Results and Discussion
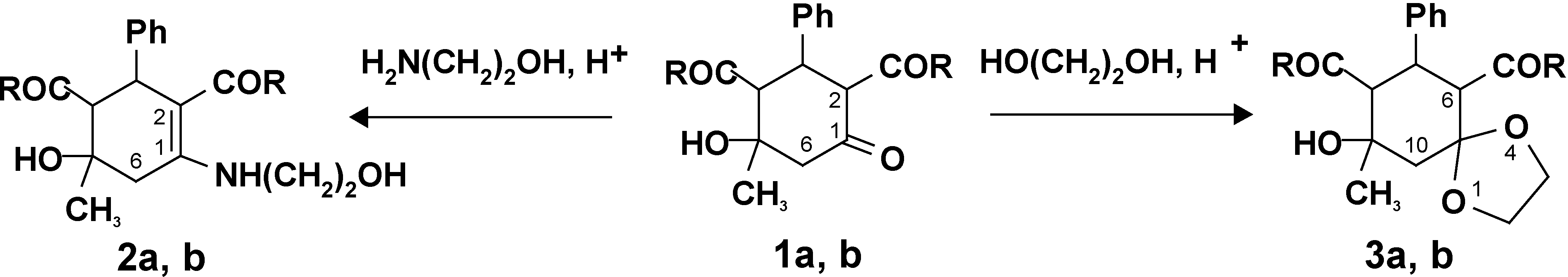
No reactions of the β-cycloketols with 1,4-binucleophilic reagents where ethanolamine is used as an amination agent have been studied. Several reaction paths may be expected: one or two reactive centers of the substrate and reagent may be involved. Enamines or the products of heterocyclization or spirocyclization may be produced. We established that when 2,4-diacetyl(diethoxycarbonyl)-5-hydroxy-5-methyl-3-phenylcyclohexanones (
1a,
b) [
2] are refluxed with ethanolamine in benzene in the presence or absence of acetic acid as a catalyst, 2,4-diacetyl(diethoxycarbonyl)-5-hydroxy-5-methyl-3-phenyl-N-oxyethylcyclohexenylamines (
2a,
b) were formed in 62-89% yield as the products of the selective amination of the carbonyl group of the alicycle (
Scheme 1).
Scheme 1.
Compounds 1-3: R = CH3(a); R = OEt(b)
Scheme 1.
Compounds 1-3: R = CH3(a); R = OEt(b)
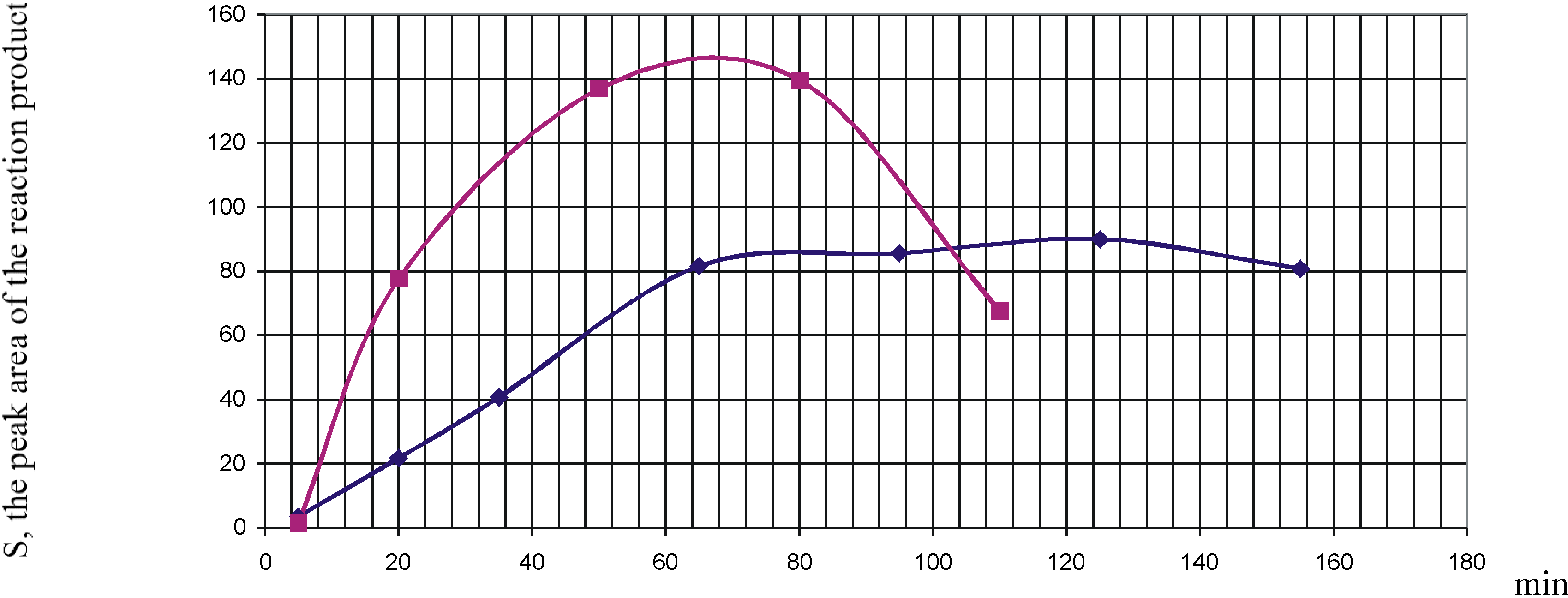
Using HPLC we found that the duration of the reaction depended on the presence of the acidic catalyst (3% CH
3COOH). E.g., with comparable yields, the concentration of product
2a,
b reaches its maximum in ~65 and ~110 min with and without the catalyst, respectively (
Figure 1).
Figure 1.
Kinetics of formation of 2,4-diacetyl-5-hydroxy-5-methyl-3-phenyl-N-oxyethyl-1-cyclohexenylamine (2a); boxes - the catalyst added, diamonds - no catalyst.
Figure 1.
Kinetics of formation of 2,4-diacetyl-5-hydroxy-5-methyl-3-phenyl-N-oxyethyl-1-cyclohexenylamine (2a); boxes - the catalyst added, diamonds - no catalyst.
The reactions of 3-R-2,4-diacetyl(diethoxycarbonyl)-5-hydroxy-5-methylcyclohexanones with binucleophilic nitrogen-free reagents have not been well studied. The reaction between 3-phenyl-2,4-dimethoxycarbonyl-5-hydroxy-5-methylcyclohexanone and dithioethylene glycol is the single example reported [
3]. The interaction of ethylene glycol with carbonyl compounds is known to produce dioxolanes as a rule, a reaction that is often used for protection of oxo-groups in a variety of syntheses.
To examine possible ketalization pathways, first we performed the reaction of 3-phenyl-2,4-diacetyl-5-hydroxy-5-methylcyclohexanone (1a) with ethylene glycol. The chosen substrate contains several different reactive centers susceptible to nucleophilic attack. The reaction has been found to proceed regioselectively via the carbonyl group of the alicycle to give 6,8-diacetyl-9-hydroxy-9-methyl-7-phenyl-1,4-dioxaspiro[4,5]decane (3a) in 66% yield. The carbonyl group of the nearest acetyl substituent is not touched. The acetyl group at C4 of the alicycle does not enter into reaction because of spatial hindrance. β-Ketol 1b bearing ethoxycarbonyl groups at the 2 and 4 positions reacts similarly.
The composition and structures of the substances obtained were determined by means of their elemental analysis, IR, UV, and
1H-NMR spectroscopy. In the
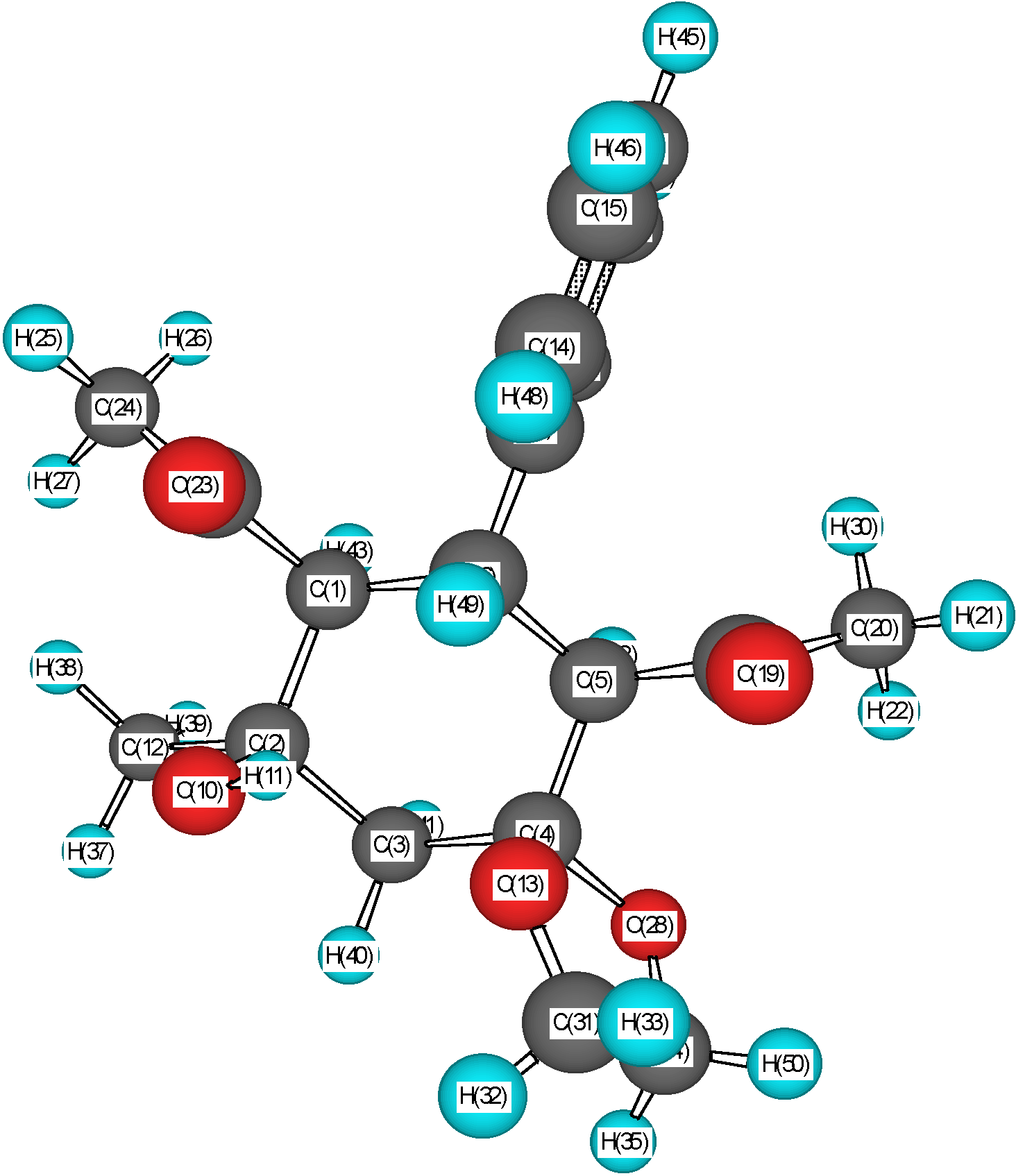
1H-NMR spectrum, the proton of the hydroxy group of the acetyl-containing spiran (
3a) manifests itself as a wide signal in a weaker field shifted (by approx. 1 ppm) in comparison with the spectrum of the initial ketol (
1a). This fact allows us to suggest the formation of intramolecular H-bonds (IHB) in the initial ketol and ketal. In the former case, as was established earlier [
4], the IHB appears between the hydrogen of the hydroxyl group and the oxygen of the acetyl substituent at C4. In the latter case, the hydrogen of the OH group may be bound to the oxygen atom of the dioxolane cycle as well. Such a supposition is proven by PM3 quantum-chemical calculations of the molecular geometry. The proton of the hydroxy group must be positioned between the oxygen atoms of the dioxolane fragment and the neighboring acetyl group (
Figure 2).
Figure 2.
Geometry of the heterospiran molecule (3a)
Figure 2.
Geometry of the heterospiran molecule (3a)
Experimental
General
The IR spectra were recorded on a Specord M80 in vaseline oil and hexachlorobutadiene (as a suspension). The 1H-NMR spectra were recorded on a Varian FT-80A with a resonance frequency of 80 MHz at 20-25°C, using 10-20% solutions in deuterated chloroform of the substances under study and tetramethylsilane as the internal reference. The UV spectra were recorded on a Specord M40 in 1 cm quartz cells in solutions in absolute ethanol or acetonitrile. Thin-layer chromatography was performed on Silufol-UV-254 plates; eluent 2:2:1 hexane-ether-chloroform; development with iodine vapours. HPLC was carried out on a Milichrom-5 device equipped with an Ultrasep 100 ES RP-18 column, 4 μm, 80 x 2 mm. The eluent was 30 and 50% acetonitrile.
2,4-diacetyl-5-hydroxy-5-methyl-3-phenyl-N-oxyethyl-1-cyclohexenylamine (2a).
A round-bottomed flask with a reflux condenser and a Dean-Stark trap was charged with β-cycloketol (1a, 0.014 mol), benzene (56 ml), and acetic acid (1 ml). After ketol dissolution by heating on a water bath, ethanolamine (1 mL, 0.014 mol) was added. The mixture was boiled for 4 hours and cooled. The precipitated product was washed with water and ethanol then recrystallized from ethanol to give 2a as white crystals; m.p. 147-149oC, 89% yield. Anal. Calcd. for C19H25O4N: С-68.86, Н-7.60, N-4.23; Found: С-69.03, Н-7.40, N-4.41; IR (thin film) cm-1: 3150-3100 (broad), 3328, 1704, 1540, 1568, 1252-1248; 1Н-NМR (CDCl3) δ: 1.24 (s, 3Н, C5-CH3), 1.60 (s, 3Н, C2COCH3), 1.64 (s, 3Н, C4COCH3), 2.48 (s, 1Н, CH2OH), 2.56 (d, 1Н, J=16 Hz, C6-Ha), 2.72 (d, 1Н, J=16 Hz, C6-Hе), 2.73 (d, 1Н, J=10 Hz, C4-H), 3.46 (t, 2Н, J=5.2 Hz, OCH2CH2N), 3.6 (s, 1Н, C5-OH), 3.81 (t, 2Н, J=5.2 Hz, OCH2CH2N), 4.12 (d, 1Н, J=10 Hz, C3-H), 7.15-7.26 (m, 5Н, C6H5), 9.04 (s, 1Н, N-H); UV nm: λmax=320.
2,4-diethoxycarbonyl-5-hydroxy-5-methyl-3-phenyl-N-oxyethyl-1-cyclohexenylamine (2b)
The same method was applied to β-cycloketol (1b) to obtain 2b. m.p. 149-150oC, 62% yield. Anal. Calcd. for C21H29O6N: С-64.45, Н-7.47, N-3.58; Found: С-65.02, Н-7.33, N-3.63; IR (thin film) cm-1: 3450-3210 (broad), 1734, 1580, 1640-1610, 1218; 1Н NМR (CDCl3) δ: 0.63 (t, 3Н, C4COCH2CH3), 0.73 (t, 3Н, C2COCH2CH3), 1.17 (s, 3Н, C5-CH3), 1.97 (d, 1Н, J=17 Hz, C6-Ha), 2.48 (d, 1Н, J=11 Hz, C4-H), 2.57 (d, 1Н, J=17 Hz, C6-Hе), 2.84 (s, 1Н, CH2OH), 2.91 (t, 2Н, J=5.0 Hz, OCH2CH2N), 3.34 (t, 2Н J=5.0 Hz, OCH2CH2N), 3.55-3.94 (m, 5Н, C2COCH2CH3, C4COCH2CH3 and C5-OH), 4.51 (d, 1Н, J=11 Hz, C3-H), 7.05-7.28 (m, 5Н, C6H5), 9.49 (s, 1Н, N-H); UV nm: λmax=298.
6,8-diacetyl(diethoxycarbonyl)-9-hydroxy-9-methyl-7-phenyl-1,4-dioxa-spiro[4,5]decane (3a,b).
The same technique was used but with different amounts of the reagents: ketol (1a,b, 0.023 mole), benzene (50 mL), and acetic acid (1.6 mL). After ketol dissolution, ethylene glycol (3 mL, 0.048 mole) was added. The system was boiled and cooled. The precipitated product was recrystallized from ethanol to afford 3a,b as white crystals. 3a: m.p. 138-139oC, 66% yield. Anal. Calcd. for C19H24O5: С-68.66, Н-7.28; Found: С-68.72, Н-7.45; IR (thin film) cm-1: 3432, 1716-1694, 1156, 1120, 1076, 1040; 1Н NМR (CDCl3) δ: 1.23 (s, 3Н, C9-CH3), 1.51 (s, 3Н, C8COCH3), 1.94 (s, 3Н, C6COCH3), 2.51 (d, 1Н, J=15 Hz, C10-Ha), 2.59 (d, 1Н, J=12 Hz, C8-H), 2.89 (d, 1Н, 12 Hz) , C6-H, 3.14-3.49 (m, 4Н, ОCH2CH2O), 3.90 (d, 1Н, J=15 Hz, C10-He), 4.03 (t, 1Н, 12 Hz, C7-H), 4.45 (s, 1Н, C9-OH), 6.91-7.15 (m, 5Н, C6H5); 3b: m.p. 129-130oC, 63% yield. Anal. Calcd. for C21H28O7: С-64.27, Н-7.19; Found: С-64.66, Н-7.29; IR (thin film) cm-1: 3508-3400, 1730-1726, 1706, 1170, 1145, 1090, 1036; 1Н NМR (CDCl3) δ: 0.48 (t, 3Н, J=6,77 Hz, C8COCH2CH3), 0.79 (t, 3Н, J=6,77 Hz, C6COCH2CH3), 1.02 (s, 3Н, C9-CH3), 1.81 (d of d, 1Н, J=15 Hz, J=1.8 Hz, C10-Ha), 2.62 (d, 1Н, J=15 Hz, C10-Hе) 2.64 (d, 1Н, J=12 Hz, C8-H), 3.38-3.66 (m, 5Н, ОCH2CH2O and C6-H), 3.74 (d, 1Н, J=1.8 Hz, C9-OH), 3.70-3.98 (m, 4Н, C6COCH2CH3 and C8COCH2CH3), 4.23 (t, 1Н, J=12 Hz, C7-H), 7.0-7.2 (m, 5Н, C6H5).