Synthesis and Characterization of New 3,5-Dinaphthyl Substituted 2-Pyrazolines and Study of Their Antimicrobial Activity
Abstract
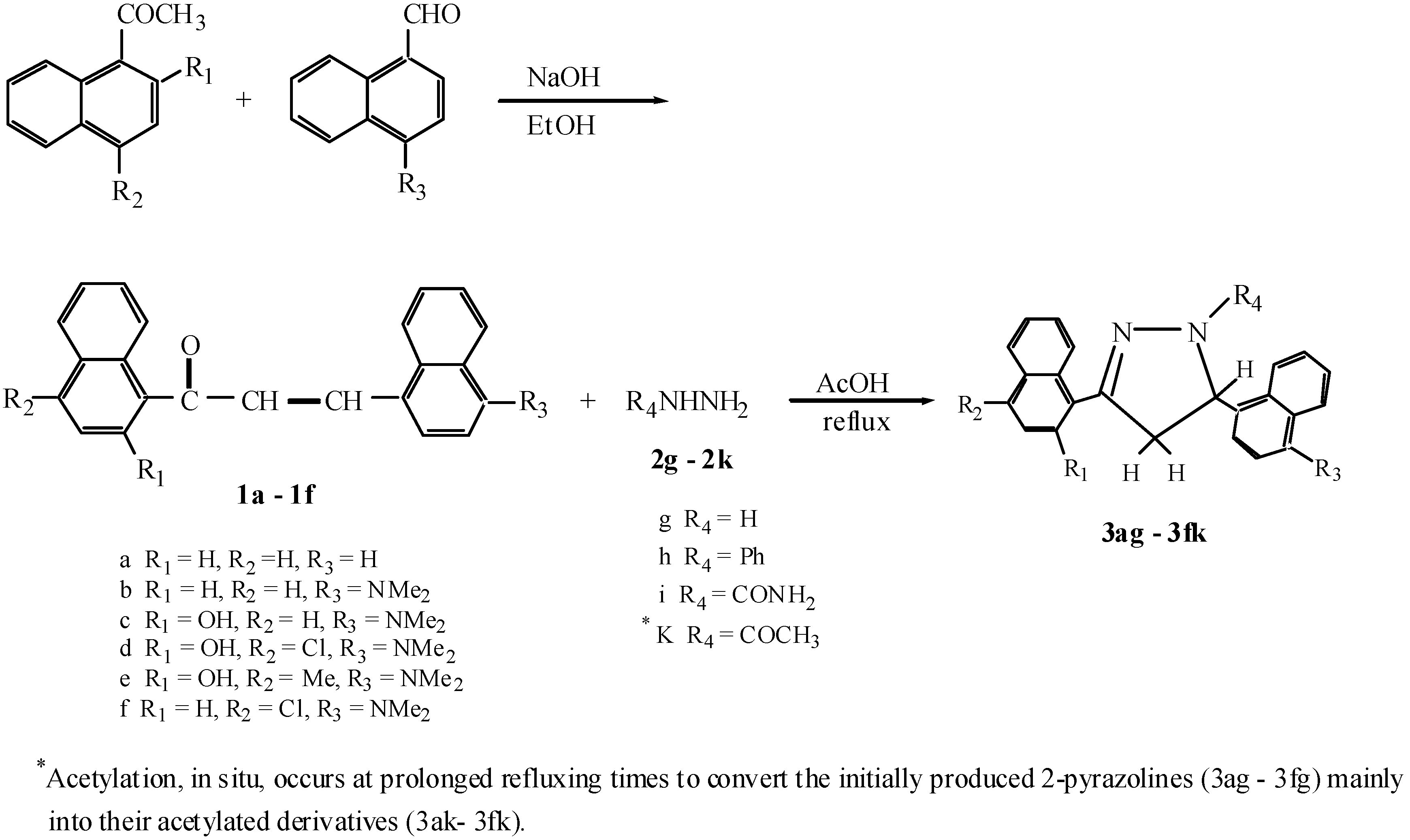
:Introduction
Results and Discussion
Antimicrobial activity
Experimental
General
General procedure for the preparation of 3,5-dinaphthyl-2-pyrazoline derivatives (3ag-3fk).
| Compounds | Molecular Formula | M.P. (˚C) | Yield (%) | Found (Calcd. %) | ||
| C | H | N | ||||
| 3ag | C23H18N2 | 195 - 196 | 82 | 85.68 (85.71) | 5.53 (5.59) | 8.48 (8.69) |
| 3bg | C25H23N3 | 232 - 234 | 73 | 82.34 (82.19) | 6.18 (6.30) | 11.37 (11.51) |
| 3cg | C25H23N3O | 285 - 286 | 65 | 78.57 (78.74) | 6.13 (6.04) | 10.87 (11.02) |
| 3dg | C25H22ClN3O | 249 (decomp.) | 82 | 72.12 (72.20) | 5.18 (5.29) | 9.76 (10.11) |
| 3eg | C26H25ON3 | 278 - 280 | 68 | 78.75 (78.99) | 6.25 (6.33) | 10.49 (10.63) |
| 3fg | C25H22N3 Cl | 237 - 239 | 55 | 74.98 (75.09) | 5.53 (5.51) | 10.27 (10.51) |
| 3ah | C29H22N2 | 225 - 226 | 78 | 87.05 (87.44) | 5.57 (5.53) | 7.21 (7.04) |
| 3bh | C31H27N3 | 238(decomp.) | 75 | 84.32 (84.35) | 6.11 (6.12) | 9.48 (9.52) |
| 3ch | C31H27ON3 | 259 - 261 | 54 | 81.43 (81.40) | 5.85 (5.91) | 9.13 (9.19) |
| 3dh | C31H26ON3 Cl | 264 (decomp.) | 73 | 75.52 (75.69) | 5.15 (5.29) | 8.52 (8.54) |
| 3eh | C32H29ON3 | 238 - 239 | 74 | 81.51 (81.53) | 5.74 (6.16) | 8.81 (8.92) |
| 3fh | C31H26N3 Cl | 242 - 244 | 67 | 78.21 (78.23) | 5.34 (5.47) | 8.64 (8.83) |
| 3ai | C24H19ON3 | 257 - 258 | 73 | 78.84 (78.90) | 5.32 (5.20) | 11.48 (11.51) |
| 3bi | C26H24ON4 | 265 - 267 | 62 | 76.42 (76.47) | 5.72 (5.88) | 13.47 (13.72) |
| 3ci | C26H24O2N4 | 272 (decomp.) | 68 | 73.46 (73.58) | 5.37 (5.66) | 13.84 (13.21) |
| 3di | C26H23O2N4Cl | 276 (decomp.) | 65 | 67.39 (68.05) | 5.08 (5.02) | 12.63 (12.21) |
| 3ei | C27H26O2N4 | 280 - 282 | 85 | 73.64 (73.97) | 5.92 (5.94) | 12.81 (12.78) |
| 3fi | C26H23ON4Cl | 270 - 272 | 74 | 70.28 (70.50) | 5.12 (5.20) | 12.53 (12.69) |
| 3ak | C25H20ON2 | 207 - 208 | 75 | 82.18 (82.42) | 5.58 (5.49) | 7.89 (7.69) |
| 3bk | C27H25ON3 | 236 - 238 | 58 | 79.33 (79.61) | 6.08 (6.14) | 10.45 (10.32) |
| 3ck | C27H25O2N3 | 243 - 245 | 72 | 76.54 (76.59) | 5.83 (5.91) | 9.65 (9.93) |
| 3dk | C27H24O2N3 Cl | 237(decomp.) | 68 | 70.85 (70.82) | 5.64 (5.25) | 9.33 (9.18) |
| 3ek | C28H27O2N3 | 226 - 228 | 78 | 76.78 (76.89) | 6.21 (6.18) | 9.67 (9.61) |
| 3fk | C27H24ON3Cl | 241 - 242 | 47 | 73.25 (73.39) | 5.32 (5.44) | 9.85 (9.51) |
| Compounds | IR (cm-1) | 1H-NMR (ppm) | Mass (m/z) |
| 3ag | 3448, 3230, 3080, 2928, 1652, 1598 | 3.35 (dd, 1H, >CHHA), 4.15 (dd, 1H, >CHBH), 5.75 (dd, 1H, >CHAr ), 6.10 (s, 1H, NH ), 6.5 - 8.2 (m, 13H, Ar), 9.45 (d, 1H, C’8 -H) | 77, 91, 127, 153, 156, 168, 169, 195, 291, 304, 322 |
| 3bg | 3180, 2925, 2812, 1585, 1578, 1310, 1205 | 2.85 (s, 6H, N(CH3)2 ), 3.32 (dd, 1H, >CHHA), 4.12 (dd, 1H, >CHBH), 5.64 (dd, 1H, >CHAr ), 6.12 (s, 1H, NH), 6.2 - 8.5 (m, 12H, Ar), 9.43 (d, 1H, C’8 -H) | 77, 91, 153, 158, 167, 170, 195, 197, 212, 291, 350, 365 |
| 3cg | 3635, 3520, 3135, 2923, 1645, 1595, 1345, 1235, 1215 | 2.80 (s, 6H, N(CH3)2 ), 3.28 (dd, 1H, >CHHA), 3.98 (dd, 1H, >CHBH), 5.32 (dd, 1H, >CHAr), 5.76 (s, 1H, NH), 6.2 - 8.5 (m, 11H, Ar), 9.38 (d, 1H, C’8 -H), 11.10 (s, 1H, ArOH) | 77, 91, 144, 169, 183, 197, 211, 350, 352, 364, 366, 381 |
| 3dg | 3589, 3485, 3165, 2918, 1687, 1582, 1327, 1258, 1218, 815 | 2.78 (s, 6H, N(CH3)2 ), 3.25 (dd, 1H, >CHHA), 3.92 (dd, 1H, >CHBH), 5.31 (dd, 1H, >CHAr), 5.65 (s, 1H, NH), 6.5 - 9.10 (m, 10H, Ar), 9.35 (d, 1H, C’8 -H), 10.92 (s, 1H, ArOH) | 77, 91, 183, 197, 203, 217, 218, 220, 245, 247, 354, 380, 386, 388, 402, 415, 417 |
| 3eg | 3610, 3285, 3105, 2889, 1652, 1594, 1325, 1232 | 2.26 (s, 3H, Ar-CH3), 2.82 (s, 6H, N(CH3)2 ), 3.26 (dd, 1H, >CHHA), 3.95 (dd, 1H, >CHBH), 5.30 (dd, 1H, >CHAr), 5.73 (s, 1H, NH), 6.2 - 8.5 (m, 10H, Ar), 9.35 (d, 1H, C’8 -H), 10.87 (s, 1H, ArOH) | 77, 91, 157, 183, 199, 225, 351, 362, 379, 380, 395 |
| 3fg | 3165, 3100, 2905, 2825, 1605, 1500, 1280, 796 | 2.83 (s, 6H, N(CH3)2 ), 3.34 (dd, 1H, >CHHA), 4.16 (dd, 1H, >CHBH), 5.67 (dd, 1H, >CHAr ), 6.15 (s, 1H, NH), 6.4 - 8.7 (m, 11H, Ar), 9.45 (d, 1H, C’8 -H) | 77, 99, 187, 189, 203, 230, 355, 384, 386, 399, 401 |
| 3ah | 3127, 2890, 1498, 1594, 1498, 1381, 1136, 772 | 3.42 (dd, 1H, >CHHA), 4.22 (dd, 1H, >CHBH), 5.91 (dd, 1H, >CHAr), 6.7- 8.3 (m, 19H, Ar), 9.56 (d, 1H, C´8-H) | 77, 91, 127, 152, 165, 195, 241, 242, 291, 304, 398 |
| 3bh | 3178, 2830, 1592, 1505, 1410, 1315, 1225 | 3.02 (s, 6H, N(CH3)2 ), 3.58 (dd, 1H, >CHHA), 4.35 (dd, 1H, >CHBH), 6.15 (dd, 1H, >CHAr), 6.2 - 8.5 (m, 18H, Ar), 9.56 (d, 1H, C´8-H) | 77, 91, 153, 167, 170, 195, 197, 244, 271, 334, 350, 441 |
| 3ch | 3515, 3150, 2875, 1618, 1518, 1423, 1345, 1223 | 2.93 (s, 6H, N(CH3)2 ), 3.64 (dd, 1H, >CHHA), 4.67 (dd, 1H, >CHBH), 6.18 (dd, 1H, >CHAr), 6.5-8.2 (m, 17H, Ar), 8.95 (d, 1H, C´8-H), 11.38 (s, 1H, ArOH) | 77, 91, 169, 170, 183, 197, 211, 260, 350, 364, 366, 457, 459 |
| 3dh | 3498, 3118, 2820, 1598, 1573, 1500, 1411, 1332, 1215, 825 | 2.91 (6H, N(CH3)2 ), 3.63 (dd, 1H, >CHHA), 4.67 (dd, 1H, >CHBH), 6.15 (dd, 1H, >CHAr), 6.3 - 8.5 (m, 16H, Ar), 9.02 (d, 1H, C´8-H), 11.12 (s, 1H, ArOH) | 77, 99, 217, 219, 245, 247, 321, 384, 400, 402, 456, 474, 491, 493 |
| 3eh | 3603, 3250, 2935, 2790, 1650, 1593, 1480, 1330, 1215 | 2.25 (s, 3H, Ar-CH3), 2.92 (s, 6H, N(CH3)2 ), 3.64 (dd, 1H, >CHHA), 4.65 (dd, 1H, >CHBH), 6.21 (dd, 1H, >CHAr), 6.5 - 8.0 (m, 16H, Ar), 8.92 (d, 1H, C´8-H), 11.42 (s, 1H, ArOH) | 77, 99, 183, 197, 225, 274, 301, 364, 454, 471 |
| 3fh | 3135, 2878, 1612, 1585, 1434, 1325, 1218, 834 | 2.95 (s, 6H, N(CH3)2 ), 3.49 (dd, 1H, >CHHA), 4.25 (dd, 1H, >CHBH), 5.82 (dd, 1H, >CHAr), 6.5 - 8.7 (m, 16H, Ar), 9.47 (d, 1H, C´8-H) | 69, 77, 99, 201, 203, 228, 230, 278, 280, 368, 384, 386, 440, 475, 477 |
| 3ai | 3456, 3200, 3085, 1677, 1650, 1582, 1505, 1468, 1391, 1106, 782 | 3.27 (dd, 1H, >CHHA), 4.32 (dd, 1H, >CHBH), 6.16 (dd, 1H, >CHAr), 6.61 (s, 2H, NH2), 7.3 - 8.3 (m, 14H, Ar), 9.25 (d, 1H, C´8-H) | 77, 115, 127, 141, 153, 168, 195, 196, 290, 292, 305, 323, 351, 366, 367 |
| 3bi | 3465, 3250, 3100, 2815, 1668, 1635, 1580, 1500, 1458, 1117 | 2.86 (s, 6H, N(CH3)2 ), 3.28 (dd, 1H, >CHHA), 4.34 (dd, 1H, >CHBH), 6.12 (dd, 1H, >CHAr), 6.65 (s, 2H, NH2), 6.80 - 8.30 (m, 12H, Ar), 9.25 (d, 1H, C´8-H) | 77, 91, 127, 153, 168, 182, 184, 195, 238, 364, 393, 408 |
| 3ci | 3632, 3410, 3182, 3110, 2865, 1670, 1610, 1480, 1455, 1238 | 2.82 (s, 6H, N(CH3)2 ), 3.23 (dd, 1H, >CHHA), 4.31 (dd, 1H, >CHBH), 6.08 (dd, 1H, >CHAr), 6.65 (s, 2H, NH2), 6.70 - 8.20 (m, 11H, Ar), 9.22 (d, 1H, C´8-H), 10.95 (s, 1H, ArOH) | 69, 97, 126, 143, 169, 184, 198, 211, 380, 409, 424 |
| 3di | 3532, 3434, 3325, 3102, 2830, 1673, 1644, 1589, 1495, 1382, 1211, 815 | 2.82 (s, 6H, N(CH3)2 ), 3.23 (dd, 1H, >CHHA), 4.33 (dd, 1H, >CHBH), 6.12 (dd, 1H, >CHAr), 6.54 (s, 2H, NH2), 7.0 - 8.5 (m, 11H, Ar), 9.12 (d, 1H, C´8-H), 11.02 (s, 1H, ArOH) | 77, 91, 101, 177, 193, 197, 203, 217, 244, 384, 423, 441, 442, 443, 458, 460 |
| 3ei | 3537, 3428, 3322, 2965, 2873, 1671, 1643, 1590, 1505, 1379, 1218 | 2.26 (s, 3H, Ar-CH3), 2.88 (s, 6H, N(CH3)2 ), 3.24 (dd, 1H, >CHHA), 4.33 (dd, 1H, >CHBH), 6.11 (dd, 1H, >CHAr), 6.57 (s, 2H, NH2), 7.2 - 8.5 (m, 11H, Ar), 9.16 (d, 1H, C´8 - H), 10.98 (s, 1H, ArOH) | 77, 91, 157, 183, 197, 211, 364, 396, 421, 423, 424, 438, 440 |
| 3fi | 3462, 3238, 3095, 2824, 1665, 1630, 1612, 1498, 1448, 1120, 785 | 2.85 (s, 6H, N(CH3)2 ), 3.29 (dd, 1H, >CHHA), 4.36 (dd, 1H, >CHBH), 6.14 (dd, 1H, >CHAr), 6.68 (s, 2H, NH2), 6.80 - 8.50 (m, 11H, Ar), 9.28 (d, 1H, C´8 - H) | 77, 99, 126, 161, 187, 202, 216, 229, 398, 427, 442, 444 |
| 3ak | 3033, 1667, 1506, 1415, 1390, 1150, 775 | 2.64 (s, 3H, COCH3), 3.39 (dd, 1H, >CHHA ), 4.13 (dd, 1H, >CHBH), 6.28 (dd, 1H, >CHAr), 7.2 - 8.2 (m, 14H, Ar), 9.36 (d, 1H, C´8 - H) | 83, 97, 127, 141, 152, 165, 167, 168, 194, 195, 290, 291, 321, 322, 349, 364 |
| 3bk | 3028, 2850, 1659, 1610, 1485, 1432, 1280 | 2.62 (s, 3H, COCH3), 2.95 (s, 6H, N(CH3)2 ), 3.36 (dd, 1H, >CHHA ), 4.14 (dd, 1H, >CHBH), 6.18 (dd, 1H, >CHAr), 6.80 - 8.00 (m, 12H, Ar), 9.32 (d, 1H, C´8 - H) | 77, 91, 127, 153, 168, 224, 237, 364, 392, 407 |
| 3ck | 3564, 3018, 2890, 1675, 1652, 1498, 1442, 1365, 1365, 1250 | 2.57 (s, 3H, COCH3), 2.98 (s, 6H, N(CH3)2 ), 3.28 (dd, 1H, >CHHA), 4.25 (dd, 1H, >CHBH), 6.04 (dd, 1H, >CHAr), 6.8 - 8.5 (m, 12H, Ar), 9.29 (d, 1H, C´8 - H), 11.13 (s, 1H, ArOH) | 69, 97, 126, 143, 169, 170, 183, 210, 350, 366, 380, 381, 408, 423 |
| 3dk | 3549, 3032, 2925, 1670, 1635, 1503, 1483, 1437, 1325, 1238, 780 | 2.63 (s, 3H, COCH3), 2.92 (s, 6H, N(CH3)2 ), 3.25 (dd, 1H, >CHHA), 4.22 (dd, 1H, >CHBH), 6.12 (dd, 1H, >CHAr), 7.10 - 8.6 (m, 11H, Ar), 9.17 (d, 1H,C´8 - H), 11.08 (s, 1H, ArOH) | 77, 91, 177, 203, 217, 225, 244, 384, 414, 422, 442, 457, 459 |
| 3ek | 3583, 3112, 2813, 1656, 1500, 1495, 1429, 1318, 1245 | 2.28 (s, 3H, ArCH3), 2.59 (s, 3H, COCH3), 2.92 (dd, 1H, >CHBH), 6.02 (dd, 1H, >CHAr), 6.2 - 8.5 (m, 11H, Ar), 9.23 (d, 1H, C´8 - H), 11.10 (s, 1H, ArOH) | 77, 91, 157, 183, 197, 225, 364, 380, 394, 420, 422, 437 |
| 3fk | 3050, 2836, 1665, 1606, 1510, 1455, 1232, 795 | 2.58 (s, 3H, COCH3), 2.97 (s, 6H, N(CH3)2 ), 3.38 (dd, 1H, >CHHA ), 4.16 (dd, 1H, >CHBH), 6.20 (dd, 1H, >CHAr), 7.00 - 8.20 (m, 11H, Ar), 9.35 (d, 1H, C´8 - H) | 77, 99, 126, 161, 187, 202, 215, 383, 398, 426, 441, 443 |
Antimicrobial activity tests of the 2-pyrazolines
| Compounds | MIC values (in mg/mL) against test organisms | |||||
|---|---|---|---|---|---|---|
| E. coli | S. aureus | K. pneumonae | S.typhii | S. dysentary | P. mirabilis | |
| 3,5-Bis (naphthalene -1- yl)-2-pyrazoline (3ag) | - | 125 | 63 | - | 125 | 125 |
| 5-(4-dimethylaminonaphthalene -1- yl)-3-(naphthalene -1- yl)-2 -pyrazoline (3bg) | 125 | 63 | 125 | 63 | 125 | - |
| 3-(2-Hydroxynaphthalene -1- yl)-5- (4-dimethylaminonaphthalene-1- yl)- 2-pyrazoline (3cg) | 63 | 63 | 31 | 63 | 125 | 63 |
| 3-(2-Hydroxy-4-chloronaphthalene- 1- yl)-5-(4 -dimethylamino-naphthalene-1- yl)-2 -pyrazoline (3dg) | 63 | 31 | 63 | 125 | - | 31 |
| 3 - (2-Hydroxy- 4 -methylnaphthalene -1 -yl ) -5- (4 –dimethylamino-naphthalene-1- yl) - 2 - pyrazoline (3eg) | 63 | 31 | 63 | - | 125 | 31 |
| 3 - (4 -Chloro-naphthalene-1- yl) - 5 - (4 -dimethylamino-naphthalene-1-yl) - 2 - pyrazoline (3fg) | 125 | 31 | 125 | 31 | 63 | - |
| 3,5 - Bis (naphthalene -1- yl) -1-phenyl- 2 -pyrazoline (3ah) | - | 125 | 125 | - | - | 125 |
| 5 -(4 -Dimethylamino-naphthalene-1-yl) - 3 -(naphthalene -1-yl) - 1 -phenyl - 2 - pyrazoline (3bh) | 125 | 125 | 63 | - | - | 63 |
| 3 - (2 - Hydroxy-naphthalene -1- yl) - 5 - (4 - dimethylamino-naphthalene- 1- yl) -1- phenyl- 2 -pyrazoline (3ch) | - | 63 | 31 | 125 | - | 125 |
| 3 - (2 -Hydroxy - 4 -chloro-naphthalene -1-yl ) -5 - (4 –dimethyl-aminonaphthalene -1-yl ) -1- phenyl - 2 - pyrazoline (3dh) | 63 | 31 | 31 | - | 125 | 63 |
| 3 - (2-Hydroxy-4-methyl-naphthalene -1-yl ) -5 - (4 -dimethylamino-naphthalene-1 - yl)-1-phenyl- 2 -pyrazoline (3eh) | 125 | 63 | 125 | 31 | 63 | 63 |
| 3 - (4 -Chloro-naphthalene -1- yl) -5 -(4 - dimethylamino-naphthalene -1-yl) - 1- phenyl- 2 – pyrazoline (3fh) | 63 | 125 | 31 | 63 | - | 125 |
| 1-Acetyl -3,5- bis (naphthalene -1 -yl) -2 - pyrazoline (3ak) | - | 63 | 63 | 125 | 63 | - |
| 1 - Acetyl - 5 - (4-dimethylamino-naphthalene -1- yl) -3 -(naphthalene -1 - yl) - 2 - pyrazoline (3bk) | 125 | 125 | 31 | 63 | 125 | - |
| 1 - Acetyl -3 - (2 - hydroxy -naphthalene -1- yl)-5 - (4 - dimethylamino-naphthalene -1- yl) - 2 -pyrazoline (3ck) | 63 | 63 | 31 | - | 125 | 63 |
| 1 - Acetyl -3 - (2 - hydroxy - 4 - chloro - naphthalene -1-yl) -5 - (4 -dimethylamino - naphthalene - 1- yl) - 2 - pyrazoline (3dk) | - | 31 | 31 | 63 | 125 | 31 |
| 1 - Acetyl -3 - (2 - hydroxy - 4 -methyl - naphthalene -1- yl) - 5 - (4 - dimethylamino - naphthalene - 1-yl) - 2 - pyrazoline (3ek) | - | 125 | 63 | 31 | 125 | 31 |
| 1 - Acetyl - 5 - (4 - dimethylamino- naphthalene -1- yl) - 3 - (4 - chloronaphthalene -1- yl) - 2 - pyrazoline (3fk) | 125 | 31 | 63 | 125 | 31 | - |
| 1 - Carboxamido -3,5 - bis (naphthalene -1- yl) - 2 - pyrazoline (3ai) | 125 | 63 | 31 | - | 63 | 125 |
| 1 - Carboxamido - 5 - (4 - dimethylaminonaphthalene -1- yl) - 3 - (naphthalene - 1- yl) - 2 - pyrazoline (3bi) | 63 | 63 | 31 | 63 | - | 125 |
| 1- Carboxamido -3 - (2 - hydroxy -naphthalene -1- yl) -5- (4 - dimethylamino - naphthalene -1- yl) - 2 - pyrazoline (3ci) | 63 | 31 | 16 | 125 | 63 | 31 |
| 1-Carboxamido -3- (2-hydroxy - 4 -chloro - naphthalene -1- yl) -5- (4 -dimethylamino - naphthalene -1- yl) - 2 - pyrazoline (3di) | 63 | 16 | 16 | 63 | 31 | 63 |
| 1-Carboxamido - 3 - (2 - hydroxy -4 - methyl naphthalene -1- yl) - 5 -(4 - dimethylaminonaphthalene -1- yl) - 2 -pyrazoline (3ei) | 125 | 31 | 63 | 31 | 63 | 63 |
| 1 - Carboxamido - 5 - (4 - dimethylaminonaphthalene -1- yl) - 3 - (4 - chloronaphthalene -1- yl) - 2 - pyrazoline (3fi) | 63 | 16 | 63 | 63 | 125 | - |
| Chloramphenicol (standard antibiotic) | - | 25 | 6 | 12 | 25 | 50 |
Acknowledgements
References
- Roelfvan, S. G.; Arnold, C.; Wellnga, K. J. Agric. Food Chem. 1979, 84, 406.
- Keats, G. H. Brit. Pat. 1,209,631, 1970.
- Kedar, R. M.; Vidhale, N. N.; Chincholkar, M. M. Orient. J. Chem. 1997, 13, 143.
- Singh, A.; Rathod, S.; Berad, B. N.; Patil, S. D.; Dosh, A. G. Orient. J. Chem. 2000, 16, 315.
- Katri, H. Z.; Vunii, S. A. J. Ind. Chem. Soc. 1981, 58, 168.
- Das, N. B.; Mittra, A. S. Ind. J. Chem. 1978, 16B, 638.
- Garge, H. G.; Chandraprakash. J. Pharm. Sc. 1971, 14, 649.
- Regaila, H. A.; El-Bayonk, A. K.; Hammad, M. Egypt. J. Chem. 1979, 20, 197.
- Krishna, R.; Pande, B. R.; Bharthwal, S. P.; Parmar, S. S. Eur. J. Med. Chem. 1980, 15, 567.
- Husain, M. I.; Shukla, S. Ind. J. Chem. 1986, 25B, 983.
- Tomilovi, Yu. V.; Okonnishnikova, G. P.; Shulishov, E. V.; Nefedov, O. M. Russ.Chem. Bt 1995, 44, 2114.
- Klimova, E. I.; Marcos, M.; Klimova, T. B.; Cecilio, A. T.; Ruben, A. T.; Lena, R. R. J. Organometallic Chem. 1999, 585, 106.
- Bhaskarreddy, D.; Padmaja, A.; Ramanareddy, P. V.; Seenaiah, B. Sulfur Lett. 1993, 16, 227.
- Padmavathi, V.; Sumathi, R. P.; Chandrasekhar, B. N.; Bhaskarreddy, D. J. Chem.Research 1999, 610.
- Bhaskarreddy, D.; Chandrasekhar, B. N.; Padmavathi, V.; Sumathi, R. P. Synthesis 1998, 491.
- Knorr, L. Ber. Dt. Chem. Ges. 1893, 26, 100.
- Thakare, V. G.; Wadodkar, K. N. Ind. J. Chem. Sect. B 1986, 25, 610.
- Ankhiwala, M. D.; Hathi, M. V. J. Ind. Chem. Soc. 1994, 71, 587.
- Vounauwers, K.; Muller. Ber. Dt. Chem. Ges. 1908, 41, 4230.
- Baker, A.; Butt, V. S. J. Chem. Soc. 1949, 2142.
- Kadu, V. B.; Dashi, A. G. Orient. J. Chem. 1997, 13(3), 285.
- Borkhade, K. T.; Marathey, M. G. Ind. J. Chem. 1972, 10, 48.
- Egorov, N. S. Antibiotics, A Scientific Approach; Mir Publishers: Moscow, 1985; pp. 170–177. [Google Scholar]
- Sample availability: Available from the authors.
© 2002 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Azarifar, D.; Shaebanzadeh, M. Synthesis and Characterization of New 3,5-Dinaphthyl Substituted 2-Pyrazolines and Study of Their Antimicrobial Activity. Molecules 2002, 7, 885-895. https://doi.org/10.3390/71200885
Azarifar D, Shaebanzadeh M. Synthesis and Characterization of New 3,5-Dinaphthyl Substituted 2-Pyrazolines and Study of Their Antimicrobial Activity. Molecules. 2002; 7(12):885-895. https://doi.org/10.3390/71200885
Chicago/Turabian StyleAzarifar, Davood, and Maseud Shaebanzadeh. 2002. "Synthesis and Characterization of New 3,5-Dinaphthyl Substituted 2-Pyrazolines and Study of Their Antimicrobial Activity" Molecules 7, no. 12: 885-895. https://doi.org/10.3390/71200885
APA StyleAzarifar, D., & Shaebanzadeh, M. (2002). Synthesis and Characterization of New 3,5-Dinaphthyl Substituted 2-Pyrazolines and Study of Their Antimicrobial Activity. Molecules, 7(12), 885-895. https://doi.org/10.3390/71200885




