A Simple Synthesis of Some New Thienopyridine and Thienopyrimidine Derivatives
Abstract
:Introduction
Results and Discussion
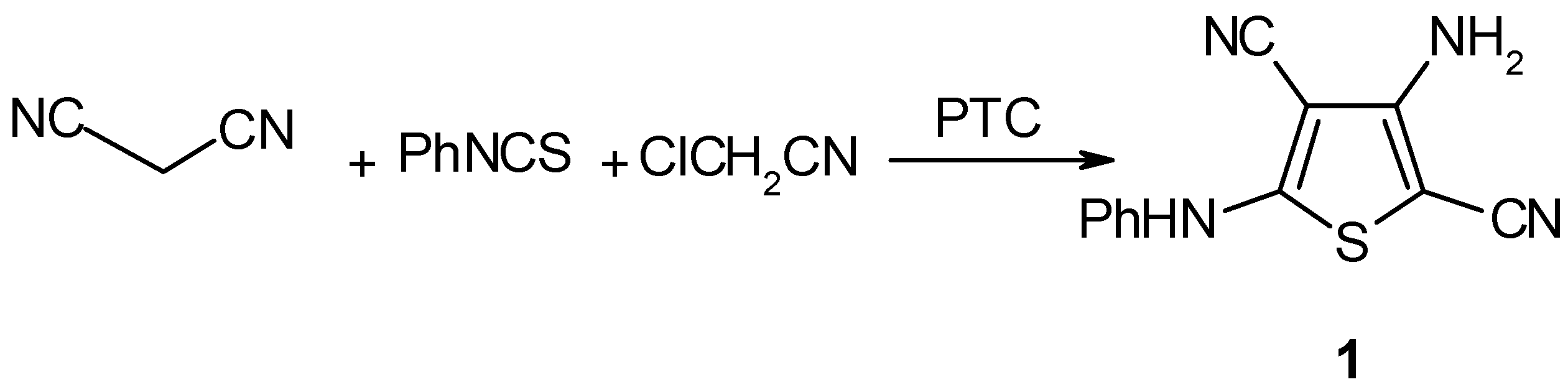
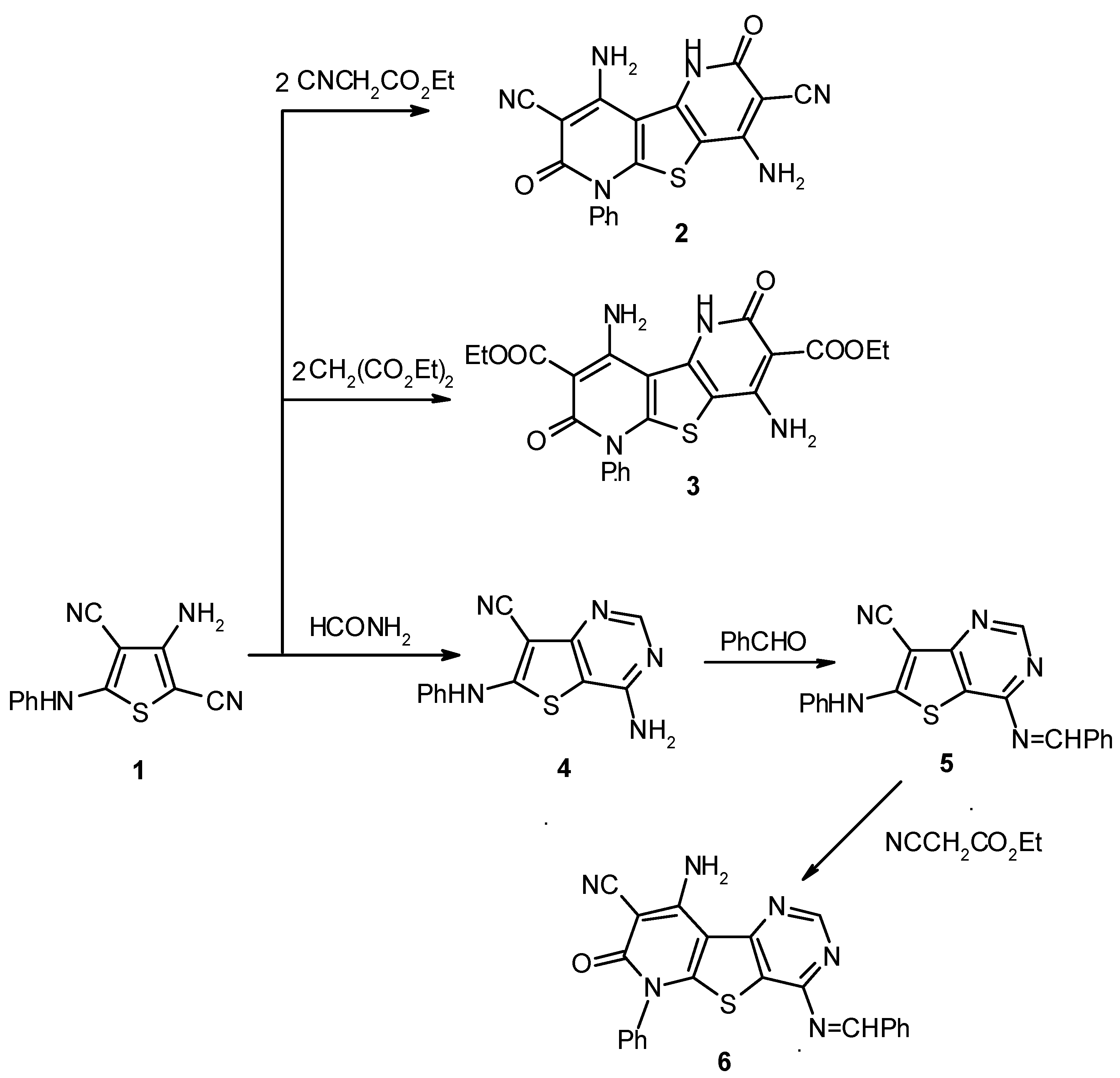
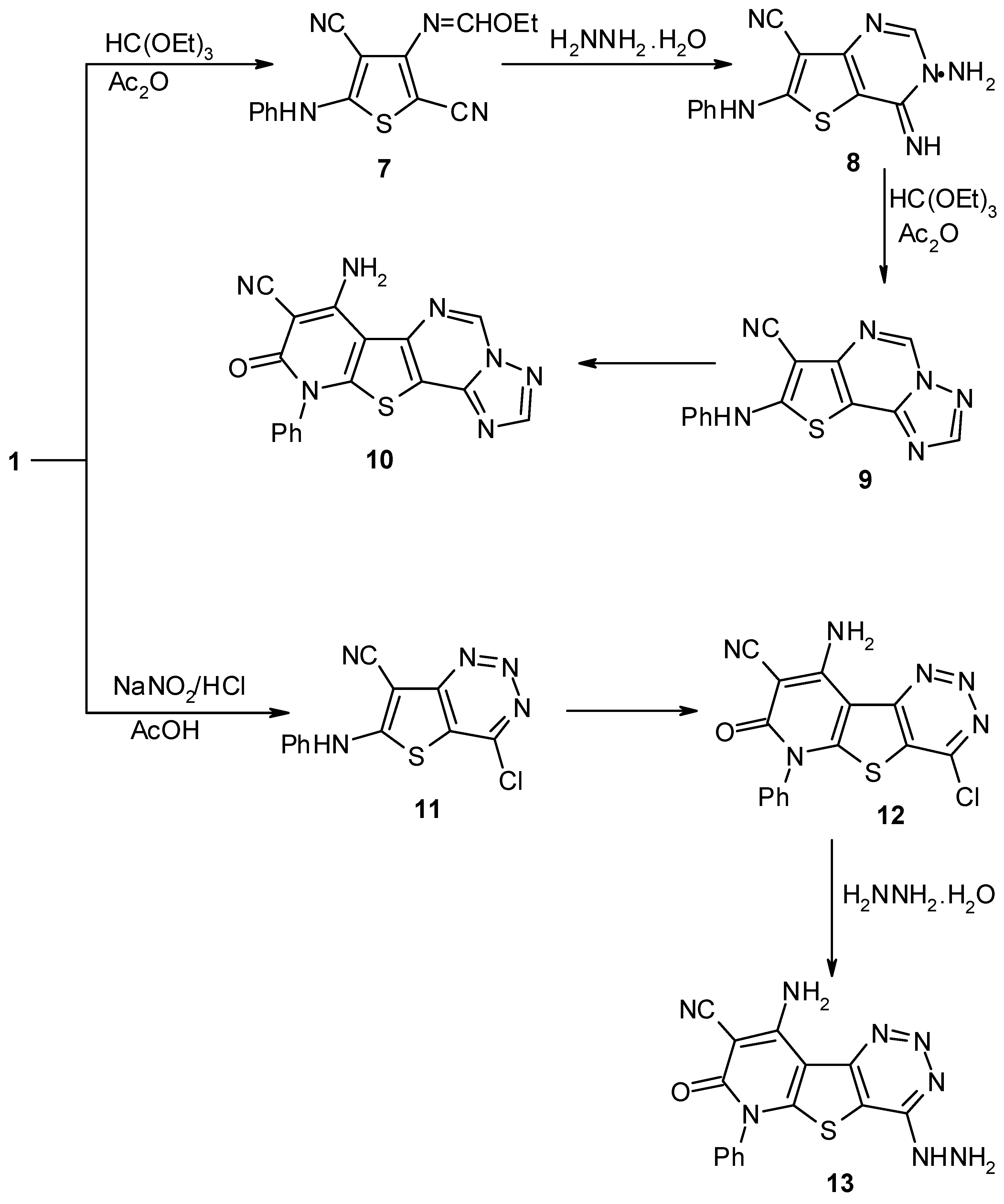
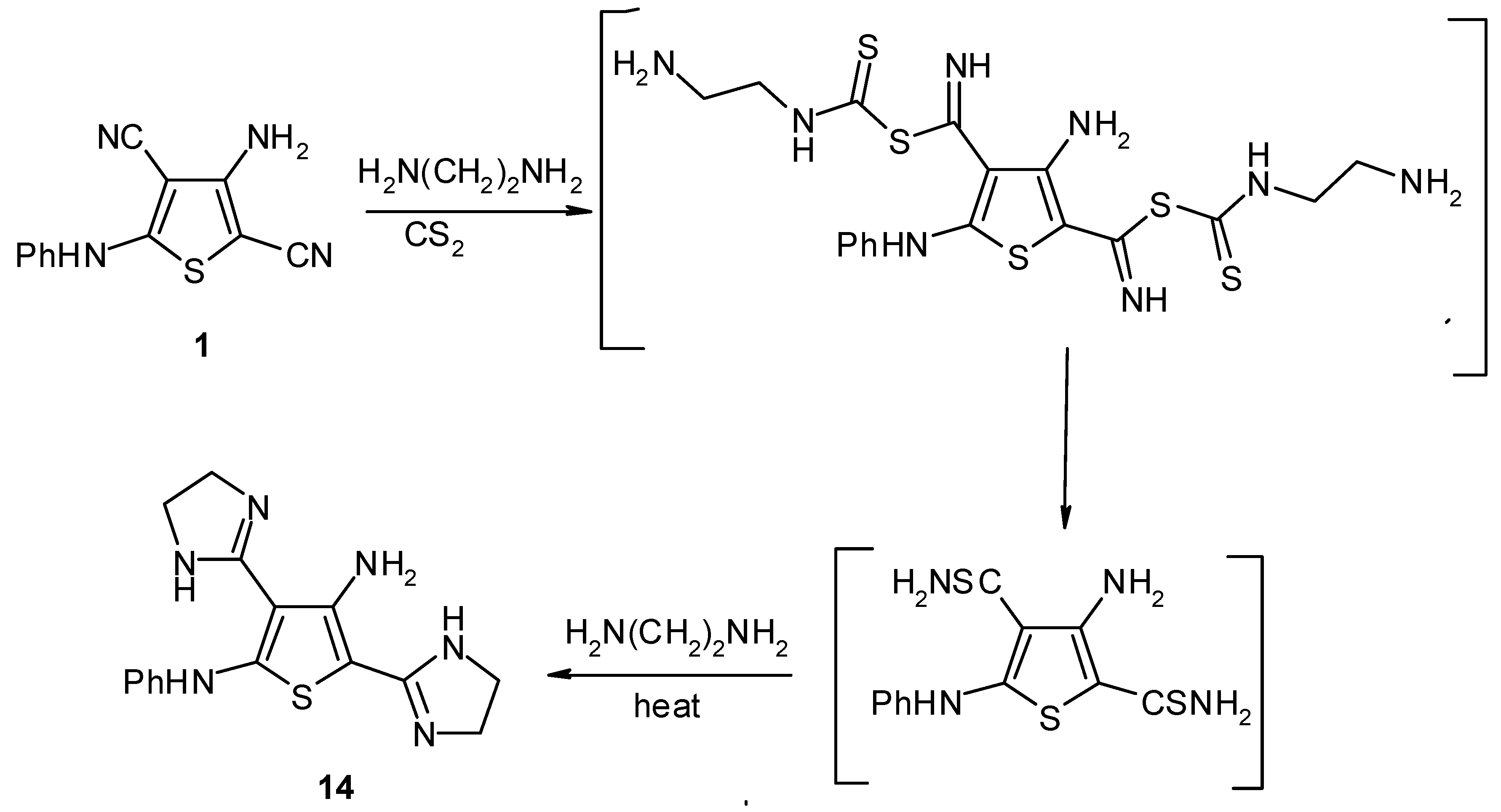
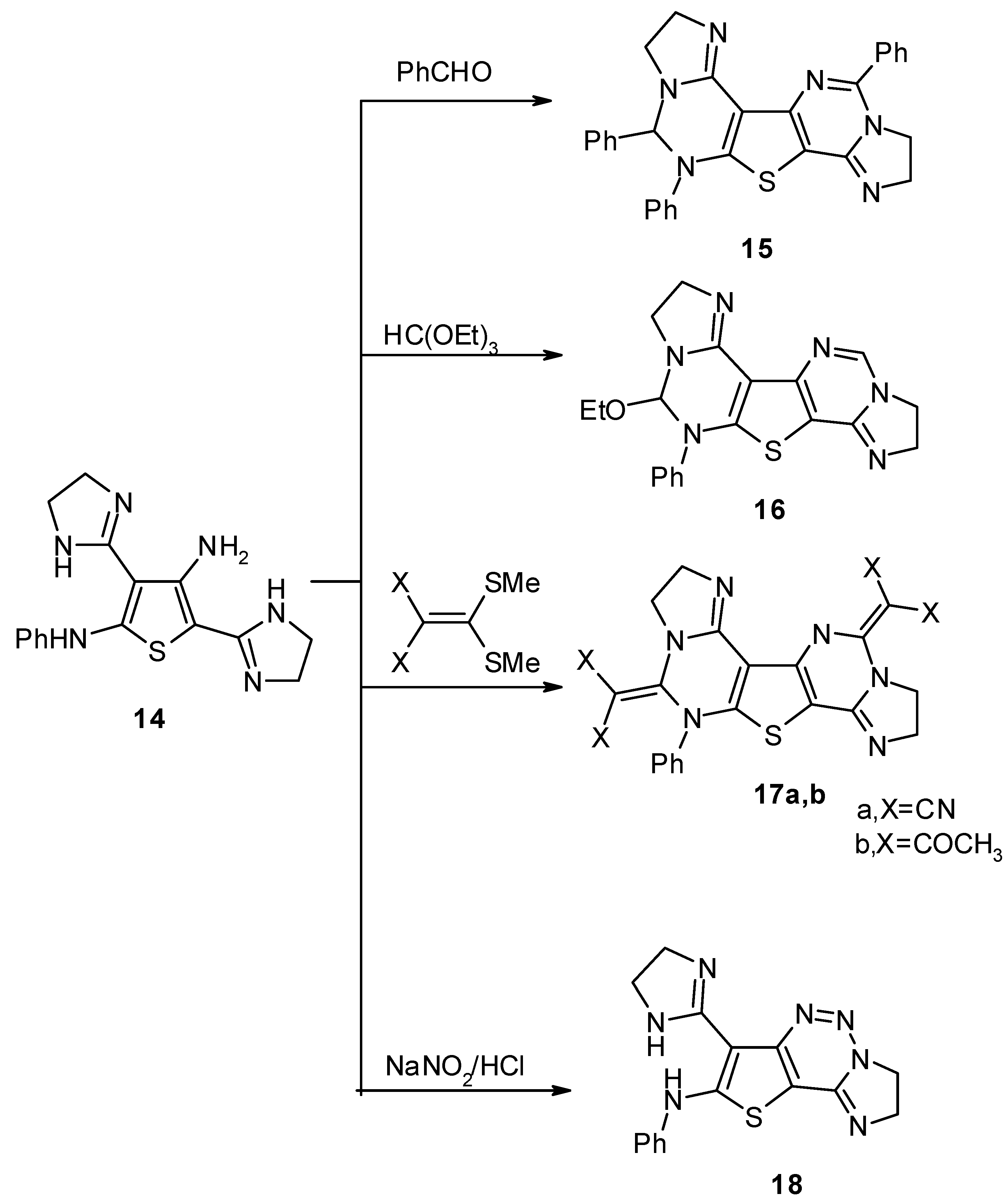
Experimental
General
| Product | Yield % | Mol.Form. Mol. Wt. | Analysis(%) Calcd./Found | |||
|---|---|---|---|---|---|---|
| C | H | N | S | |||
| 2 | 76 | C18H10N6O2S 374.37 | 57.75 57.64 | 2.67 2.73 | 22.44 22.55 | 8.56 8.62 |
| 3 | 81 | C22H20N4O6S 468.48 | 56.40 56.48 | 4.27 4.05 | 17.93 17.85 | 6.83 6.77 |
| 4 | 92 | C13H9N5S 267.30 | 58.41 58.56 | 3.63 3.55 | 26.28 25.99 | 11.97 11.88 |
| 5 | 85 | C20H13N5S 355.41 | 67.58 67.67 | 3.66 3.77 | 19.69 19.58 | 9.00 8.98 |
| 6 | 77 | C23H14N6 OS 422.46 | 65.38 65.44 | 3.31 3.23 | 19.88 19.92 | 7.57 7.61 |
| 7 | 89 | C15H12N4OS 269.21 | 60.82 61.02 | 4.05 3.98 | 18.90 19.03 | 10.82 1076 |
| 8 | 90 | C13H10N6S 282.32 | 55.30 55.22 | 3.54 3.46 | 29.75 29.66 | 11.33 11.43 |
| 9 | 78 | C14H8N6S 292.31 | 57.52 57.33 | 2.74 2.87 | 28.74 28.65 | 11.94 11.99 |
| 10 | 66 | C17H9N7OS 359.36 | 56.81 56.77 | 2.50 2.56 | 27.27 27.30 | 8.90 9.12 |
| 11 | 75 | C12H6N5SCl 287.72 | 50.10 49.99 | 2.09 1.98 | 24.33 24.15 | 11.12 10.98 |
| 12 | 81 | C15H7N6OSC 354.77 | 50.77 50.85 | 1.97 2.01 | 23.68 23.44 | 9.02 8.94 |
| 13 | 64 | C15H10N8OS 350.35 | 51.42 51.61 | 2.85 2.82 | 31.97 31.99 | 9.13 8.99 |
| 14 | 91 | C16H18N6S 326.41 | 58.87 58.84 | 5.61 5.54 | 25.73 25.62 | 9.80 9.91 |
| 15 | 89 | C30H24N6S 500.62 | 71.97 71.89 | 4.79 4.81 | 211.40 21.32 | 6.39 6.41 |
| 16 | 78 | C20H20N6OS 392.47 | 61.20 60.32 | 5.10 4.94 | 21.40 21.22 | 8.15 8.00 |
| 17a | 85 | C24H14N10S 474.50 | 60.75 60.56 | 2.95 3.02 | 22.50 22.65 | 6.74 6.61 |
| 17b | 82 | C28H26N6O4S 542.61 | 61.97 62.13 | 4.79 4.81 | 15.48 15.50 | 5.89 5.75 |
| 18 | 89 | C16H15N7S 337.40 | 56.95 57.02 | 4.44 4.32 | 29.04 30.12 | 9.48 9.51 |
| Product | IR (KBr)b | 1H-NMRc (DMSO-d6) |
|---|---|---|
| 2 | 3430,3330,3300 (2NH2), 3180 (NH), 2222, 2210 (2CN), 1640, 1630 (C=O) | 8.20 (s,1H,NH), 7.70-7.20 (m,5H,arom.), 6.20-5.90 (br,2H,NH2) |
| 3 | 3420,3300,3250(2NH2), 3190 (NH), 1720,1710 (C=O ester), 1630 (C=O). | 8.30-7.80 (m,5H,arom.), 6.30-6.10 (br,2H,NH2 ), 4.80-4.60 (br,2H,NH2), 2.80 (s,3H,COCH3) , 2.10 (s,3H,CH3). |
| 4 | 3340,3300, (NH2), 3200 (NH), 2218 (CN). | 9.10 (s,1H,NH), 8.40 (s,1H,CH-Pyridine), 7.70-7.20 (m,5H,arom.), 5.50 (s,2H,NH2). |
| 5 | 3200(NH), 2210 (CN). | 9.10 (s,1H,NH), 8.40 (s,1H,CH-Pyridine), 7.90-7.30 (m,10H,arom.), 5.50-5.10 (br,2H,NH2). |
| 6 | 3350,3240 (NH2), 2220 (CN), 1640 (C=O). | 8.40 (s,1H,CH-Pyridine), 8.10 (s,1H,N=CH), 7.90-7.00 (m,10H,arom.), 6.20 (s,2H,NH2). |
| 7 | 3180 (NH), 2220,2210 (CN), 1630 (C=N). | 8.50 (s,1H,NH), 7.70 (s,1H,CH), 7.70-7.00 (m,5H,arom.), 3.80-3.40 (q,2H,OCH2), 1.25-1.00 (t,3H,CH3). |
| 8 | 3430,3330 (NH2), 3200,3180 (2NH), 2190 (CN). | 8.20-8.00 (br,2H,2NH), 7.80 (s,1H,CH), 7.70-7.20 (m,5H,arom.), 5.20 (s,2H,NH2). |
| 9 | 3180 (NH), 2220 (CN), 1600 (C=N). | 8.80 (s,1H,NH), 8.40 (s,1H,CH-Pyridine), 8.10 (s,1H,CH-triazole), 7.50-7.00 (m,5H,arom.), 6.20 (s,2H,NH2). |
| 10 | 3400, 3330, 3300 (2NH2), 2217 (CN), 1640 (C=O), 1600 (C=N). | 8.50 (s,1H,CH-Pyridine), 8.20 (s,1H,CH-triazole), 7.50-7.00 (m,5H,arom.), 6.20 (s,2H, NH2). |
| 11 | 3320 (NH), 2210 (CN), 1640 (C=N). | 8.40 (s,1H,NH), 7.70-7.20 (m,5H,arom.). |
| 12 | 3340,3300, 3270(2NH2), 2220 (CN), 1640(C=O), 1620(C=N). | 7.70-7.00 (m,5H,arom.), 6.20-6.00 (br,2H, (NH2). |
| 13 | 3350,3330,3210 (2NH2), 3180, (NH), 2220 (CN), 1640 (C=O) | 8.00 (s,1H,NH), 7.80-7.20 (m,5H,arom.), 6.10 (s,2H,NH2), 5.00 (s,2H,NH2). |
| 14 | 3390,3260 (NH2), 3200,3180,3100 (3NH). | 8.40 (s,1H,NH), 8.10 (s,1H,NH), 7.70-7.10 (m,5H,arom.), 5.60 (s,2H,NH2), 4.00-3.50 (m, 8H,4CH2-imidazole). |
| 15 | 3080 (CH-arom.), 1640 (C=N) | 7.90-7.10 (m,15H,arom.), 4.00-3.40 (m,9H, 4CH2-imidazole + 1H, CH-Ph) |
| 16 | 3050 (CH-arom.), 1640 (C=N). | 9.10 (s,1H,CH), 7.70-7.20 (m,5H,arom.), 4.00-3.40 (m,10H,4CH2-imidazole+ OCH2), 1.25-1.00 (t,3H,CH3). |
| 17a | 3200 (NH), 2220,2210,2197 (4CN), 1620 (C=N). | 9.00 (s,1H,NH), 7.70-7.20 (m,5H,arom.), 4.00-3.40 (m,8H,4CH2-imidazole). |
| 17b | 3200 (NH), 1690,1670 (C=O), 1640 (C=N). | 8.90 (s,1H,NH), 7.70-7.00 (m,5H,arom.), 4.00-3.40 (m,,8H,4CH2-imidazole), 2.70-2.60 (ss, 12H,2COCH3). |
| 18 | 3050 (CH-arom.), 1640 (C=N). | 8.70 (s,1H,NH), 8.40 (s,1H,NH), 7.70-7.20 (m, 5H,arom.), 4.00-3.50 (m,8H,4CH2-imidazole). |
References
- Mongevega, A.; Aldama, I.; Robbani, M.M.; Fernandez-Alvarez, E. J. Heterocycl. Chem. 1980, 17, 77.
- Bellary, J.H.; Badiger, V.V. Indian J. Chem. 1981, 20B, 654.
- Joshi, K.C.; Chand, P. J. Heterocycl. 1980, 17, 1783.
- Yossef, M.S.K.; Hssan, Kh.M.; Atta, F.M.; Abbady, M.S. J. Heterocycl. Chem. 1984, 21, 1565.
- Pottus, K.T.; Husain, S. J. Org. Chem. 1971, 36, 10. [CrossRef]
- Bridson, P.K.; Davis, R.A.; Renner, L.S. J. Heterocycl. Chem. 1985, 22, 753. [CrossRef]
- Saito, Y.; Yasushi, M.; Sakoshita, M.; Toyda, K.; Shibazalti, T. European Patent Appl. 1993, 535, 548, [Chem. Abstr., 1993, 119, 117112e].
- Furuya, S.; Takeru, N.; Matsumoto, H. Jpn Kokai Tokkyo Koho JP 09,169,766; [Chem. Abstr., 1997, 127, 176416v],
- Dave, C.G.; Shah, P.R.; Shah, A.B.; Dave, K.C.; Patel, V.J. J. Indian Chem. Soc. 1989, 66, 48.
- El-Saghier, A.M.M. Bull. Chem. Soc. Jpn. 1993, 66, 2011. [CrossRef]
- El-Shafei, A.K.; Abdel-Ghany, H.A.; Sultan, A.; El-Saghier, A.M.M. Phosphorus, Sulfur Silicon Relat. Elem. 1992, 73, 15. [CrossRef]
- El-Shafei, A.K.; El-Saghier, A.M.M.; Soliman, A.M.; Sultan, A. Phosphorus, Sulfur Silicon Relat. Elem. 1992, 80, 72.
- Khodairy, A.; Abdel-Ghany, H. Phosphorus, Sulfur Silicon Relat. Elem. 2000, 162, 259.
- Abdel-Ghany, H.; Khodairy, A. Phosphorus, Sulfur Silicon Relat. Elem. 2000, 166, 45.
- El-Shafei, A.K.; El-Saghier, A.M.M.; Ahmed, E.A. Synthesis 1994, 152.
- Bakhite, E.A.; Radwan, S.M.; El-Saghier, A.M.M. Indian J. Chem. 1995, 34B, 97.
- Al-Omran, F.; Abdel Khalik, M.M.; Al-Awadhi, H.; Elnagdi, M. H. Tetrahedron 1996, 52, 11915. [CrossRef]
- Abdel-Rahman, A. E.; Bakhite, E. A.; Mohamed, O.S.; Thabet, E. A. 37th IUPAC Congress. 1999. Berlin, 14-19 August. [Google Scholar]
- Sample Availability: Available from the author.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
El-Saghier, A.M.M. A Simple Synthesis of Some New Thienopyridine and Thienopyrimidine Derivatives. Molecules 2002, 7, 756-766. https://doi.org/10.3390/71000756
El-Saghier AMM. A Simple Synthesis of Some New Thienopyridine and Thienopyrimidine Derivatives. Molecules. 2002; 7(10):756-766. https://doi.org/10.3390/71000756
Chicago/Turabian StyleEl-Saghier, Ahmed M. M. 2002. "A Simple Synthesis of Some New Thienopyridine and Thienopyrimidine Derivatives" Molecules 7, no. 10: 756-766. https://doi.org/10.3390/71000756
APA StyleEl-Saghier, A. M. M. (2002). A Simple Synthesis of Some New Thienopyridine and Thienopyrimidine Derivatives. Molecules, 7(10), 756-766. https://doi.org/10.3390/71000756




