, H4’), 6.33 (d, 1H, J = 3.1 Hz, H3’), 6.98 (m, 2H, ArH), 7.35 (m, 2H, ArH), 7.33 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C): δ 15.0, 41.4, 55.2, 63.1, 63.7, 66.7, 103.4, 108.7, 110.2, 113.5, 128.7, 130.8, 142.2, 150.4, 158.6.
Anal. Calcd. for C17H21NO4 (303.35): C, 67.31; H, 6.98; N, 4.62. Found C, 67.34; H, 6.80; N, 4.45.
(3S*,5S*)-5-Ethoxy-3-(2-furyl)-2-(4-metoxybenzyl)isoxazolidine 5d
Rf (hexane-Et2O, 7:3) = 0.29; 1H NMR (CDCl3, 55°C): δ 1.20 (t, 3H, J = 5.7 Hz, CH3CH2O), 2.55 (ddd, 1H, J = 3.1, 8.0, 13.4 Hz, H4a), 2.74 (ddd, 1H,J = 6.5, 8.0, 13.4 Hz, H4b), 3.76 (s, 3H, OCH3), 4.08 (s, 2H, NCH2Ph), 4.24 (q, 2H, J = 5.7 Hz, CH3CH2O), 4.30 (t, 1H, J = 8.0 Hz, H3), 5.17 (dd, 1H, J = 3.1, 6.5 Hz, H5), 6.15 (d, 1H, J = 3.1 Hz, H3’), 6.26 (dd, 1H, J = 1.5, 3.1 Hz, H4’), 6.88 (m, 2H, ArH), 7.27 (m, 2H, ArH), 7.33 (d, 1H, J= 1.5 Hz, H5’); 13C NMR (CDCl3, 55°C): δ 15.0, 41.8, 55.5, 62.4, 64.0, 66.9, 102.8, 107.2, 110.3, 113.4, 126.7, 132.2, 142.6, 148.5, 158.8.
Anal. Calcd. for C17H21NO4 (303.35): C, 67.31; H, 6.98; N, 4.62. Found C, 67.16; H, 7.02; N, 4.77.
General Procedure 1,3-Dipolar Cycloaddition of Nitrones 2 with methyl fumarate 6 and methyl maleate 7
To a solution of the corresponding nitrone (1 mmol) in toluene was added the corresponding alkene (0.72 g, 5.0 mmol) and the resulting solution was heated under an inert atmosphere (Ar) at reflux for 12 h at which time the mixture was cooled to ambient temperature and concentrated under reduced pressure. The diastereomeric ratio (d.r. %) was determined on the residue by 1H NMR analysis. The crude material was purified with the Chromatotron® (2 mm layer thickness).
(3S*,4S*,5S*)-2-Benzyl-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 8a
Rf (hexane-Et2O, 3:2) = 0.62; 1H NMR (CDCl3, 55°C): δ 3.70 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.88 and 4.11 (2d, 2H, J = 14.7 Hz, NCH2Ph), 4.06 (dd, 1H, J = 7.1, 8.6 Hz, H4), 4.56 (d, 1H, J = 8.6 Hz, H3), 5.13 (d, 1H, J = 7.1 Hz, H5), 6.27 (d, 1H, J = 3.3 Hz, H3’), 6.29 (dd, 1H, J = 1.7, 3.3 Hz, H4’), 7.18-7.38 (m, 6H, ArH and H5’); 13C NMR (CDCl3, 55°C): δ 52.0, 52.3, 55.7, 59.1, 64.5, 66.5, 109.3, 110.4, 127.3, 128.1, 128.7, 136.6, 142.4, 149.4, 169.1, 171.1.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found C, 62.43; H, 5.67; N, 4.12.
(3S*,4R*,5R*)-2-Benzyl-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 9a
Rf (hexane-Et2O, 3 : 2) = 0.52; 1H NMR (CDCl3, 55°C): δ 3.70 (s, 3H, OCH3), 3.77 (s, 3H, OCH3), 4.03 and 4.09 (2d, 2H, J = 14.4 Hz, NCH2Ph), 4.16 (dd, 1H, J = 4.0, 7.8 Hz, H4), 4.20 (d, 1H J = 7.8 Hz, H3), 4.89 (d, 1H, J = 4.0 Hz, H5), 6.29 (dd, 1H, J = 1.7, 3.3 Hz, H4’), 6.35 (d, 1H, J = 3.3 Hz, H3’), 7.18-7.38 (m, 6H, ArH and H5’); 13C NMR (CDCl3, 55°C): δ 52.0, 52.4, 54.8, 59.1, 64.5, 66.5, 109.3, 110.4, 127.0, 128.0, 128.4, 136.4, 143.0, 149.4, 170.8, 170.9.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found C, 62.47; H, 5.71; N, 4.13.
(3S*,4S*,5S*)-2-(4.Methoxybenzyl)-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 8b
Rf (hexane-Et2O, 7 : 3) = 0.11; 1H NMR (CDCl3, 55°C): δ 3.70 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.82 and 4.04 (2d, 2H, J = 14.5 Hz, NCH2Ph), 4.12 (dd, 1H, J = 4.4, 7.6 Hz, H4), 4.19 (d, 1H, J = 7.6 Hz, H3), 4.89 (d, 1H, J = 4.4 Hz, H5), 6.30 (dd, 1H, J = 1.8, 3.2 Hz, H4’), 6.33 (d, 1H, J = 3.2 Hz, H3’), 6.79 (m, 2H, ArH), 7.23 (m, 2H, ArH), 7.35 (dd, 1H, J = 0.8, 1.8 Hz, H5’); 13C NMR (CDCl3, 55°C): δ 52.3, 52.5, 55.1, 55.7, 58.5, 66.1, 77.5, 109.3, 110.5, 113.6, 128.5, 129.7, 142.9, 149.5, 159.0, 170.9, 171.1.
Anal. Calcd. for C19H21NO7 (375.37): C, 60.79; H, 5.64; N, 3.73. Found C, 60.63; H, 4.94; N, 3.79.
(3S*,4R*,5R*)-2-(4-Methoxybenzyl)-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 9b
Rf (hexane-Et2O, 7 : 3) = 0.16; 1H NMR (CDCl3, 55°C): δ 3.70 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.76 (s, 3H, OCH3), 3.83 and 4.02 (2d, 2H, J = 14.4 Hz, NCH2Ph), 4.03 (t, 1H, J = 7.3 Hz, H4), 4.52 (d, 1H, J = 7.5 Hz, H3), 5.10 (d, 1H, J = 7.1 Hz, H5), 6.25 (d, 1H, J = 3.1 Hz, H4’), 6.31-6.35 (m 1H, H3’), 6.79 (m, 2H, ArH), 7.19 (m, 2H, ArH), 7.31 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C): δ 52.0, 52.3, 54.8, 55.1, 58.5, 66.1, 77.5, 109.2, 110.4, 113.7, 128.4, 130.0, 142.4, 149.5, 159.1, 169.1, 170.9.
Anal. Calcd. for C19H21NO7 (375.37): C, 60.79; H, 5.64; N, 3.73. Found C, 60.94; H, 5.48; N, 3.78.
(3S*,4R*,5S*)-2-Benzyl-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 8c
Rf (hexane-Et2O, 3 : 2) = 0.43; mp 75°C; 1H NMR (CDCl3, 55°C): δ 3.64 (s, 3H, OCH3), 3.72 (s, 3H, OCH3), 4.02 (dd, 1H, J = 8.8, 9.0 Hz, H4), 4.05 (s, 2H, NCH2Ph), 4.40 (d, 1H, J = 8.8 Hz, H3), 4.88 (d, 1H, J = 9.0 Hz, H5), 6.31 (dd, 1H, J = 1.7, 3.3 Hz, H4’), 6.33 (d, 1H, J = 3.3 Hz, H3’), 7.18-7.32 (m, 5H, ArH), 7.39 (d, 1H, J = 1.7 Hz, H5’); 13C NMR (CDCl3, 55°C): δ 51.8, 52.0, 52.1, 55.5, 60.3, 66.0, 109.3, 110.4, 127.1, 128.0, 128.1, 136.9, 143.0, 149.2, 168.9, 169.5.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found 62.72; H, 5.43; N, 3.98.
(3S* 4S*,5R*)-2-Benzyl-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 9c
Rf (hexane-Et2O, 3 : 2) = 0.38; mp 91-93 •C; 1H NMR (CDCl3, 55°C): δ 3.40 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.86 and 4.16 (2d, 2H, J = 14.5 Hz, NCH2Ph), 4.04 (dd, 1H, J = 8.1, 8.8 Hz, H4), 4.38 (d, 1H, J = 8.1 Hz, H3), 4.76 (d, 1H, J = 8.8 Hz, H5), 6.30 (dd, 1H, J = 1.7, 3.1 Hz, H4’), 6.39 (d, 1H, J = 3.1 Hz, H3’), 7.16-7.40 (m, 6H, ArH and H5’); 13C NMR (CDCl3, 55°C): δ 51.8, 52.0, 52.2, 55.2, 58.8, 65.0, 109.5, 110.6, 127.3, 128.1, 129.0, 135.8, 142.3, 148.4, 168.6, 169.6.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found C, 62.47; H, 5.67; N, 4.10.
(3S*,4R*,5S*)-2-(4.Methoxybenzyl)-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 8d
Rf (hexane-Et2O, 3 : 2) = 0.18; mp 74-76°C; 1H NMR (CDCl3, 55°C): δ 3.63 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 3.72 (s, 3H, OCH3), 3,97 and 4.03 (2d, 2H, J = 13.9 Hz, NCH2Ph), 3.99 (t, 1H, J = 8.9 Hz, H4), 4.40 (d, 1H, J = 8.9 Hz, H3), 4.85 (d, 1H, J = 8.9 Hz, H5), 6.29-6.36 (m, 2H, H4’ and H3’), 6.78 (m, 2H, ArH), 7.21 (m, 2H, ArH), 7.37 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C): δ 51.9, 52.0, 55.0, 55.3, 59.5, 65.7, 76.8, 109.2, 110.3, 113.5, 128.8, 130.0, 142.9, 149.1, 158.9, 168.8, 169.5.
Anal. Calcd. for C19H21NO7 (375.37): C, 60.79; H, 5.64; N, 3.73. Found C, 60.67; H, 5.75; N, 3.65.
(3S*,4S*,5R*)-2-(4-Methoxybenzyl)-3-(2-furyl)-4,5-bis(methoxycarbonyl)isoxazolidine 9d
Rf (hexane-Et2O, 3 : 2) = 0.14; mp 91-93°C; 1H NMR (CDCl3, 55°C): δ 3.42 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.78 (s, 3H, OCH3), 4.05 (t, 1H, J = 8.0 Hz, H4), 4.25 and 4.75 (2d, 2H, J = 13.5 Hz, NCH2Ph), 4.38 (d, 1H, J = 8.0 Hz, H3), 4.55 (d, 1H, J = 8.0 Hz, H5), 6.34 (m, 1H, H4’), 6.41 (m, 1H, H3’), 6.84 (m, 2H, ArH), 7.24 (m, 2H, ArH), 7.38 (bs, 1H, H5’); 13C NMR (CDCl3, 55°C): δ 51.8, 52.0, 55.2, 55.3, 58.5, 64.6, 75.8, 109.4, 110.7, 113.7, 127.7, 130.5, 142.5, 148.5, 159.2, 168.7, 169.6.
Anal. Calcd. for C19H21NO7 (375.37): C, 60.79; H, 5.64; N, 3.73. Found C, 60.91; H, 5.51; N, 3.79.
General Procedure for the reduction of isoxazolidines 8 and 9. Synthesis of pyrrolidin-2-ones 10 and 11
To a solution of the corresponding isoxazolidine (1 mmol) in THF (10 mL) were added acetic acid (20 mL) and water (10 mL). The resulting solution was then treated with Zn dust (0.4 g, 6.1 mmol) and heated at 60 °C for 5 h. The reaction mixture was cooled to room temperature and then filtered through a short pad of Celite. The filtrate was neutralized with saturated aqueous sodium carbonate until pH = 8-9 and then extracted with CH2Cl2 (3 x 20 mL). The combined organic extracts were joined, washed with brine, dried over MgSO4 and evaporated under reduced pressure. The obtained crude material was purified with the Chromatotron® (2 mm layer thickness).
(3S*,4S*,5S*)-1-Benzyl-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 10a
Rf (Et2O ) = 0.49; mp 129-131°C; 1H NMR (CDCl3): δ 3.57 (dd, 1H, J = 3.8, 8.1 Hz, H4), 3.59 and 4.96 (2d, 2H, J = 15.0 Hz, NCH2Ph), 3.65 (s, 3H, OCH3), 4.50 (bs, 1H, OH), 4.79 (d, 1H, J = 3.8 Hz, H5), 4.90 (d, 1H, J = 8.1 Hz, H3), 6.22 (d, 1H, J = 3.2 Hz, H3’), 6.30 (dd, 1H, J = 1.8, 3.2 Hz, H4’), 7.18-7.39 (m, 6H, ArH and H5’); 13C NMR (CDCl3): δ 44.8, 48.6, 52.6, 54.4, 69.9, 110.2, 110.5, 127.7, 128.4, 128.6, 134.9, 143.5, 149.5, 169.7, 172.6.
Anal. Calcd. for C17H17NO5 (315.32): C, 64.75; H, 5.43; N, 4.44. Found C, 64.53; H, 5.60; N, 4.54.
(3R*,4R*,5S*)-1-Benzyl-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 11a
Rf (Et2O ) = 0.33; 1H NMR (CDCl3): δ 3.52 (t, 1H, J = 7.0 Hz, H4), 3.54 (s, 3H, OCH3), 3.60 (bs, 1H, OH), 3.71 and 3.78 (2d, 2H, J = 14.9 Hz, NCH2Ph), 4.51 (d, 1H, J = 7.0 Hz, H5), 4.67 (d, 1H, J = 7.0 Hz, H3), 6.30 (d, 1H, J = 2.2 Hz, H3’), 6.36 (dd, 1H, J = 1.8, 2.8 Hz, H4’), 7.01-7.38 (m, 6H, ArH and H5’); 13C NMR (CDCl3): δ 45.0, 48.1, 52.2, 53.8, 70.4, 110.5, 110.8, 127.9, 128.4(2C), 135.4, 143.6, 148.0, 169.4, 172.2.
Anal. Calcd. for C17H17NO5 (315.32): C, 64.75; H, 5.43; N, 4.44. Found C, 64.89; H, 5.68; N, 4.27.
(3S*,4S*,5S*)-1-(4-Methoxybenzyl)-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 10b
Rf (Et2O ) = 0.47; mp 111-113°C; 1H NMR (CDCl3): δ 3.54 and 4.90 (2d, 2H, J = 14.7 Hz, NCH2Ph), 3.55 (dd, 1H, J = 4.0, 7.8 Hz, H4), 3.64 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 4.80 (d, 1H, J = 4.0 Hz, H5), 4.92 (d, 1H, J = 7.8 Hz, H3), 5.28 (bs, 1H, OH), 6.22 (d, 1H, J = 3.1 Hz, H3’), 6.29 (dd, 1H, J = 1.9, 3.1 Hz, H4’), 6.81 (m, 2H, ArH), 7.09 (m, 2H, ArH), 7.34 (d, 1H, J = 1.9 Hz, H5’); 13C NMR (CDCl3): δ 44.1, 48.6, 52.1, 54.2, 55.1, 69.8, 110.0, 110.3, 113.8, 126.8, 129.6, 143.4, 149.5, 159.0, 169.6, 172.7.
Anal. Calcd. for C18H19NO6 (345.35): C, 64.75; H, 5.43; N, 4.44. Found C, 64.87; H, 5.57; N, 4.29.
(3R*,4R*,5S*)-1-(4-Methoxybenzyl)-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 11b
Rf (Et2O ) = 0.30; 1H NMR (CDCl3): δ 3.49 (t, 1H, J = 7.1 Hz, H4), 3.54 (s, 3H, OCH3), 3.62 and 4.98 (2d, 2H, J = 14.6 Hz, NCH2Ph), 3.76 (s, 3H, OCH3), 3.78 (d, 1H, J = 7.1 Hz, H5), 4.47 (bs, 1H, OH), 4.66 (d, 1H, J = 7.1 Hz, H3), 6.31 (dd, 1H, J = 0.6, 3.3 Hz, H3’), 6.36 (dd, 1H, J = 1.8, 3.3 Hz, H4’), 6.77 (m, 2H, ArH), 6.93 (m, 2H, ArH), 7.37 (dd, 1H, J = 0.6, 1.8 Hz, H5’); 13C NMR (CDCl3): δ 44.3, 48.0, 52.2, 53.7, 55.2, 70.5, 110.8, 110.8, 114.0, 127.5, 129.8, 143.2, 148.2, 159.2, 169.5, 172.0.
Anal. Calcd. for C18H19NO6 (345.35): C, 64.75; H, 5.43; N, 4.44. Found C, 64.61; H, 5.33; N, 4.59.
(3R*,4S*,5S*)-1-Benzyl-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 10c
Rf (Et2O ) = 0.45; mp 133-135°C; 1H NMR (CDCl3): δ 3.45 (t, 1H, J = 8.5 Hz, H4’), 3.57 and 4.90 (2d, 2H, J = 14.8 Hz, NCH2Ph), 3.65 (s, 3H, OCH3), 4.64 (d, 1H, J = 8.5 Hz, H5), 4.69 (d, 1H, J = 8.5 Hz, H3), 5.30 (bs, 1H, OH), 6.30 (dd, 1H, J = 0.9, 3.2 Hz, H3’), 6.33 (dd, 1H, J = 1.8, 3.2 Hz, H4’), 7.05-7.12 (m, 2H, ArH), 7.20-7.30 (m, 3H, ArH), 7.40 (bs, 1H, H5’); 13C NMR (CDCl3): δ 44.8, 51.5, 52.5, 53.7, 72.0, 110.4, 111.2, 127.6, 128.2, 128.5, 135.2, 143.8, 148.5, 171.3, 172.6.
Anal. Calcd. for C17H17NO5 (315.32): C, 64.75; H, 5.43; N, 4.44. Found C, 64.83; H, 5.57; N, 4.33.
(3S*,4R*,5S*)-1-Benzyl-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 11c
Rf (Et2O) = 0.45; mp 121-123°C; 1H NMR (CDCl3): δ 3.33 (t, 1H, J = 9.4 Hz, H4), 3.49 (s, 3H, OCH3), 3.56 and 5,03 (2d, 2H, J = 14.7 Hz, NCH2Ph), 4.40 (bs, 1H, OH), 4.65 (d, 1H, J = 8.9 Hz, H5), 5.12 (d, 1H, J = 9,8 Hz, H3), 6.21 (d, 1H, J = 3.2 Hz, H3’), 6.29 (dd, 1H, J = 1.8, 3.2 Hz, H4’), 7.16-7.50 (m, 6H, ArH and H5’); 13C NMR (CDCl3): δ 45.0, 50.9, 52.2, 52.6, 69.7, 110.4, 110.4, 128.0, 128.4, 128.9, 134.9, 143.5, 148.3, 169.0, 172.8.
Anal. Calcd. for C17H17NO5 (315.32): C, 64.75; H, 5.43; N, 4.44. Found C, 64.88; H, 5.23; N, 4.31.
(3R*,4S*,5S*)-1-(4-Methoxybenzyl)-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 10d
Rf (Et2O) = 0.43; mp 138-140°C; 1H NMR (CDCl3): δ 3.41 (t, 1H, J = 8.5 Hz, H4), 3.48 and 4.84 (2d, 2H, J = 14.6 Hz, NCH2Ph), 3.64 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 4.60 (d, 1H, J = 8.5 Hz, H5), 4.65 (dd, 1H, J = 2.8, 8.5 Hz, H3), 5.30 (d, 1H, J = 2.8 Hz, OH), 6.30 (dd, 1H, J = 0.9, 3.2 Hz, H3’), 6.32 (dd, 1H, J = 1.8, 3.2 Hz, H4’), 6.60 (m, 2H, ArH), 6.94 (m, 2H, ArH), 7.38 (bs, 1H, H5’); 13C NMR (CDCl3): δ 44.2, 51.5, 52.5, 53.5, 55.1, 72.1, 110.4, 111.1, 113.9, 127.3, 129.6, 143.7, 148.7, 159.0, 171.3, 172.5.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found C, 62.75; H, 5.65; N, 4.17.
(3S*,4R*,5S*)-1-(4-Methoxybenzyl)-5-(2-furyl)-3-hydroxy-4-(methoxycarbonyl)pyrrolidin-2-one 11d
Rf (Et2O) = 0.40; 1H NMR (CDCl3): δ 3.32 (t, 1H, J = 9.7 Hz, H4), 3.40 and 4.97 (2d, 2H, J = 14.7 Hz, NCH2Ph), 3.47 (s, 3H, OCH3), 3.77 (s, 3H, OCH3), 4.60 (bs, 1H, OH), 4.64 (d, 1H, J = 9.7 Hz, H5), 5.14 (d, 1H, J = 9.7 Hz, H3), 6.20 (d, 1H, J = 3.0 Hz, H3’), 6.28 (dd, 1H, J = 1.8, 3.0 Hz, H4’), 6.82 (m, 2H, ArH), 7.08 (m, 2H, ArH), 7.32 (d, 1H, J = 1.8 Hz, H5’); 13C NMR (CDCl3): δ 44.4, 50.9, 52.5, 52.6, 55.3, 70.0, 110.4, 110.5, 114.2, 125.5, 129.8, 143.5, 148.5, 159.4, 169.1, 173.2.
Anal. Calcd. for C18H19NO6 (345.35): C, 62.60; H, 5.55; N, 4.06. Found C, 62.55; H, 5.69; N, 3.98.
Acknowledgments
This work was supported by research funds provided by the Direccion General de Enseñanza Superior del Ministerio de Educacion and Cultura (Project PB97-1014. Madrid, Spain).
References and Notes
- Torssell, K.G.B. Nitrile oxides, nitrones and nitronates in Organic Synthesis; VCH: New York, 1988. [Google Scholar] Tuffariello, J.J. 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; Vol. 12, p. 83. [Google Scholar] Gothelf, K.V.; Jorgensen, K.A. Chem. Rev. 1999, 99, 863. Frederickson, M. Tetrahedron 1997, 53, 403. Confalone, P.N.; Huie, E.M. Org. React. 1988, 36, 1. Padwa, A. Comprehemsive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, 1991; Vol. 4, p. 1069. [Google Scholar] Wade, P.A. Comprehemsive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, 1991; Vol. 4, p. 1111. [Google Scholar] Chiacchio, U.; Resciffina, A.; Romeo, G. Targets in Heterocyclic Systems; Attanasi, O., Spinelli, D., Eds.; Italian Chemical Society: Roma, 1997; Vol.1, p. 225. [Google Scholar] Merino, P.; Tejero, T. Molecules 1999, 4, 165, and references cited therein.
- For some particular examples see: Camiletti, C.; Poletti, L.; Trombini, C. J. Org. Chem. 1994, 59, 6843. Basha, A.; Henry, R.; McLaughlin, M.A.; Ratajczyk, J.D.; Wittenberger, S.J. J. Org. Chem. 1994, 59, 6103. Moriyama, S.; Vallee, Y. Synthesis 1998, 393.
- Merchan, F.L.; Merino, P.; Rojo, I.; Tejero, T.; Dondoni, A. Tetrahedron: Asymmetry 1996, 7, 667.
- Tejero, T.; Dondoni, A.; Rojo, I.; Merchan, F.L.; Merino, P. Tetrahedron 1997, 53, 3301. Merino, P.; Anoro, S.; Franco, S.; Merchan, F.L.; Tejero, T.; Tu on, V. J. Org. Chem. 2000, in press.
- Merino, P.; Anoro, S.; Merchan, F.L.; Tejero, T. Heterocycles 2000, in press.
- Dondoni, A.; Franco, S.; Junquera, F.; Merchan, F.L.; Merino, P.; Tejero, T. Synth. Commun. 1994, 24, 2537.
- Aurich, H.-G.; Franzke, M.; Keiselheim, H.P. Tetrahedron 1992, 48, 663.
- The authors have deposited atomic coordinates for the structure of 2a with the Cambridge Crystallographic Data Centre. The coordinates can be obtained on request, from the director, Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge, CB2 1EZ, UK.
- For a study on the cycloaddition of nitrones 2 with alkyl acrylates see ref. 4b.
- Jorgensen, K.A.; Gothelf, K.V. Acta Chem. Scand.. 1996, 50, 652. Cordero, F.M.; Aniquini, B.; Goti, A.; Brandi, A. Tetrahedron 1993, 49, 9867. Bimanand, A.Z.; Houk, K.N. Tetrahedron Lett. 1983, 24, 435. Dicken, C.M.; DeShong, P. J. Org. Chem. 1982, 47, 2047. DeShong, P.; Dicken, C.M.; Staib, R.R.; Freyer, A.J.; Weinreb, S.M. J. Org. Chem. 1982, 47, 4397. Cristina, D.; DeMicheli, C.; Gandolfi, R. J. Chem. Soc. Perkin Trans 1 1979, 2891.
- Collins, I.; Nadin, A.; Holmes, A.B.; Long, M.A.; Man, J.; Baker, R. J. Chem. Soc. Perkin Trans 1 1994, 2205. In general, electron-rich alkenes are expected to give LUMO(nitrone)-HOMO(alkene) controlled 1,3-dipolar cycloadditions. See: Seerden, J.-P.G.; Kuypers, M.M.M.; Scheeren, H.W. Tetrahedron 1994, 6, 1441, and references therein.
- PM3 calculations were carried out using MOPAC 97 as implemented in the WINMOPAC 2.0 package (Fujitsu, 1998).
- Inter alia: Rispens, M.T.; Keller, E.; Lange, B.; Zijlstra, R.W.J.; Feringa, B.L. Tetrahedron: Asymmetry 1994, 5, 607. Chiacchio, U.; Casuscelli, F.; Corsaro, A.; Rescifina, A.; Romeo, G.; Uccella, N. Tetrahedron 1994, 50, 667. Chiacchio, U.; Buemi, G.; Casuscelli, F.; Procopio, A.; Rescifina, A.; Romeo, G. Tetrahedron 1994, 50, 5503.
- Semiempirical geometry optimizations for all systems were carried out at the AM1 and PM3 levels of theory as implemented in WINMOPAC 2.0. Fujitsu Limited. 1998.
- Ab initio calculations were performed using Gaussian 98, Revision A.3, M. Frisch, J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, J. A., Jr.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone, V.; Cossi, M.; Cammi, R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian, Inc.: Pittsburgh PA, 1998.
- The study was carried out with the N-methyl nitrone and vinyl acetate (or methyl vinyl ether). For a study of the same reaction with methyl acrylate see ref. 4b.
- Samples Availability: Available from MDPI.
© 2000 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.