Synthesis of New Polyfused Heterocycles of Biological Importance by Means of Pd(0) Catalysis
Abstract
:Introduction
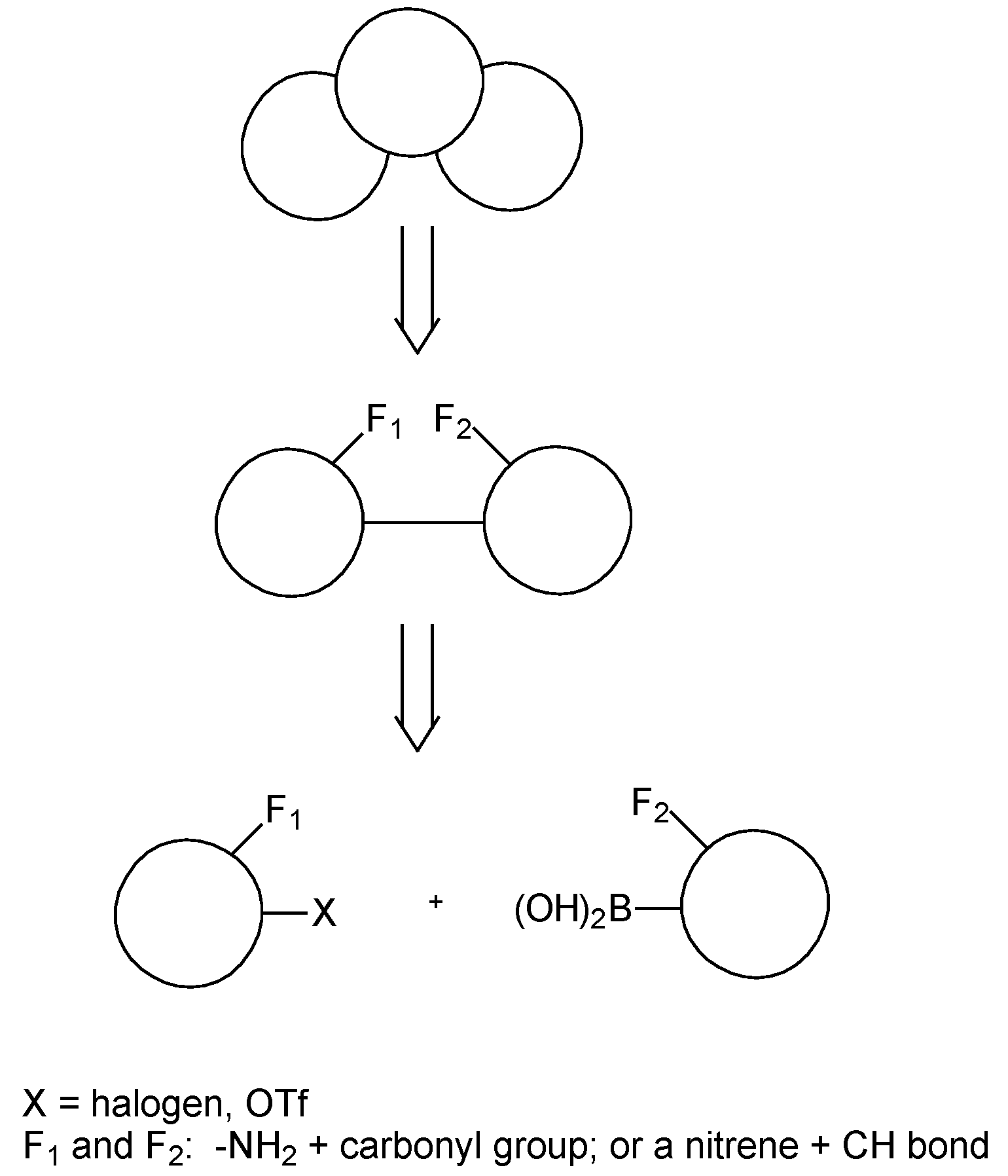
Results and Discussion
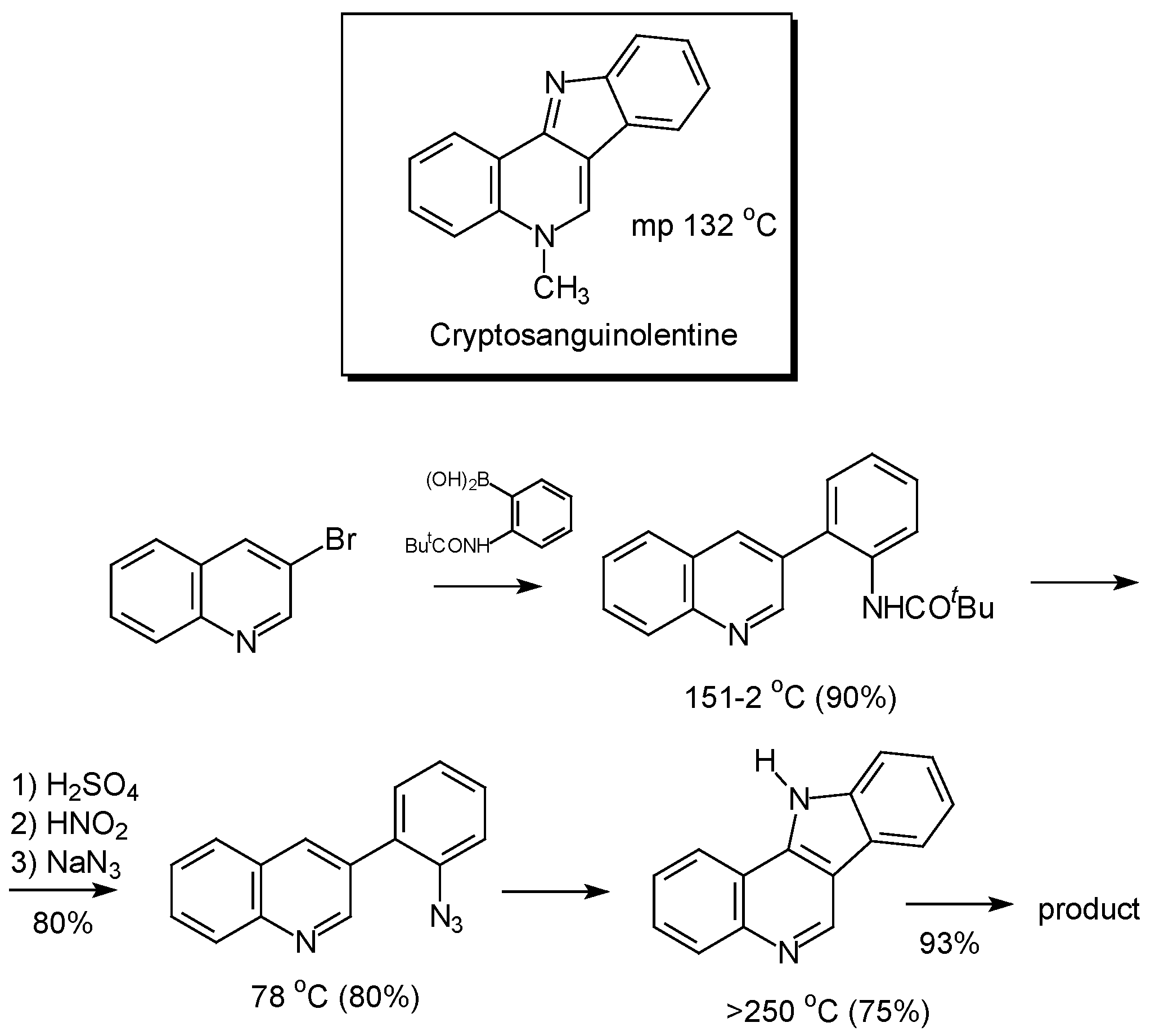


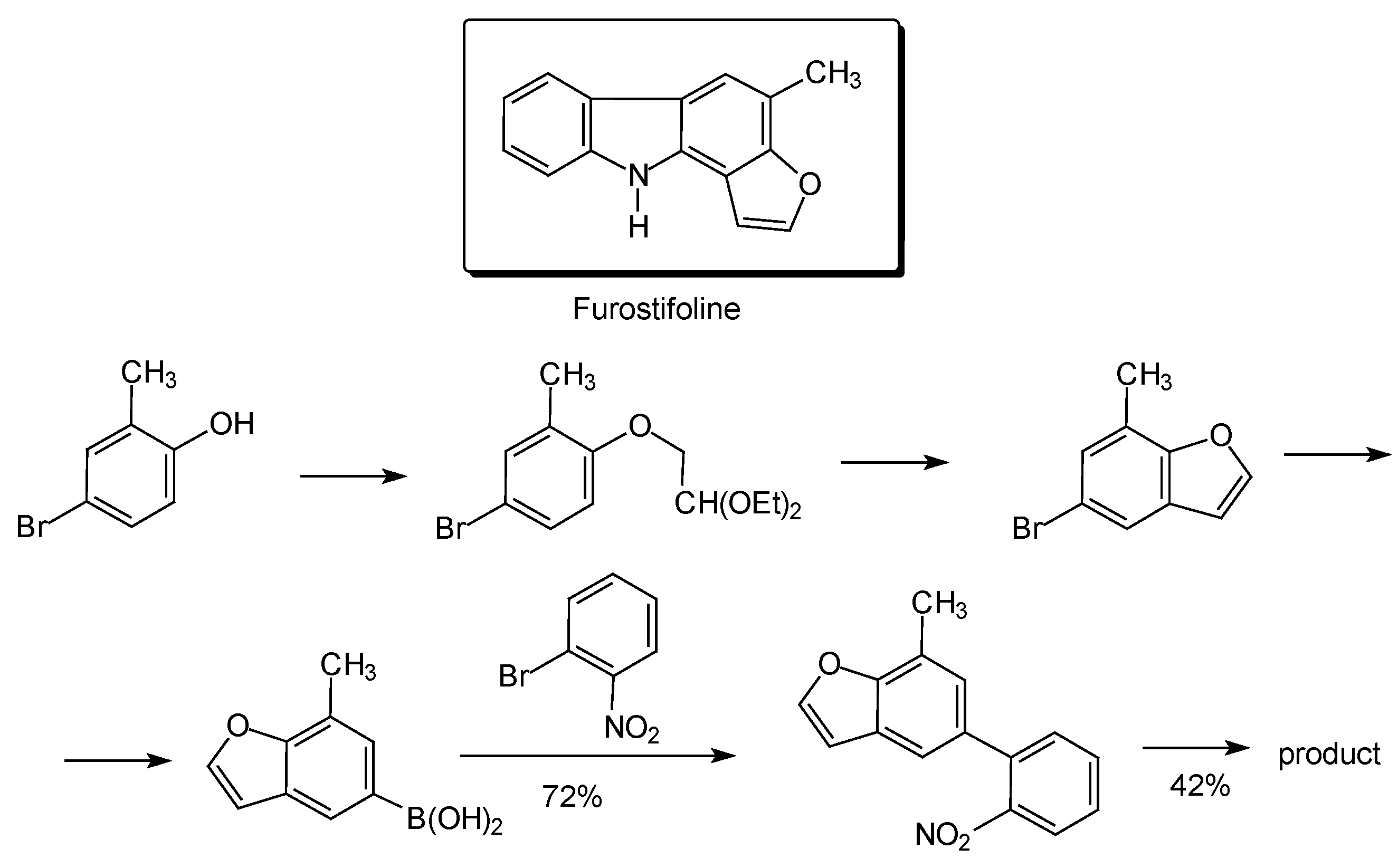
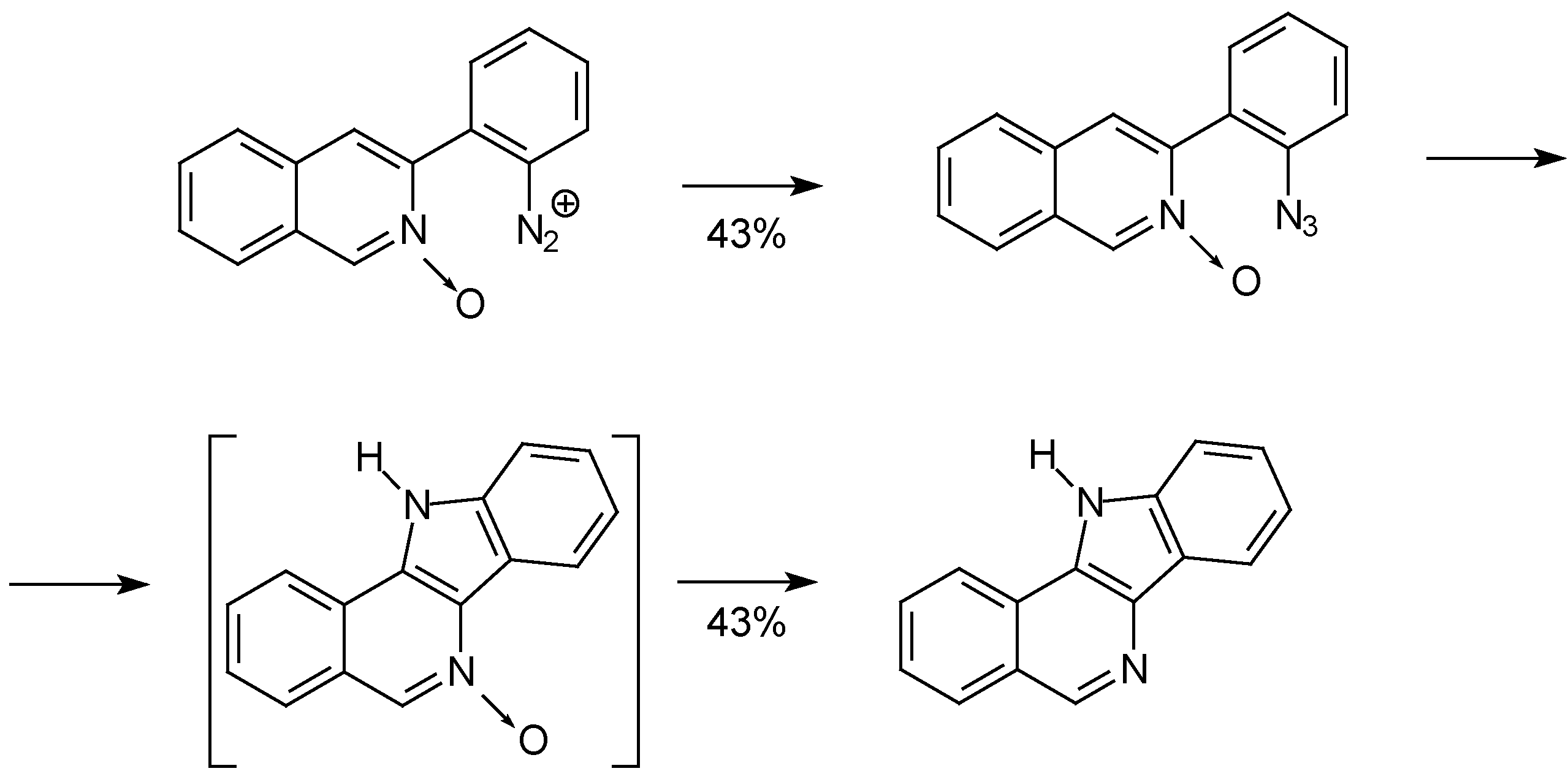
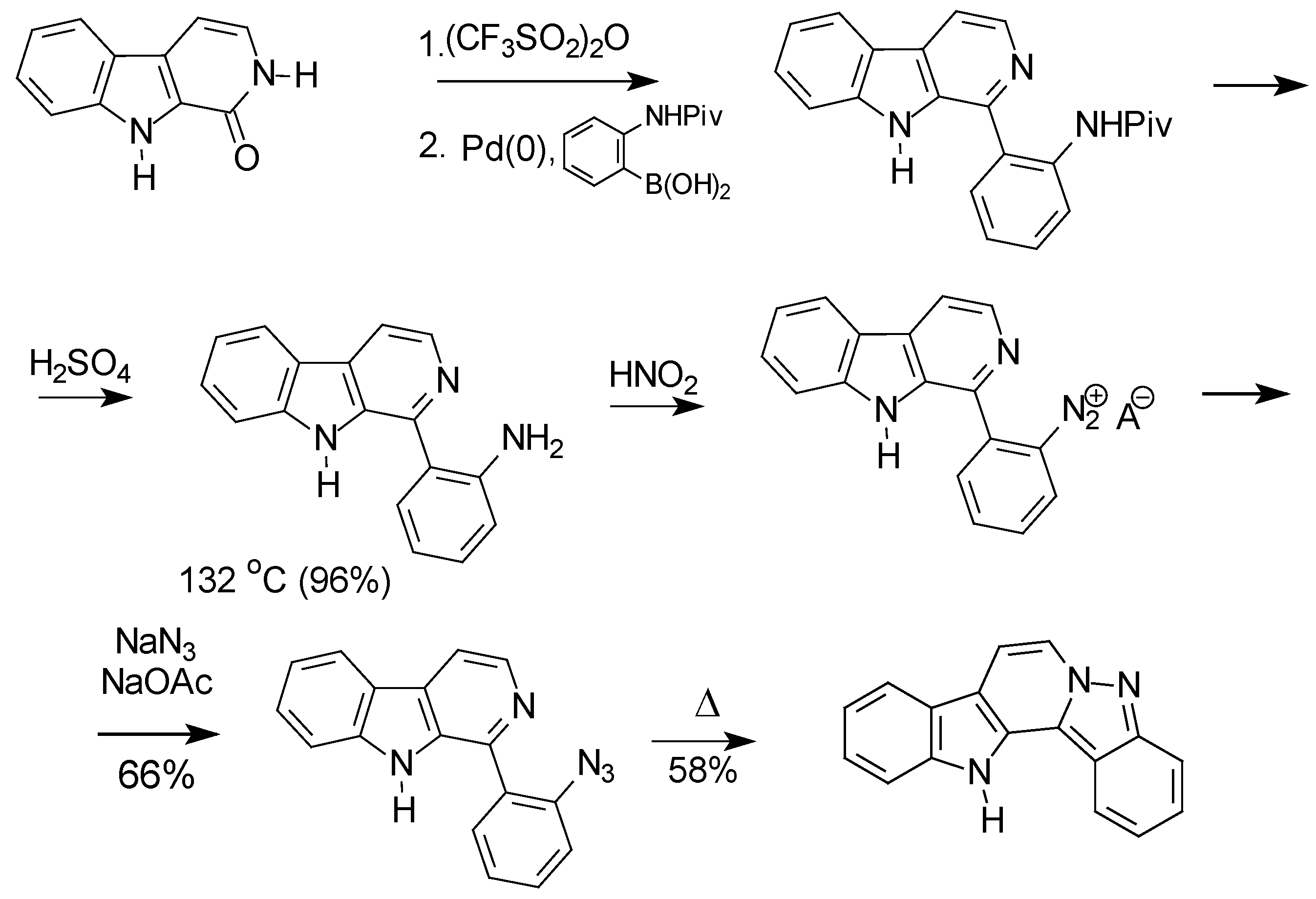
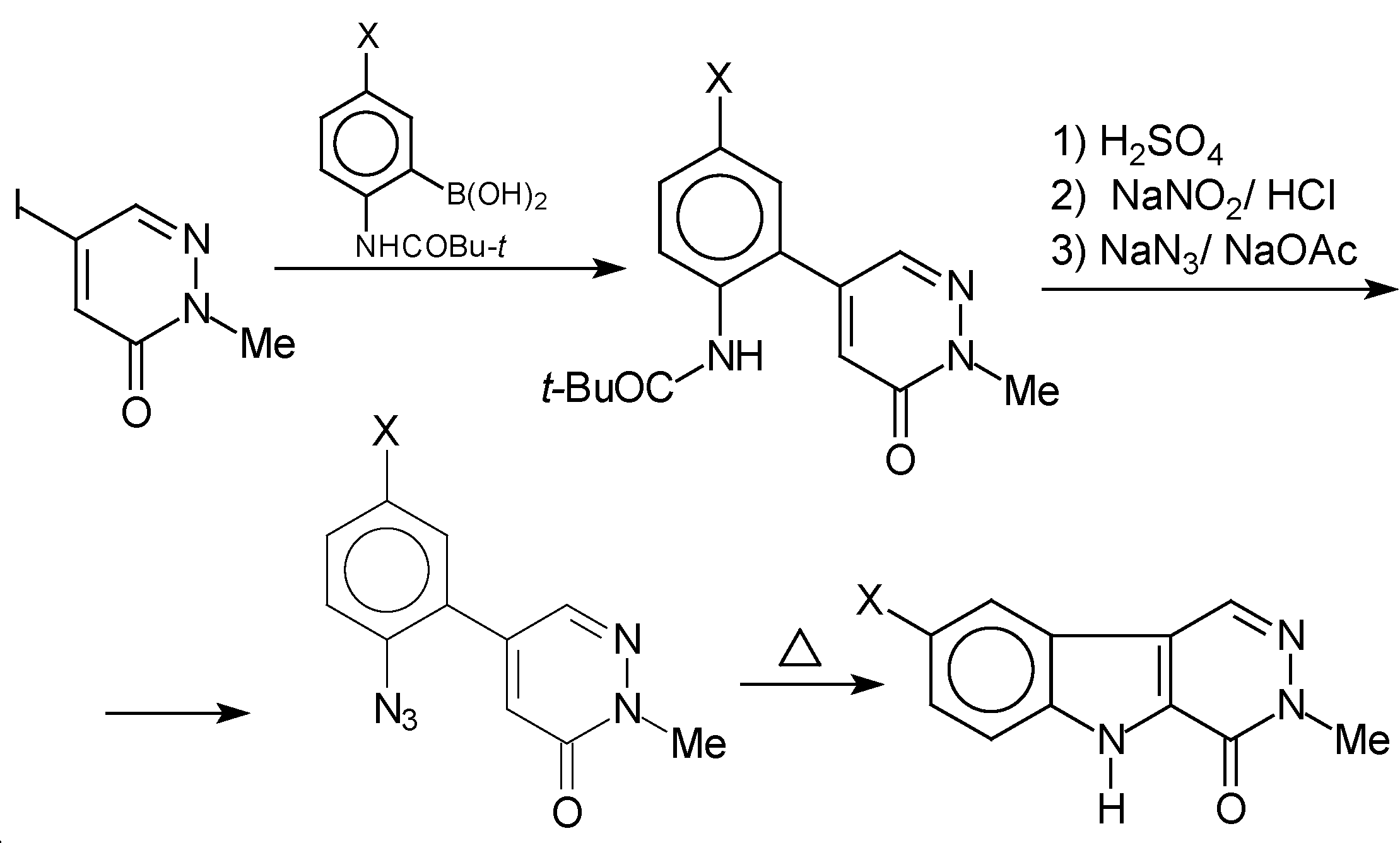
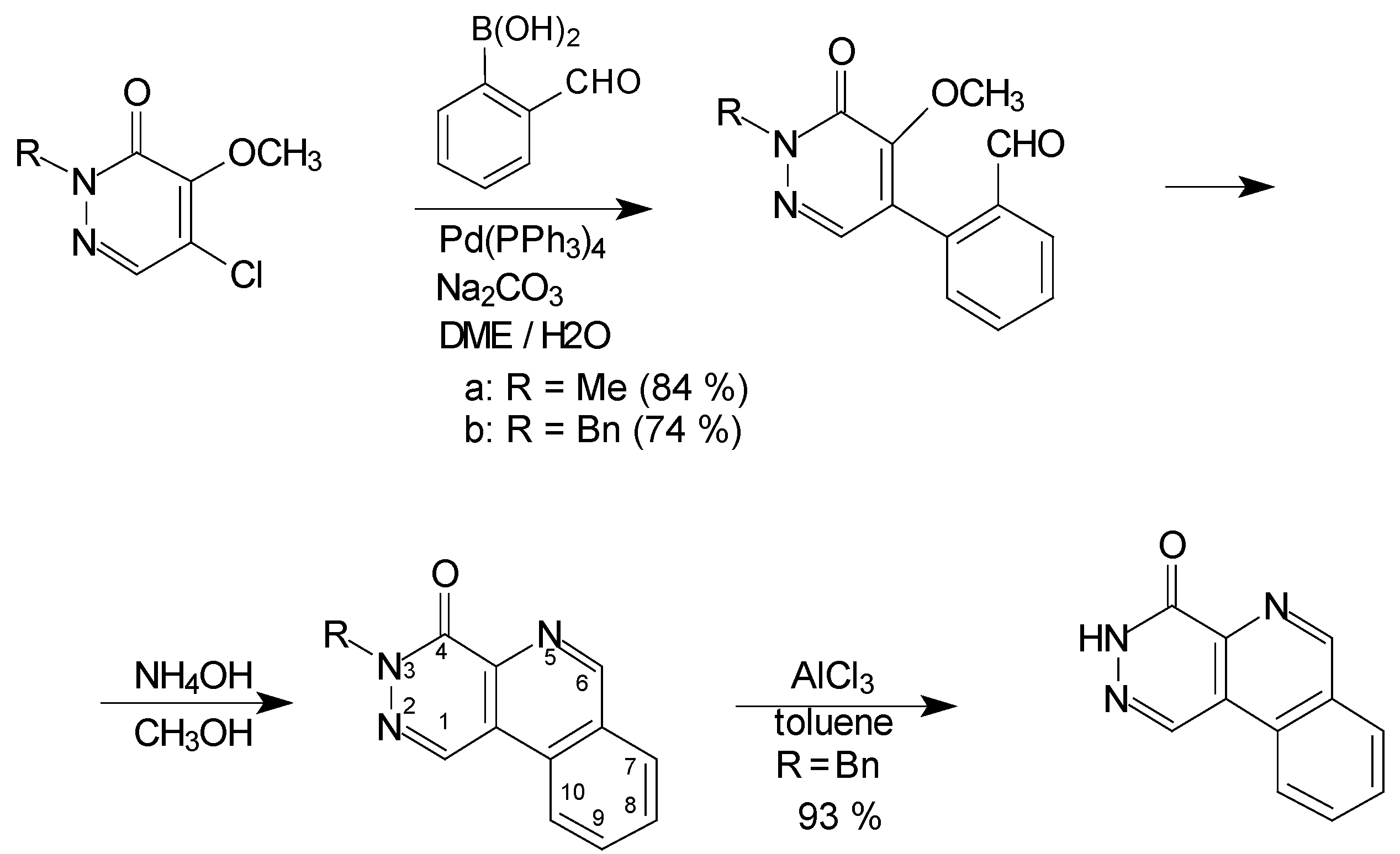
Conclusions
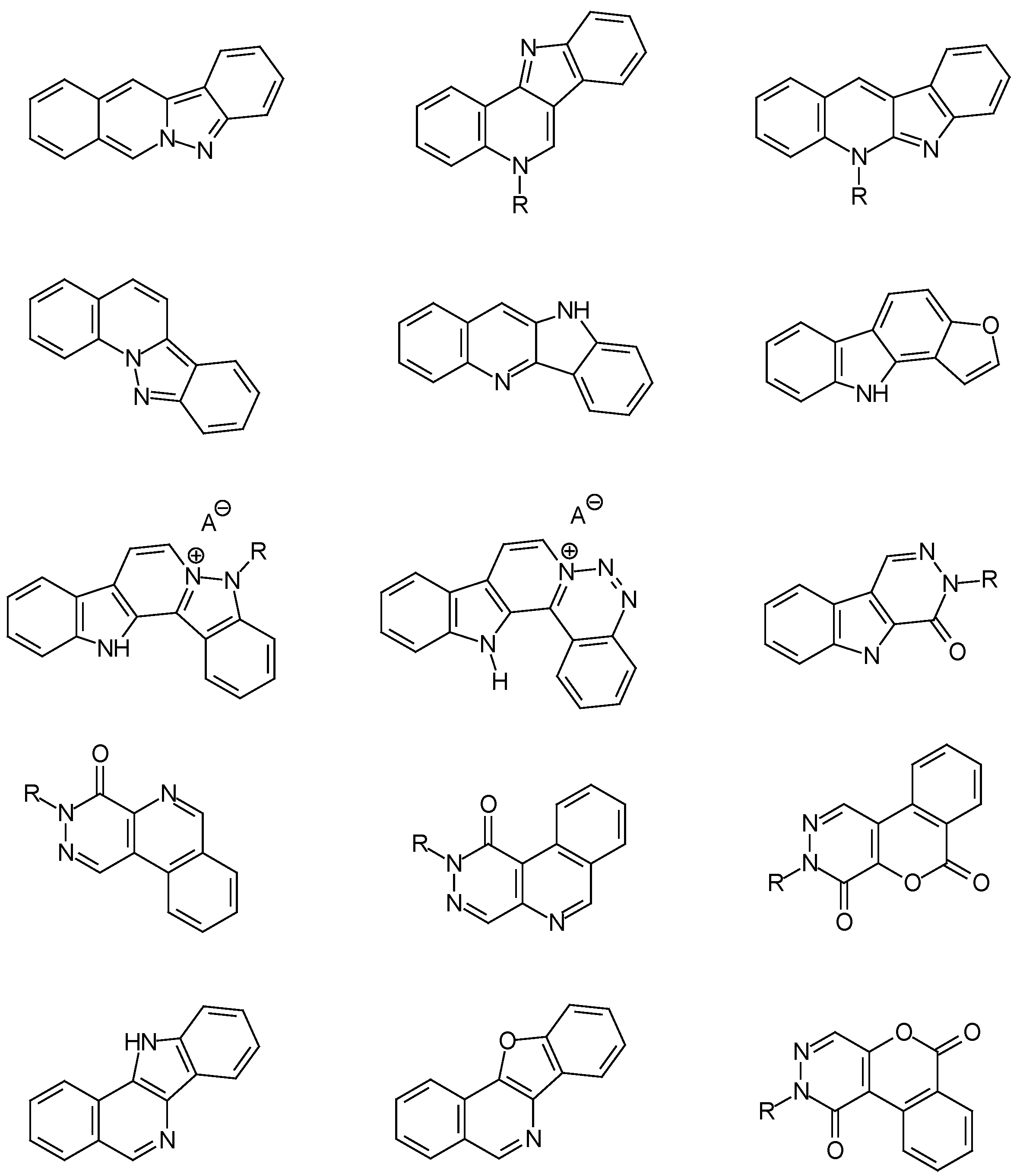
Acknowledgments
References
- Timári, G.; Soós, T.; Hajós, Gy.; Messmer, A.; Nacsa, J.; Molnár, J. Synthesis of Novel Ellipticine Analogues and Their Inhibition of Moloney Leukemia Reverse Transcriptase. Bioorg. Med. Chem. Lett. 1996, 6, 2831–2836. [Google Scholar]
- Hajós, Gy.; Riedl, Zs.; Molnár, J.; Szabó, D. Non-nucleoside reverse transcriptase inhibitors. Drugs of the Future. 2000, 25, 47–62. [Google Scholar]
- Timári, G.; Hajós, Gy.; Riedl, Zs.; Béres, M.; Soós, T.; Messmer, A. Synthesis of Polyfused Heteroaromatics by Palladium-catalysed Cross-coupling Reaction. Molecules 1996, 1, 1–5. [Google Scholar]
- Timári, G.; Soós, T.; Hajós, Gy. A Convenient Synthesis of Two New Indoloquinoline Alkaloids. Synlett 1997, 1067–1068. [Google Scholar]
- Csányi, D.; Timári, G.; Hajós, Gy. An alternative synthesis of quindoline and one of its closely related derivative. Synth. Commun. 1999, 29, 3959–3969. [Google Scholar]
- Soós, T.; Timári, G.; Hajós, Gy. A New and Concise Synthesis of Furostifoline. Tetrahedron Lett. 1999, 40, 8607–8609. [Google Scholar]
- Béres, M.; Timári, G.; Hajós, Gy. Straightforward synthesis of 11H-indolo[3,2-c]isoquinoline and benzofuro[3,2-c]isoquinoline by ring transformation. Tetrahedron Lett. 2002, 43, 6035–6038. [Google Scholar]
- Csányi, D.; Hajós, Gy.; Riedl, Zs.; Timári, G.; Bajor, Z.; Cochard, F.; Sapi, J.; Laronze, J.-Y. Synthesis of two New Heteroaromatic ß-Carboline-fused Pentacycles. Observation of a New Intercalating Agent. Bioorg. Med. Chem. Lett. 2000, 10, 1767–1769. [Google Scholar]
- Krajsovszky, G.; Mátyus, P.; Riedl, Zs.; Csányi, D.; Hajós, Gy. New Synthetic Approach to Pyridazino[4,5-b]indoles by Pd(0)-Catalyzed Cross-Coupling Reaction. Heterocycles 2001, 55, 1105–1111. [Google Scholar]
- Riedl, Zs.; Maes, B.U.W.; Monsieurs, K.; Lemiere, G.L.F.; Mátyus, P.; Hajós, Gy. Synthesis of pyridazino [4,5-c] isoquinolinones by Suzuki cross-coupling reaction. Tetrahedron 2002, 58, 5645–5650. [Google Scholar]
- Sharples, D.; Molnár, J.; Szabó, D.; Hajós, Gy.; Riedl, Zs.; Csányi, D. Ellipticine Analogues and Related Compounds as Inhibitors of Reverse Transciptase and as Inhibitors of the Efflux Pump. Arch. Pharm. 2001, 331, 269–274. [Google Scholar]
© 2003 by MDPI ( http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
Share and Cite
Hajós, G.; Riedl, Z.; Timári, G.; Mátyus, P.; Maes, B.U.W.; Lemière, G.L.F. Synthesis of New Polyfused Heterocycles of Biological Importance by Means of Pd(0) Catalysis. Molecules 2003, 8, 480-487. https://doi.org/10.3390/80600480
Hajós G, Riedl Z, Timári G, Mátyus P, Maes BUW, Lemière GLF. Synthesis of New Polyfused Heterocycles of Biological Importance by Means of Pd(0) Catalysis. Molecules. 2003; 8(6):480-487. https://doi.org/10.3390/80600480
Chicago/Turabian StyleHajós, Gy., Zs. Riedl, G. Timári, P. Mátyus, B. U. W. Maes, and G. L. F. Lemière. 2003. "Synthesis of New Polyfused Heterocycles of Biological Importance by Means of Pd(0) Catalysis" Molecules 8, no. 6: 480-487. https://doi.org/10.3390/80600480



